ISSN: 0973-7510
E-ISSN: 2581-690X
The study of efficiency of Cyathus spp. to control plant pathogenic bacteria and fungi the results showed that some Cyathus spp. effectively control plant pathogenic bacteria, such as Pectobacteria sp., Pseudomonas sp., Ralstonia solanacearum and Xanthomonas campestris pv. vesicatoria in vitro. The most effective to control Pectobacteria sp. was C. striatus KKU4 with 0.62 cm clear zone diameters. Therefore C. striatus KKU6 could control Pseudomonas sp. with 1.34 cm clear zone diameters, C. pallidus KKUITN2/KKULN2 could control R. solanacearum with 0.85 cm clear zone diameters and C.striatus KKU 5 was the most effective to control X. campestris pv. vesicatoria with 0.29 cm clear zone diameters. The study of antagonistic activity against plant pathogenic fungi by dual culture technique, the results showed that some species could control Fusarium sp. including C. striatus KKU3 and C. earlei KKULP1 with 47.94% and 56.0% inhibition, respectively when placing zero and four days before Fusarium sp. C. stercoreus KKUITP2/KKULP2 and KKUITP3/KKULP3 could control Pythium aphanidermatum when placing before P. aphanidermatum for four days. None of the isolates could control Rhizoctonia solani and Sclerotium rofsii.
Biological control, Bird’s nest fungi, Cyathus
Cyathus species are saprobic since they obtain nutrients from decomposing organic matter. They usually grow on decaying wood or woody debris, on cow and horse dung, or directly on humus – rich soil. The genus Cyathus Haller belongs to the family Nidulariaceae, which is included in the agaricoid clade of Basidiomycota1,2 and it is morphologically characterized by a three-layered bell or vase-shaped basidiomata with lenticular structures (peridioles) fixed to the inner wall by the funicular cord. Several Cyathus species produce bioactive compounds, some with medicinal properties, and several lignin degrading enzymes from the genus may be useful in bioremediation and agriculture. C. olla has the potential of being developed into a microbial inoculant aimed at reducing the incidence of stubble-borne disease of canola. Isolates were cultured from field collected specimens and were also obtained from culture collections for morphological and molecular studies3. Cyathus striatus No.12 is highly active against fungi imperfecti and a variety of gram-positive bacteria, as well as against some gram-negative bacteria4. El-Fallal and Moussa5 reported that eight basidiomyces, including Cyathus stercoreus, were tested to antagonize Ralstonia solanacearum (causal agent of brown rot disease of potato) in vitro. All of these fungi inhibited the growth of R. solanacearum, and the largest inhibition zones were recorded with C. stercoreus Egyptian strain and Agaricus campester Egyptian strain. Sutthisa and Sanoamuang6 studied the ability of bird’s nest fungi (Cyathus sp.) to inhibit the mycelial growth of soil-borne plant pathogenic fungi by the dual culture technique. The results showed that Cyathus sp. inhibited the mycelial growth of Fusarium oxysporum f.sp. lycopersici (FOL), but did not inhibit Pythium aphanidermatum, Sclrotium rolfsii and Rhizoctonia sp., which had 62.22 – 63.64 %inhibition of mycelial growth and 21.84 – 22.46 %overgrowth. The culture filtrate of Cyathus showed no effect on FOL conidial germination but did affect the mycelium growth.
The objective of this research is to study antagonistic ability of bird’s nest fungi (Cyathus sp.) against plant pathogenic bacteria and fungi for basic information to applied in the further research.
Organisms
Twelve isolates of Cyathus spp. including Cyathus striatus (KKU1, KKU2, KKU3, KKU4, KKU5 and KKU6), Cyathus berkeleyanus (KKUNN1), Cyathus earlei (KKULP1), Cyathus pallidus (KKUITN2/KKULN2 and KKITN3/KKULN3) and Cyathus stercoreus (KKUITP2/KKULP2 and KKITP3/KKULP3) were obtained from Khon Kaen University Culture Collection (KKU). Plant pathogenic bacteria and fungi such as Pectobacterium sp., Pseudomonas sp., Ralstonia solanacearum, Xanthomonas campestris pv. vesicatoria, Fusarium sp., Pythium aphanidermatum, Rhizoctonia solani and Sclerotium rofsii were obtained from the Branch of Plant Pathology, Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University, Thailand.
Efficacy of Cyathus spp. controlling plant pathogenic bacteria
The agar plug technique was used to test the ability of Cyathus spp. to control plant pathogenic bacteria, such as Pectobacterium sp., Pseudomonas sp., Ralstonia solanacearum and Xanthomonas campestris pv. vesicatoria. Spread bacterial cell suspension (104 cfu/ml) on PDA plate after placing a 0.7 cm diameter agar disc of 10 days old Cyathus spp. at four points in a cross pattern and incubated at 28 ± 2°C, and after 24-48 hr observe and measure clear zone. There were five replications per treatment.
Efficacy of Cyathus spp. controlling plant pathogenic fungi
Cyathus spp. isolates were tested in vitro for their antagonistic activity against Fusarium sp., Pythium aphanidermatum, Rhizoctonia solani and Sclerotium rofsii using the dual culture technique. Two experiments were conducted: first, placing a 0.7 cm diameter disc of 10 days old Cyathus sp. on PDA plate and then placing a 0.7 cm diameter disc of each plant pathogenic fungi. The discs were put a 1.5 cm distance from the edge so they opposed each other on the same diagonal line. Second, placing Cyathus sp. four days before placing plant pathogenic fungi. Five plates were considered for each fungus-fungus interaction and daily measurements of the radial distance of Cyathus sp. and the plant pathogenic fungi were made. The percentage of inhibition and the percentage of overgrowth were calculated following Landum et al.7
Statistical analysis of data
All the data were subject to analysis of variance (ANOVA) and treatment mean comparisons were performed using least significant differences at P=0.05 by (LSD).
Efficacy of Cyathus spp. controlling plant pathogenic bacteria
The ability of Cyathus spp. to control plant pathogenic bacteria was tested by the dual culture technique. The results showed the best inhibitors included C. striatus KKU4 that could inhibit Pectobacteria sp. with a 0.62 cm clear zone diameter, which was significantly different from the others isolates. C. striatus KKU6 could inhibit Pseudomonas sp. with a 1.34 cm clear zone diameter, which was significantly different from the others isolates. C. pallidus KKUITN2/KKULN2 and KKUITN3/KKULN3 could inhibit R. solanacearum with 0.85 cm and 0.83 cm clear zone diameters, respectively. C. striatus KKU3 and KKU5 had significantly different inhibitions of X. campestris pv. vesicatoria with 0.26 cm and 0.29 cm clear zone diameters, respectively (Table 1, Fig. 1).
Table (1):
Efficacy of Cyathus spp. to control plant pathogenic bacteria by dual culture technique.
| Isolates | Clear zone diameter (cm) | |||
|---|---|---|---|---|
| Pectobacterium sp. | Pseudomonas sp. | Ralstonia solanacearum | Xanthomonas campestris pv. vesicatoria | |
| C. striatus | ||||
| KKU1 | 0.27±0.06 de | 0.69±0.13 de | 0.31±0.03 g | 0.23±0.01 c |
| KKU2 | 0.38±0.14 c | 0.91±0.16 b | 0.71±0.23 bc | 0.23±0.10 c |
| KKU3 | 0.21±0.01 e | 0.65±0.05 e | 0.29±0.04 g | 0.26±0.01 ab |
| KKU4 | 0.62±0.10 a | 0.66±0.11 e | 0.58±0.09 de | 0.00±0.00 d |
| KKU5 | 0.51±0.08 b | 0.96±0.18 b | 0.40±0.09 fg | 0.29±0.05 a |
| KKU6 | 0.00±0.00 f | 1.34±0.13 a | 0.53±0.09 e | 0.24±0.06 bc |
| C. berkeleyanus | ||||
| KKUNN1 | 0.28±0.03 de | 0.29±0.13 g | 0.33±0.22 g | 0.00±0.00 d |
| C. pallidus | ||||
| KKUITN2/KKULN2 | 0.21±0.06 de | 0.38±0.10 fg | 0.85±0.17 a | 0.00±0.00 d |
| KKUITN3/KKULN3 | 0.33±0.10 cd | 0.43±0.10 f | 0.83±0.09 ab | 0.00±0.00 d |
| C. earlei | ||||
| KKULP1 | 0.27±0.09 de | 0.78±0.05 d | 0.69±0.23 cd | 0.24±0.03 bc |
| C. stercoreus | ||||
| KKUITP2/KKULP2 | 0.00±0.00 f | 0.66±0.14 e | 0.00±0.00 h | 0.00±0.00 d |
| KKUITP3/KKULP3 | 0.30±0.10 d | 0.85±0.09 bc | 0.48±0.01 ef | 0.00±0.00 d |
| CV (%) | 27.70 | 16.95 | 26.82 | 30.57 |
Note: Different superscripts within same column indicated significant difference (P < 0.05).
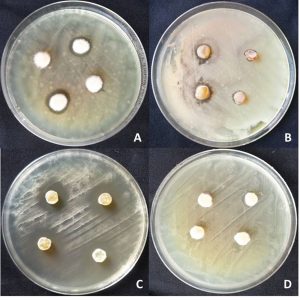
Fig. 1. Efficacy of Cyathus sp. controlling plant pathogenic bacteria by dual culture technique.
A: C. striatus KKU4 + Pectobacteria sp.,
B: C. striatus KKU6 + Pseudomonas sp.,
C: C. pallidus KKUITN3/KKULN3 + Ralstonia solanacearum,
D: C. striatus KKU3 + Xanthomanas campestris pv. vesicatoria
Efficacy of Cyathus sp. controlling plant pathogenic fungi
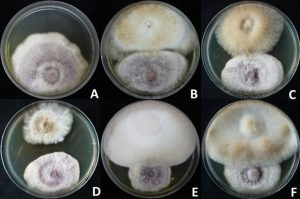
Cyathus spp. isolates were tested in vitro for their antagonistic activity against Fusarium sp., P. aphanidermatum, R. solani and S. rofsii using the dual culture technique. The results show that none of the isolates could inhibit P. aphanidermatum, Rhizoctonia sp. and S. rofsii, due to the plant pathogenic fungi mycelial growing rapidly and covered the Cyathus spp. colony. It was found that C. earlei KKULP1, C. stercoreus KKUITP2/KKULP2 and KKUITP3/KKULP3 could inhibit the mycelial growth of P. aphanidermatum with 54.31, 48.28% and 47.41 %inhibition and 20%, 21.67% and 20 %overgrowth, respectively (Fig. 2). All isolates could inhibit the mycelial growth of Fusarium sp. in both experiments. Except, C. berkeleyanus KKUNN1, C. pallidus KKUITN2/KKULN2 and KKUITN3/KKULN3 because they grew slowly. The most effective control of Fusarium sp. in experiment I was C. striatus KKU3 with 47.94 %inhibition and 10 %overgrowth, and in experiment II it was C. earlei KKULP1 with 56.90 %inhibition and a clear zone (Table 2, Fig. 3).
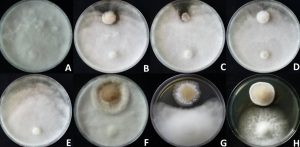
Fig. 2. Efficacy of Cyathus sp. controlling Pythium aphanidermatum. Top- Placing Cyathus sp. and P. aphanidermatum on same day. Bottom- Placing Cyathus sp. four days before P. aphanidermatum.
A, E: Control; B, F: C. striatus KKU1; C, G: C. earlei KKULP1; D, H: C. stercoreus KKUITP3/KKULP3
Table (2):
Efficacy of Cyathus spp. controlling Fusarium sp. by dual culture technique.
| Isolates | Inoculation Cyathus spp. before pathogen | |||
|---|---|---|---|---|
| 0 days | 4 days | |||
| % Inhibition | % Overgrowth | % Inhibition | % Overgrowth | |
| C. striatus | ||||
| KKU1 | 11.38±3.24 c | 27.50±3.54 a | 27.59±14.63 f | 15.00±7.07 e |
| KKU2 | 18.22±9.11 cd | Clear zone | 34.48±0.00 e | Clear zone |
| KKU3 | 47.94±23.97 a | 10.00±0.00 d | 44.83±0.00 bc | 42.50±10.61 a |
| KKU4 | 31.85±15.93 b | 20.00±14.14 bc | 41.83±0.00 cd | 35.00±0.00 b |
| KKU5 | 13.64±6.82 d | 17.50±3.54 c | 39.66±7.32 d | 20.00±0.00 d |
| KKU6 | 22.76±11.38 c | 12.50±3.54 d | 48.28±4.87 b | 17.50±3.54 de |
| C. berkeleyanus | ||||
| KKUNN1 | 0.00±0.00 e | 0.00±0.00 e | 0.00±0.00 g | 0.00±0.00 f |
| C. pallidus | ||||
| KKUITN2/KKULN2 | 0.00±0.00 e | 0.00±0.00 e | 0.00±0.00 g | 0.00±0.00 f |
| KKUITN3/ KKULN3 | 0.00±0.00 e | 0.00±0.00 e | 0.00±0.00 g | 0.00±0.00 f |
| C. earlei | ||||
| KKULP1 | 9.09±0.00 cd | Clear zone | 56.90±2.44 a | Clear zone |
| C. stercoreus | ||||
| KKUITP2/ KKULP2 | 11.38±3.24 c | 22.50±3.54 b | 31.04±4.87 ef | 32.50±3.54 b |
| KKUITP3/ KKULP3 | 9.09±0.00 cd | 22.50±3.54 b | 34.48±0.00 e | 27.50±10.61 c |
| CV (%) | 34.39 | 42.34 | 17.31 | 31.57 |
Note: Different superscripts within same column indicated significant difference (P < 0.05).
Efficacy tests of bird’s nest fungi to control plant pathogenic bacteria and fungi by the dual culture technique were performed, and we found that some isolates could inhibit Pectobacteria sp., Pseudomonas sp., R. solanacearum and X. campestris pv. vesicatoria with different clear zone diameters. Some isolates could control Fusarium sp. when both placing the bird’s nest fungi and Fusarium sp. on the same date and when placing the Fusarium sp. four days before with overgrowth and clear zone mechanism. Except C. berkeleyanus KKUNN1, C. pallidus KKUITN2/KKULN2 and KKUITN3/KKULN3 because of their slow growth. Only C. stercoreus KKUITP2/KKULP2 and KKITP3/KKULP3 could control P. aphanidermatum when placing four days before P. aphanidermatum. Moreover, we found that after P. aphanidermatum was covered with bird’s nest fungi the day after that bird’s nest fungi was placed it recover growth and covered P. aphanidermatum. None of the isolates could inhibit Rhizoctonia sp. and S. rofsii due to the plant pathogenic fungi’s rapid mycelial growth that covered the bird’s nest fungi colony. El-Fallal and Moussa5 reported that eight basidiomyces, including Cyathus stercoreus, were tested to antagonize Ralstonia solanacearum (causal agent of brown rot disease of potato) in vitro. All of these fungi inhibited the growth of R. solanacearum and the largest inhibition zones were recorded with C. stercoreus Egyptian strain and Agaricus campester Egyptian strain. Suwanpitak8 investigated the antagonistic activities in vitro of wood rotting fungi on two important wilt pathogens (Fusarium oxysporum f.sp. lycopersici and R. solanacearum) in which 45 isolates of wood rotting fungi were screened individually against both pathogens using the dual culture technique in vitro and measuring the width of the colony growth or zone of inhibition. Bird’s nest fungi (Cyathus spp.) isolates KK1, KK2, KK3, KK4 and Shiitake (Lentinula edodes) showed the best activities against F. oxysporum f.sp. lycopersici. All isolates of the bird’s nest fungi, KK1, KK2, KK3, KK4 and NN inhibited the growth of R. solanacearum with large inhibition zones. Moreover, the bird’s nest fungi, KK1, KK2, KK3, KK4 and NN inhibited the growth of Pythium sp. and Rhizoctonia solani, but they could not inhibit the growth of S. rolfsii. This is consistent with our previous study on the efficiency of bird’s nest fungi (Cyathus sp.) to inhibit the mycelial growth of soil-borne plant pathogenic fungi carried out by the dual culture technique. The results showed that Cyathus sp. inhibited the mycelial growth of Fusarium oxysporum f.sp. lycopersici (FOL), but did not inhibit P. aphanidermatum, S. rolfsii and Rhizoctonia sp. The greatest inhibitions were obtained from isolates KKU1 and KKU2 with 63.64% and 62.22 %inhibition of mycelial growth and 22.46% and 21.84 %overgrowth, respectively. The culture filtrate of Cyathus showed no effect on the FOL conidial germination but did affect the mycelium growth. The ability of Cyathus sp. to control tomato wilt disease caused by F. oxysporum f.sp. lycopersici under greenhouse conditions was done. At twenty-eight days after inoculation, KKU1 could reduce the disease incident more than KKU2. Cyathus sp. KKU1and KKU2 enhanced the growth of tomato that caused increasing tomato dry weight and height6. Lui and Zhang9 reported that 12 selected Cyathus species were tested for their abilities to produce antimicrobial metabolites. Most of them were found to produce secondary exo-metabolites that could induce morphological abnormalities in the rice pathogenic fungi Pyricularia oryzae. Moreover, Dong et al.10 study about nematicidal effect of freshwater fungal cultures against the pine-wood nematode (Bursaphelenchus xylophilus). The results showed that the nematicidal effect only occurred in the broken hyphal extracts and not in filtrates. The nematicide is secreted within the fungi hyphae (i.e. endotoxin). Natural and neutrally adjusted extracts of 13 fungal hyphae were pathogenic to the nematodes. They were species of Annulatascus, Caryospora, Cyathus, Ophioceras and Phomatospora.
Acknowledgements
Grateful acknowledgement is made to Dr. Jolyon Dodgson from the Faculty of Science, Mahasarakham University for proofreading.
Conflict Of Interest
The author declares that there is no conflict of interest.
- Matheny, P.B., Curtis, J.M., Hofstetter, V., Aime, M.C., Moncalvo ,J.M., Ge Z.W., Yang, Z.L., Slot, J.C., Mmrati, J.F., Baroni, T.J., Bougher, N.L., Hughes, K.W., Lodge, D.J., Kerrigan, R.W. Seidi, M.T., Aanen, D.K., DeNitis, M., Danniele, G.M., Desjardin, D.E., Kropp, B.R., Norvell, L.N., Parker, A., Vellinga, E.C., Vilgalys, R., and Hibbett, D.S. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia, 2006; 98: 984-997.
- Barbosa, M.M.B., Cruz, R.H.S.F., Calonge, F.D., Baseia, I.G. Two new records of Cyathus species for South America. Mycosphere, 2014; 5(3): 425–428.
- Shinners, T.C., Tewari, J.P. Morphological and RAPD Analyses of Cyathus olla from Crop Residue. Mycologia ,1998; 90(6): 980-989.
- Anke, T., Oberwinkler, F. The striatins-new antibiotic from the basidiomycete Cyathus striatus (Huds ex Pers.) willd. The Journal of Antibiotics, 1977; 30: 221-225.
- El-Fallal, A.A., Moussa, A. Prospects for biocontrol of brown rot disease of potato in vitro and under greenhouse conditions. Plant Pathology, 2008; 7: 54-64.
- Sutthisa, W., Sanoamuang, N. Efficiency of bird’s nest fungi (Cyathus sp.) for biological control of soil-borne plant pathogenic fungi. Khon Kaen Agricultural Journal, 2014; 42(4): 539-546.
- Landum, M.C., do Rosário, F.M., Alho, J, Garcia, R., Cabrita, M.J., Rei, F., Varanda, C.M.R. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiological Research, 2016; 183: 100–108.
- Suwanpitak P. Reaction of Wood decay fungi particular bird’s nest fungi on Fusarium and bacteria wilt disease of tomato. Master of Science Thesis in Agricultural Microbiology, Graduate school, Khon Kaen University. 2010.
- Lui Y.J., Zhang, K.Q. Antimicrobial activities of selected Cyathus species. Mycopathologia, 2004; 157: 185–89.
- Dong, J.Y., Zhao,Z.X., Cai, L., Liu, S.Q.,Zhang, H.R., Duan, M., Zhang, K.Q. Nematicidal effect of freshwater fungal cultures against the pine-wood nematode, Bursaphelenchus xylophilus. Fungal Diversity, 2013; 125-135.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.