ISSN: 0973-7510
E-ISSN: 2581-690X
This study presents an integrated approach for solvent free recovery of total phenolic compounds (TPC) from the biomass of double mutant microalga Chlorococcum humicola MCH4 using microwave-assisted pretreatment (MAPT) followed by submerged fermentation (SmF) with Bacillus subtilis. This microalga strain was developed through UV-C and EMS-induced mutagenesis and cultivated in ultrasonically pretreated municipal wastewater to obtain biomass, which was then subjected to MAPT to improve the release of TPC. Key fermentation conditions such as temperature, pH, fermentation time, agitation speed and zinc oxide nanoparticles (ZnO-NPs) concentration were optimized to maximize TPC yield. Under optimal conditions-microwave power of 600 W, pH 7.0, temperature 35 °C, agitation at 150 rpm, fermentation time of 48 h, and ZnO-NPs supplementation at 150 mgL-1, the process achieved a maximum TPC yield of 60.72 ± 0.11 mg GAE g-1 DW. The IC50 values of 38.7 µgmL-1 and 41.2 µgmL-1 were obtained for antioxidant activity of the recovered TPC by DPPH and ABTS assay, respectively, indication strong antioxidant potential. This work highlights the synergistic effect of MAPT and bacterial fermentation for effective solvent free recovery of bioactive phenolics from microalgal biomass. The process is both scalable and environmentally sustainable, indicating robust possible for use in the nutraceutical and pharmaceutical industries.
Antioxidant Activity, Chlorococcum humicola, Microwave-assisted Pretreatment, Submerged Fermentation, Total Phenolic Compounds
Human metabolic processes and environmental exposures result in the production of considerable levels of reactive oxygen species (ROS). Although ROS play vital roles in normal physiological functions, their overproduction can contribute to the development of numerous health issues such as cancer, diabetes, Alzheimer’s disease, etc.1 Antioxidants can protect from these harmful effects by neutralizing ROS through electron donation, thereby preventing oxidative stress-a condition that damages biomolecules such as proteins, nucleic acids and lipids. By mitigating this impairment, antioxidants help maintain cellular integrity and reduce the risk of the aforementioned chronic diseases. To counteract oxidative damage, synthetic antioxidants viz butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), ethoxyquin (EQ), and tertiary butylhydroquinone (TBHQ) have been widely employed in foods, pharmaceuticals, and cosmetics since the 1970s to inhibit oxidation and extend shelf life. However, concerns have emerged regarding their potential toxicity, including risks of liver damage and carcinogenic effects, which have prompted growing interest in safer, natural alternatives.2
Rising health awareness has boosted demand for natural antioxidants, which safely neutralize ROS, reduce oxidative stress, and support cellular balance.3 As a result, plant-based antioxidants are gaining research interest because of their abundant content of bioactive compounds like polyphenols and carotenoids, offering strong antioxidant properties for use in food, cosmetics, nutraceuticals, and pharmaceuticals.4 Despite growing global demand for natural health-promoting products, comprehensive data on the practical application of many plant-derived antioxidants remain limited. Consequently, the search for sustainable sources of natural antioxidants has gained significant attention, particularly for nutraceutical and pharmaceutical use. Among emerging candidates, microalgae stand out due to their rich biochemical composition, fast growth rates, and overall sustainability.5-7 Microalgae are promising sources of natural antioxidants due to their strong activity, safety, and sustainability, with studies showing efficacy comparable to or exceeding that of terrestrial plants.2,8 However, further exploration of robust microalgal species for antioxidant production is still needed.
As of 2024, the global market for algae-based products was estimated at USD 2.03 billion, with forecasts suggesting it could grow to approximately USD 3.03 billion by 2033, driven by demand for health foods, pharmaceuticals, and plant-based lifestyles.9 Microalgae, known for their sustainable cultivation via CO2 fixation and wastewater utilization, are gaining attention as scalable bioresources.10 However, downstream processing, particularly the extraction of bioactive compounds from microalgal biomass, remains a significant bottleneck limiting large-scale application. Solvent-free extraction through fermentation is increasingly favored over chemical methods due to cost and regulatory concerns.11,12 Fermentation not only improves phenolic content but also enhances the antioxidant action of microalgal extracts. This remains attributed toward the increased level of polyphenols, peptides, and other antioxidant metabolites generated during microbial metabolism.13,14 Although research on the extraction of total phenolic compounds (TPC) from mutant microalgal biomass under optimized fermentation conditions remains limited, the integration of microwave-assisted pretreatment (MAPT) with fermentation presents a promising strategy for enhancing antioxidant recovery for functional food and nutraceutical applications.
In recent years, the use of nanoparticles to enhance fermentation processes has gained considerable attention due to their unique physicochemical and electrical properties at the nanoscale.15 Nanoparticles such as magnetic materials, carbon nanotubes, and metal oxides have demonstrated potential in improving microbial stability and enzymatic efficiency, particularly in bioenergy production and the bioconversion of algal biomass.16-19 However, there is no research has focused on their role in TPC recovery from microalgal biomass by microbial fermentation. This presents a promising avenue for future studies aimed at boosting the recovery of valuable antioxidants from sustainable algal sources.
Based on the aforementioned insights, the present study aimed to utilize ultrasonically pretreated municipal wastewater-grown double mutant Chlorococcum humicola MCH4 biomass as a substrate for natural antioxidant production. As far as we are aware, this represents the first documented study to employ microwave-assisted pretreatment for the preparation of hydrolysate from C. humicola MCH4 biomass, with the goal of enhancing solvent-free extraction of TPC through fermentation using B. subtilis, supplemented with ZnO-NPs. Furthermore, the antioxidant potential of extracted TPC was evaluated through free radical scavenging assay with half-maximal inhibitory concentration (IC50) values determined to evaluate their efficacy. This study pioneers the use of microwave-assisted pretreatment of double mutant C. humicola biomass for enhanced, solvent-free antioxidant production via B. subtilis fermentation, offering a sustainable approach to bioactive compound extraction.
Materials
Nutrient agar, media components, and reagent compounds used in this study were of analytical grade and high purity. They were procured from reputed suppliers, including Himedia Laboratories and Merck Life Science Pvt. Ltd. (Mumbai, India).
Double mutant green microalga
Green microalga Chlorococcum humicola KMS2 (GenBank Accession No.: PQ650940), was originally isolated from an ancient freshwater lake located in Mamandur (12.75°N, 79.99°E), Cheyyar Taluk, Tiruvannamalai District, Tamil Nadu, India, and made as a pure culture. To produce a double mutant strain, wild microalga C. humicola KMS2 was first treated with UV-C radiation for 10 min then with 1.5 M ethyl methanesulfonate (EMS). This sequential mutagenesis yielded a double mutant strain, designated as C. humicola MCH4 and which was routinely subcultured in Chu13 medium.20 The mother culture was maintained under continuous white fluorescent light (30 µE m-2s-1) with 12:12 h at 25 ± 2 °C. This double mutant microalga was cultivated in ultrasonically pretreated municipal wastewater (UPMWW) for biomass production.
Ultrasonic pretreatment of MWW
The untreated MWW was taken from Kanchipuram municipal wastewater treatment plant, Tamil Nadu, India. Then, ultrasonic pretreatment of untreated MWW was performed using improved method of Dhandayuthapani et al.21 Approximately 400 mL of 75% (v/v) untreated MWW was taken in a 500 mL stainless steel container and exposed to ultrasonic irradiation for 20 min at 0.35 W mL-1. The resulting treated MWW, designated as ultrasonically pretreated municipal wastewater (UPMWW) and used as a sole culture medium for biomass production by C. humicola MCH4.
Biomass production
Batch cultivation was conducted in 1000 mL Erlenmeyer flasks contained 500 mL UPMMW. Then the flask was inoculated (8% v/v) with actively grown C. humicola MCH4 and incubated at 30 ± 1 °C with continuous shaking at 150 rpm. During cultivation, illumination was provided at 90 µE m-2s-1 under a 10:14 h light-dark photoperiod for 10 days. End of the cultivation, the biomass was separated by centrifugation using a laboratory benchtop centrifuge at 14,000 rpm for 15 min (Remi Model R-8C BL, Mumbai, Maharashtra, India). The resulting pellet was washed twice by deionized water then recentrifuged to eliminate residual scums. The cleaned biomass was then dehydrated by 40 °C using a hot air oven. The dried biomass (DB) was subsequently used for hydrolysate preparation.
Hydrolysate preparation from DB by MAPT
MAPT was employed to prepare hydrolysate from DB of C. humicola MCH4 for subsequent extraction of TPC. The pre-treatment was carried out using a domestic microwave oven (Model: IFB 17PM-MEC1, 17L Solo Microwave Oven, IFB Industries Ltd., India). For each experiment, 25 g of DB was mixed in 100 mL of deionized water and then exposed to microwave irradiation at varying power levels ranging from 400 W to 700 W, with 100 W increments. The treatment was conducted for 5 minutes at a temperature of 80 °C for 400-600 W and 120 °C for ≥700 W. Following MAPT, the samples were cooled at ambient temperature and estimated TPC. A non-treated biomass hydrolysate served as the control. The condition that resulted in the maximum TPC harvest was identified and chose as the optimal microwave setting for hydrolysate preparation from DB of C. humicola MCH4 using MAPT.
Bacterial strain and inoculum preparation
The bacterial strain Bacillus subtilis KT28131 was isolated from sago industry effluent polluted soil sample and was utilized for the fermentation of hydrolysate to TPC. The strain was well-maintained on nutrient agar at 4 °C and sub-cultured monthly to maintain viability. Fresh inoculum was prepared by transferring a loopful of the strain into nutrient broth and incubating it for 24 h at 120 rpm in an orbital shaking incubator (REMI, Model CIS-18 Plus, Mumbai, Maharashtra, India). The resulting culture was used as the seed inoculum for subsequent fermentation experiments.
Optimization of batch fermentation conditions
Submerged fermentation (SmF) was performed in 250 mL Erlenmeyer flasks with 100 mL of hydrolysate added with a defined basal medium comprising the following components (per liter): 5 g yeast extract, 5 g peptone, 1 g monopotassium phosphate, 1 g ammonium sulfate, 0.5 g magnesium sulfate heptahydrate, 0.5 g sodium chloride, and 0.1 g calcium chloride. To optimize the fermentation parameters for enhanced extraction of TPC from the hydrolysate, four key process factors were assessed individually via the one-factors-at-a-time (OFAT) approach: initial pH (6.0 to 8.0, in 0.5 pH intervals), incubation temperature (30 °C to 50 °C, in 5 °C intervals), agitation speed (100 to 300 rpm, in 50 rpm intervals), and fermentation time (0 to 72 h, at 24 h intervals).
Fermentation medium initial pH was adjusted by 1N NaOH or HCl prior to sterilization. The media was then autoclaved at 121 °C for 15 min at 15 psi and subsequently cooled at room temperature. Fermentation was initiated by inoculating the sterile medium with 5% (v/v) of a freshly prepared B. subtilis inoculum. Cultures were incubated under aerobic conditions in an orbital shaking incubator. End of the SmF, clear supernatant was obtained from fermented broth by centrifugation at 14,000 rpm for 15 min. Subsequently, it was used for TPC estimation, while the raw hydrolysate was used as a control sample. The condition yielding the highest TPC concentration in each parameter set was identified and selected as optimal for further experimentation.
Effect of ZnO-NPs concentration on TPC extraction
To study the effect of ZnO-NPs on the recovery of TPC, ZnO-NP with an average particle size of ≤100 nm was procured from AdNano Technologies Pvt. Ltd., Karnataka, India (CAS No.: 1314-13-2). As per the manufacturer’s data, the nanoparticles exhibited a near-spherical morphology, a bulk density of 0.54 g.cm-3, and a specific surface area ranging between 100 and 120 m²g-1. The crystalline nature of the ZnO-NP was confirmed via X-ray Diffraction (XRD) analysis.
SmF medium amended with different concentrations of ZnO-NPs ranging from 50 to 250 mgL-1 with an increment of 50 mgL-1. Each flask was inoculated with 5% (v/v) of a freshly prepared B. subtilis culture and incubated under previously optimized fermentation conditions using an orbital shaking incubator. A parallel control experiment without ZnO-NPs supplementation was also included. End of SmF, TPC was quantified, and the nanoparticle concentration yielding the highest TPC was determined and selected for subsequent studies.
Validation of TPC extraction under optimized SmF conditions
To confirm the effectiveness of the optimized SmF parameters, a validation experiment was conducted under the best conditions identified through the classical OFAT approach. The hydrolysate pH was adjusted to its optimum level and then inoculation with 5% (v/v) of freshly prepared B. subtilis culture. The fermentation medium was then supplemented with the optimal concentration of ZnO-NPs. SmF was carried out at the optimized temperature, agitation speed, and incubation time. A control was maintained at pH 7 and room temperature for 48 h without ZnO-NPs. End of SmF, supernatant was collected and used for TPC estimation. The validated setup yielding the maximum TPC concentration was confirmed and TPC was extracted from fermented broth.
Estimation of TPC
The TPC was quantified by Folin-Ciocalteu colorimetric method22 with slight modifications. About 1 mL of diluted Folin-Ciocalteu reagent was added to 200 µL sample and then mixed well. After a 4-minute incubation, 800 µL sodium carbonate solution (0.075 gmL-1) was mixed and again the mixture was incubated for 2 h at dark condition. Finally, the supernatant was obtained from the reaction mixture by centrifugation at 6000 rpm for 10 min and its absorbance was measured at 765 nm using a UV-visible spectrophotometer (Model 2206, Systronics India Ltd., Gujarat, India). Standard graph was prepared using gallic acid with the concentration range from 0-500 mgL-1. TPC of the test samples was quantified by interpolated the absorbance value of sample in the standard graph and reported as gallic acid equivalents per gram dry weight (mg GAE g-1 DW).
Extraction of TPC using ethyl acetate
TPC extraction from the fermented broth was performed using ethyl acetate, following a modified method of Xue et al.23 At the end of the fermentation, the broth was collected and then centrifuged at 9000 rpm for 15 min at 4 °C to remove bacterial biomass and insoluble materials. The resulting supernatant was carefully collected and acidified (pH 2.5) using 1N HCl to convert phenolic compounds into their protonated forms, thereby enhancing their partitioning into the organic phase. Equal volumes of acidified supernatant and ethyl acetate were transferred into a separatory funnel and thoroughly mixed by shaking for 30 minutes. After settling for 30 minutes to allow clear phase separation, the upper ethyl acetate layer-containing the extracted phenolics was carefully separated. The aqueous layer was re-extracted two additional times using fresh portions of ethyl acetate, following the same procedure. All ethyl acetate extracts were pooled and concentrated using a rotary evaporator (BUCHI R-210 with B-491 heating bath) until the volume was reduced to approximately 5 mL. To prevent thermal degradation, the concentrated extract was then air-dried at ambient temperature. The resulting dry residue was reconstituted in a small amount of methanol and stored at 4 °C until further analysis.
DPPH assay
In vitro antioxidant activity of the TPC extracted from double mutant C. humicola MCH4 biomass was assessed by DPPH (2,2-diphenyl-1-picrylhydrazyl) assay via procedure adapted from Qureshi et al.24 1 mL of 0.2 mM DPPH solution was added to 1 mL of various concentration (50 to 300 µgmL-1) of extract and vortexed for 30 seconds. The reaction mixture was incubated for 30 min in the dark at room temperature. After incubation, the sample absorbance was measured at 517 nm using UV-visible spectrophotometer. Methanol and DPPH solution without sample was used as blank and control, respectively. Ascorbic acid was utilized as a reference. The percentage of DPPH radical scavenging activity was calculated using the following formula:
DPPH scavenging activity (%) = Abs.control – Abs.sample / Abs.control × 100
Where,
Abs.control = absorbance of control, Abs.sample = absorbance of sample (DPPH + TPC extract).
ABTS assay
ABTS 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) assay for determining radical scavenging ability of TPC extracted from the double mutant C. humicola MCH4 was carried out using standard procedure.25 ABTS radical cation (ABTS•+) was prepared by mixing equal volume of 7 mM ABTS solution with 2.45 mM potassium persulfate, followed by incubation for 12 h in the dark at room temperature. Before use, the ABTS•+ was diluted with ethanol until it reached an absorbance of 0.700 ± 0.02 at 734 nm, as measured using a UV-Visible spectrophotometer. For the assay, 180 µL of ABTS•+ was mixed with 10 µL of various concentration of sample range from 50-300 µgmL-1 and then incubated for 10 min in the dark at room temperature. The absorbance of the mixture was measured at 734 nm using UV-Visible spectrophotometer. Methanol and ABTS•+ solution without sample were used as blank and control, respectively. Ascorbic acid served as the reference standard. The percentage of ABTS•+ scavenging activity was calculated using the following formula:
ABTS scavenging activity (%) = Abs.control – Abs.sample / Abs.control × 100
Where
Abs.control = absorbance of control, Abs.sample = absorbance of sample
The IC50 value was determined separately for each assay by plotting the percentage of radical scavenging activity against the concentrations of the TPC extract (50-300 µgmL-1). Linear regression analysis was applied to the data, and the extract concentration corresponding to 50% inhibition was calculated from the resulting regression equation.
Cellulase assay
Dinitrosalicylic acid (DNS) method26 was used to estimate the cellulase (EC 3.2.1.4) activity in the fermented broth. In this assay, 1% carboxymethyl cellulose (CMC) was prepared in 50 mM sodium citrate buffer (pH 4.8) and used as substrate. Approximately 0.5 mL of supernatant obtained from fermented broth was mixed with 0.5 mL CMC solution, mixed well, and incubated at 50 oC for 30 min. End of incubation, reaction was stopped by addition of 1 mL of DNS reagent. The mixture was immediately kept in a boiling waste bath for 5 min for color development, then cooled to room temperature. At 540 nm, sample absorbance was measured using a UV-Visible spectrophotometer. A glucose standard curve (0-500 µgmL-1) was used to estimate the amount of reducing sugar released. One unit (U) of cellulase activity is defined as the amount of enzyme that releases 1 µmol of glucose per minute under the specified assay conditions.
Statistical analysis
In this study, each experiment was performed in triplicate, and the results are presented as the mean ± standard deviation (SD). In every case, the SD remained within 5% of the corresponding mean. One-way analysis of variance (ANOVA) also performed with MINITAB version 12 software to determine the statistically significant differences among various treatments. Differences were considered statistically significant at a p ≤ 0.05. Correlation analysis was performed to assess the relationship between TPC and free radical scavenging activity. The Pearson correlation coefficient (r) was calculated using MINITAB version 12 to determine the strength and direction of the association.
Hydrolysate preparation and extraction of TPC under various microwave power
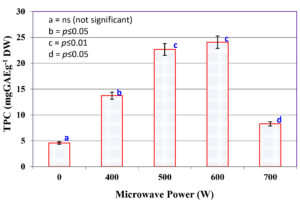
The effect of varying microwave power levels (0-700 W) on the extraction efficiency of TPC from C. humicola MCH4 biomass is illustrated in Figure 1. The TPC yield was significantly influenced by the applied microwave power at 0 W (control), where no microwave irradiation was applied, the TPC yield was lowest at 4.57 ± 0.28 mg GAE g-1 DW. Application of microwave power at 400 W sharply increased the TPC yield to 13.72 ± 0.09 mg GAE g-1 DW. Further increase to 500 W and 600 W resulted in higher yields of 22.67 ± 0.73 mg GAE g-1 DW and 24.06 ± 0.11 mg GAE g-1 DW, respectively. The highest TPC values were obtained at 500 W and 600 W, which did not differ significantly from each other (p ≤ 0.05), but both were significantly higher than the control and other treatments. At 700 W, TPC (8.27 ± 0.18 mg GAE g-1 DW) decreased significantly compared to 500-600 W. Bars with different letters indicate significant differences (p ≤ 0.05) according to Tukey’s test. The maximum TPC yield of 24.06 ± 0.11 mg GAE g-1 DW was recorded at 600 W. Hence, microwave power 600 W was used for hydrolysate preparation from C. humicola MCH4 biomass.
Influence of pH on TPC extraction
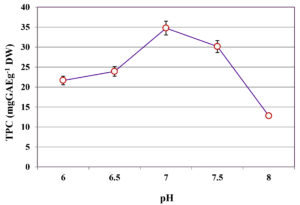
The influence of different pH levels (6.0-8.0) on the extraction of TPC from C. humicola MCH4 hydrolysate, prepared using MAPT at 600 W and fermented by B. subtilis under SmF, was evaluated (Figure 2). TPC yield was lowest at pH 6.0, with a value of 21.65 ± 0.12 mg GAE g-1 DW. A gradual increase in TPC yield was observed with rising pH, reaching a maximum of 34.76 ± 0.22 mg GAE g-1 DW at pH 7.0. Further elevation of pH beyond this optimum resulted in decreased phenolic content: 30.15 ± 0.09 mg GAE g-1 DW at pH 7.5 and 12.82 ± 0.14 mg GAE g-1 DW at pH 8.0. The one-way ANOVA showed a statistically significant difference in TPC among the different pH levels (p ≤ 0.05). This indicates that pH has a significant effect on extraction of TPC. The highest TPC was observed at pH 7, hence it was used as an optimal pH for further study.
Influence of temperature on TPC extraction
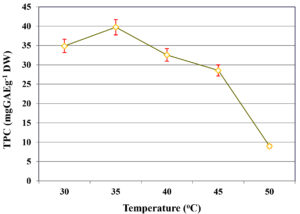
The influence of incubation temperature on TPC extraction during SmF of C. humicola MCH4 hydrolysate using B. subtilis was assessed across a temperature range of 30-50 °C (Figure 3). At 30 °C, the TPC yield was 34.88 ± 0.11 mg GAE g-1 DW. An increase in temperature to 35 °C resulted in a significant enhancement in phenolic content, reaching the maximum yield of 39.71 ± 0.21 mg GAE g-1 DW. However, further increases in temperature led to a progressive decline in TPC yield, with values of 32.56 ± 0.13 mg GAE g-1 DW at 40 °C and 28.55 ± 0.11 mg GAE g-1 DW at 45 °C. The lowest yield was recorded at 50 °C, with only 8.98 ± 0.09 mg GAE g-1 DW. This indicates that temperature significantly influence on extraction of TPC (p ≤ 0.05). The highest TPC was recorded at 35 °C, hence it was used as an optimal temperature for further study.
Influence of agitation speed on TPC extraction
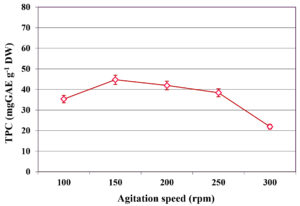
The impact of agitation speed on TPC extraction during SmF of C. humicola MCH4 hydrolysate using B. subtilis was assessed across a range of 100 to 300 rpm (Figure 4). At 100 rpm, the TPC yield was 35.33 ± 0.15 mg GAE g-1 DW. Increasing the agitation rate to 150 rpm significantly enhanced phenolic yield to a maximum of 44.72 ± 0.11 mg GAE g-1 DW. However, further increases in agitation speed led to a gradual decline in TPC content: 41.94 ± 0.10 mg GAE g-1 DW at 200 rpm, 38.41 ± 0.12 mg GAE g-1 DW at 250 rpm, and a pronounced reduction to 21.29 ± 0.21 mg GAE g-1 DW at 300 rpm. This suggests that agitation speed has a significant effect on extraction of TPC (p ≤ 0.05). The highest TPC was observed at 150 rpm, hence it was used as an optimum agitation speed for further study.
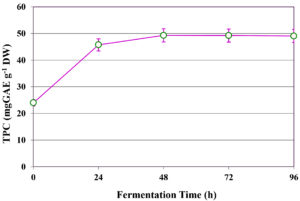
Influence of fermentation time on TPC extraction
The influence of fermentation duration on the extraction of TPC from C. humicola MCH4 hydrolysate using B. subtilis was evaluated over a time range of 0 to 96 hours (Figure 5). At the initial time point (0 h), the TPC content was 24.00 ± 0.13 mg GAE g-1 DW, representing the baseline phenolic concentration in the absence of microbial fermentation. A substantial increase in TPC yield was observed after 24 h, reaching 45.75 ± 0.12 mg GAE g-1 DW. The highest phenolic yield was recorded at 48 h, with 49.34 ± 0.12 mg GAE g-1 DW. Beyond this point, the TPC levels remained relatively stable, with minor decreases observed at 72 h (49.26 ± 0.15 mg GAE g-1 DW) and 96 h (49.09 ± 0.11 mg GAE g-1 DW). The one-way ANOVA showed a statistically significant difference in TPC extraction with respect to fermentation time (p ≤ 0.05). TPC increased substantially from 0 to 48 h, after which it plateaued. This suggests that 48 h is likely the optimal fermentation time for maximizing TPC extraction under the conditions tested.
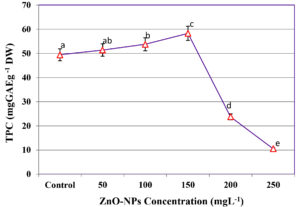
Influence of ZnO-NPs on TPC extraction
The effect of ZnO-NPs supplementation on TPC extraction during SmF of C. humicola MCH4 hydrolysate using B. subtilis was investigated at various concentration (Figure 6). In the absence of ZnO-NPs (0 mg L-1), the TPC yield was 49.43 ± 0.12 mg GAE g-1 DW. A progressive increase in TPC content was observed with rising ZnO-NPs concentrations, reaching a maximum of 58.29 ± 0.09 mg GAE g-1 DW at 150 mgL-1. This represents an approximate 18% improvement compared to the control. However, further increases in ZnO-NPs concentration led to a sharp decline in TPC yield, with values decreasing to 23.73 ± 0.12 mg GAE g-1 DW at 200 mg L-1 and 10.49 ± 0.11 mg GAE g-1 DW at 250 mg L-1. In Figure 6, different letters (a-e) indicate statistically significant differences among treatments according to one-way ANOVA followed by Tukey’s test (p < 0.05). The 150 mg L-1 ZnO-NPs treatment (c) showed the highest TPC (58.29 ± 0.09 mg GAE g-1 DW), which was significantly different from the control and other treatments. Since the highest TPC was observed at 150 mg L-1, this concentration was selected as the optimum ZnO-NPs level for further study.
Validation of optimized conditions and enzyme activity assessment
Under the optimized conditions such as pH 7, temperature 35 oC, agitation speed 150 rpm, fermentation time 48 h, and ZnO-NPs concentration 150 mg L-1– SmF of C. humicola MCH4 hydrolysate using B. subtilis yielded a maximum TPC of 60.72 ± 0.11 mg GAE g-1 DW. This yield was approximately 2.14 times greater than that obtained under non-optimized conditions (28.36 ± 0.11 mg GAE g-1 DW). Cellulase activity measured under these optimized fermentation parameters was 74.07 ± 0.22 UmL-1. Whereas, very low cellulase activity of 9.13 ± 0.05 UmL-1 was recorded under non-optimized conditions. Cellulase activity measured under the optimized fermentation parameters was 74.07 ± 0.22 UmL-1, whereas significantly lower activity (9.13 ± 0.05 UmL-1) was observed under non-optimized conditions. The process was carried out without the use of organic solvents, supporting its environmentally friendly nature. The fermented broth was subsequently used for TPC extraction and assessment of antioxidant activity.
Antioxidant activity of extracted TPC
Antioxidant activity of TPC extracted from C. humicola MCH4 hydrolysate, fermented under optimized conditions with B. subtilis, was assessed using DPPH and ABTS radical scavenging assays. The results revealed a clear dose-dependent increase in antioxidant activity across a concentration range of 50-300 µgmL-1 (Figure 7). At 50 µgmL-1, the TPC extract exhibited a high DPPH radical scavenging activity of 90.08%, which gradually rose to 94.03% at 300 µgmL-1. This shows a strong positive correlation between TPC concentration and DPPH scavenging activity. The IC50 value calculated from the scavenging curve was 38.7 µgmL-1, indicating potent antioxidant potential.
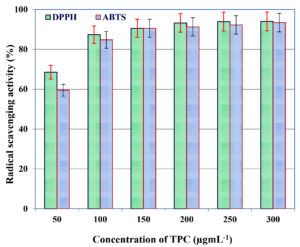 Figure 7. Antioxidant activity of TPC extracted from C. humicola MCH4 hydrolysate under optimized SmF condition
Figure 7. Antioxidant activity of TPC extracted from C. humicola MCH4 hydrolysate under optimized SmF condition
Similarly, the TPC extract also demonstrated a concentration-dependent increase in ABTS radical scavenging activity (Figure 7). At 50 µgmL-1, the extract showed 28.47% scavenging activity, which progressively increased to 63.42% at 300 µgmL-1. This shows a strong positive correlation between TPC concentration and ABTS scavenging activity. The most pronounced increase was observed between 100 µgmL-1 (41.29%) and 200 µgmL-1 (60.32%). Beyond 200 µgmL-1, the scavenging activity began to plateau. The IC50 value derived from the ABTS scavenging curve was 41.2 µgmL-1, indicating strong antioxidant capacity. The IC50 values of both the assay indicate strong antioxidant activity of the TPC extracted from the fermented hydrolysate of mutant C. humicola MCH4. The slightly lower IC50 in the DPPH assay suggests marginally higher radical scavenging efficiency against DPPH radicals compared to ABTS. This difference may be attributed to variations in the reaction mechanisms and solubility of phenolics in different assay environments. Overall, both assays confirm potent antioxidant potential of the extracted TPC.
Microwave power was a critical factor influencing TPC extraction from C. humicola MCH4 biomass, with optimal recovery achieved at 600 W. This aligns with Georgiopoulou et al.,27 demonstrating that microwave-assisted extraction (MAE) effectively disrupts microalgal cell walls and enhances solvent penetration, outperforming conventional methods and microbial fermentation yields reported by Sivakumar and Dhandayuthapani.12 However, excessive microwave energy (700 W) caused thermal degradation of phenolics, consistent with findings by Tan et al.,28 underscoring the importance of precise parameter optimization. Similarly, fermentation conditions-pH 7.0, temperature 35 °C, and agitation at 150 rpm were found optimal for maximizing enzymatic hydrolysis and phenolic release by B. subtilis, corroborating earlier studies by Malik and Javed,29 and Narmatha and Shanthi.30 Excessive agitation and temperature resulted in enzyme inactivation and microbial stress, reducing phenolic yields, while fermentation beyond 48 hours led to plateauing or decline due to phenolic degradation.31,32
The incorporation of ZnO-NPs at 150 mgL⁻¹ further enhanced phenolic recovery by stimulating microbial growth, enzyme production, and stabilizing fermentation, consistent with Babadi et al.17 However, higher concentrations induced cytotoxic effects and phenolic degradation, as reported by Hashempour et al.33 and Shahbaz et al.,34 highlighting the delicate balance required in nanoparticle-assisted bioprocesses. Under these optimized conditions, cellulase activity reached 74.07 ± 0.22 UmL-1 -exceeding values from previous harsher fermentation setups,35 and TPC yield increased 4.42-fold compared to microbial fermentation of Pseudochlorella pringsheimii EMM2.12 The solvent-free approach presents a sustainable, eco-friendly strategy for valorizing microalgal biomass into high-value phenolic extracts.
The improvement in TPC recovery observed with ZnO nanoparticles likely arises from a dual physicochemical-biocatalytic mechanism. First, ZnO surfaces provide hydroxyl/Lewis-acid sites that interact with phenolic hydroxyls and wall polysaccharides, facilitating desorption of matrix-bound phenolics; consistent with reports of phenol-ZnO interfacial binding. Concurrently, low-level ROS produced at ZnO surfaces can mildly permeabilize algal cell walls, enhancing solvent access. Second, partial ZnO dissolution supplies Zn2+, an essential micronutrient in B. subtilis that modulates metal-homeostasis regulons and supports secretion of wall-degrading enzymes, including feruloyl esterases that release ester-linked phenolic acids. Together, these effects increase the release and stabilization of phenolics during SmF, provided ZnO-NP dosage remains below levels that perturb B. subtilis growth or redox balance.36-38
The phenolic-rich extracts exhibited strong antioxidant potential, with DPPH and ABTS radical scavenging assays showing high activity (90.08% at 50 µgmL-1 and IC50 values of 38.7 and 41.2 µgmL-1, respectively), indicating efficient enzymatic liberation of potent phenolic compounds such as flavonoids and phenolic acids.12,39 Saturation of scavenging activity beyond 200 µgmL-1 reflects the maximal radical neutralization capacity typical of phenolic extracts.40,41 These findings validate the optimized microbial fermentation approach as a promising biotechnological route for producing antioxidant-rich phenolic extracts suitable for nutraceutical and functional food applications.
This study addressed the critical question of how to efficiently recover phenolic antioxidants from microalgal biomass while ensuring process sustainability and scalability. By integrating MAPT with SmF using B. subtilis, we demonstrated a highly effective strategy for enhancing TPC extraction from the double mutant C. humicola MCH4 biomass. Optimization of operational parameters-microwave power (600 W), pH 7, temperature 35 °C, agitation speed 150 rpm, fermentation time 48 h, and ZnO-NPs supplementation (150 mgL-1)-resulted in a significant increase in TPC yield, achieving 60.72 ± 0.11 mg GAE g-1 DW. Importantly, the recovered extracts exhibited potent antioxidant activities, with low IC50 values in DPPH (38.7 µgmL-1) and ABTS (41.2 µgmL-1) assays, confirming their functional potential. The findings not only establish MAPT-SmF as a promising solution for valorizing microalgal biomass into high-value nutraceutical antioxidants but also highlights the novel role of ZnO-NPs as fermentation enhancers, paving the way for greener and more efficient bioprocesses. Future research should focus on scaling up this integrated approach, evaluating its techno-economic feasibility, and exploring its applicability across diverse microalgal strains and bioactive targets to advance sustainable biotechnological production of functional compounds.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Principal and the Head of the Department, Department of Botany, and the authorities of Thiruvalluvar University for their support and for providing the facilities necessary to carry out this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
KD conceptualized the study and performed administration and supervision. EV applied methodology and performed data validation and visualization. EV and KD performed investigation, formal analysis, data curation and wrote the manuscript. KD reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- He M, Wang M, Xu T, et al. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J Control Release. 2023;356:623-648.
Crossref - Yang N, Zhang Q, Chen J, et al. Study on bioactive compounds of microalgae as antioxidants in a bibliometric analysis and visualization perspective. Front Plant Sci. 2023;14:1144326.
Crossref - Yu YQ, Zhu T. Effects of endogenous and exogenous reductants in lung fluid on the bioaccessible metal concentration and oxidative potential of ultrafine particles. Sci Total Environ. 2023;882:163652.
Crossref - Xu DP, Li Y, Meng X, et al. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int J Mol Sci. 2017;18(1):96.
Crossref - Galasso C, Gentile A, Orefice I, et al. Microalgal derivatives as potential nutraceutical and food supplements for human health:A focus on cancer prevention and interception. Nutrients. 2019;11(6):1226.
Crossref - Sansone C, Brunet C. Promises and Challenges of Microalgal Antioxidant Production. Antioxidants. 2019;8(7):199.
Crossref - Andriopoulos V, Gkioni MD, Koutra E, et al. Total phenolic content, biomass composition, and antioxidant activity of selected marine microalgal species with potential as aquaculture feed. Antioxidants. 2022;11(7):1320.
Crossref - Wang N, Pei H, Xiang W, et al. Rapid screening of microalgae as potential sources of natural antioxidants. Foods. 2023;12(14):26.
Crossref - IMARC Group. Algae products market, algae products market report by type (Lipids, Carrageenan, Carotenoids, Algal Protein, Alginate, and Others), source (Brown Algae, Blue-Green Algae, Red Algae, Green Algae), form (Solid, Liquid), distribution channel (Online, Offline), application (Food and Beverages, Nutraceuticals and Dietary Supplements, Personal Care, Feed, Pharmaceuticals, Chemicals, and Others), and region 2025-2033. IMARC Group. 2025. https://www.imarcgroup.com/algae-products-market. Accessed April 25, 2025.
- Pereira L, Cotas J, Valado A. Antioxidants from microalgae and their potential impact on human well-being. Explor Drug Sci. 2024;2(3):292-321.
Crossref - Marti-Quijal FJ, Khubber S, Remize F, Tomasevic I, Rosello-Soto E, Barba FJ. Obtaining antioxidants and natural preservatives from food by-products through fermentation:A review. Fermentation. 2021;7(3):106.
Crossref - Sivakumar N, Dhandayuthapani K. Total phenolic content and in vitro evaluation of antioxidant activity of microbial extract of defatted biomass of mutant Pseudochlorella pringsheimii EMM2. J Appl Nat Sci. 2025;17(1):31.
Crossref - Qian ZJ, Jung WK, Kang KH, et al. In vitro antioxidant activities of the fermented marine microalga Pavlova lutheri (Haptophyta) with the yeast Hansenula polymorpha. J Phycol. 2012;48(2):475-482.
Crossref - Nina N, Bressa C, de Lucas B, et al. Polyphenol metabolism, short-chain fatty acids production, and microbiota changes during in vitro digestion and fermentation of Chilean beans (Phaseolus vulgaris L.). Food Chem. 2025;486:144669.
Crossref - Hidalgo D, Martin-Marroquin JM, Corona F. The role of magnetic nanoparticles in dark fermentation. Biom Conver Bioref. 2023;13(18):16299-16320.
Crossref - Rambabu K, Bharath G, Banat F, Hai A, Show PL, Nguyen THP. Ferric oxide/date seed activated carbon nanocomposites mediated dark fermentation of date fruit wastes for enriched biohydrogen production. Inter J Hydro Ener. 2021;46(31):16631-16643.
Crossref - Babadi AA, Rahmati S, Fakhlaei R, et al. Emerging technologies for biodiesel production:Processes, challenges, and opportunities. Biom Bioener. 2022;163:106521.
Crossref - El-Sheekh M, Elshobary M, Abdullah E, Abdel-Basset R, Metwally M. Application of a novel biological-nanoparticle pretreatment to Oscillatoria acuminata biomass and coculture dark fermentation for improving hydrogen production. Microb Cell Factor. 2023;22(1):34.
Crossref - Rekha B, Saravanathamizhan R. Catalytic conversion of corncob biomass into bioethanol. Int J Energy Res. 2021;45(3):4508-4518.
Crossref - Chu SP. The influence of the mineral composition of the medium on the growth of planktonic algae. Part I. Methods and culture media. J Ecol. 1942;30(2):284-325.
Crossref - Dhandayuthapani K, Kumar PS, Chia WY, et al. Bioethanol from hydrolysate of ultrasonic processed robust microalgal biomass cultivated in dairy wastewater under optimal strategy. Energy. 2022;244:122604.
Crossref - Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144-153.
Crossref - Xue S, Jones AD, Sousa L, et al. Water-soluble phenolic compounds produced from extractive ammonia pretreatment exerted binary inhibitory effects on yeast fermentation using synthetic hydrolysate. PLoS One. 2018;13(3):e0194012.
Crossref - Qureshi MN, Kuchekar BS, Logade NA, Haleem MA. In vitro antioxidant and in vivo hepatoprotective activity of Leucas ciliata leaves. Rec Nat Prod. 2010;4(2):124-130. http://www.acgpubs.org/RNP/2010/Volume%204/Issue%201/15-RNP-0910-153.pdf
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9-10):1231-7.
Crossref - Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426-428.
Crossref - Georgiopoulou I, Louli V, Magoulas K. Comparative study of conventional, microwave-assisted and supercritical fluid extraction of bioactive compounds from microalgae:The case of Scenedesmus obliquus. Separations. 2023;10(5):290.
Crossref - Tan YH, Chai MK, Wong LS, Ong MY, Rajamani R. Optimizing microwave-assisted extraction of carbohydrate from Scenedesmus sp. cultivated in domestic wastewater. Int J Technol. 2025;16(1):255-274.
Crossref - Malik WA, Javed S. Enhancement of cellulase production by cellulolytic bacteria SB125 in submerged fermentation medium and biochemical characterization of the enzyme. Int J Biol Macromol. 2024;263(Pt 2):130415.
Crossref - Narmatha R, Shanthi K. Optimization of microbial fermentation of defatted biomass hydrolysate of mutant green microalga Tetradesmus dimorphus EMS2 for production of total phenolic content and antioxidant activity. J Xi’an Univ Archit Technol. 2022;14(12):44-56.
- Punia S, Sandhu KS, Grasso S, et al. Aspergillus oryzae fermented rice bran: A byproduct with enhanced bioactive compounds and antioxidant potential. Foods. 2020;10(1):70.
Crossref - Zofia N, Aleksandra Z, Tomasz B, et al. Effect of fermentation time on antioxidant and anti-ageing properties of green coffee kombucha ferments. Molecules. 2020;25(22):5394.
Crossref - Hashempour Y, Mortezazadeh F, Rezaei S, et al. Co-immobilization of laccase and zinc oxide nanoparticles onto bacterial cellulose to achieve synergistic effect of photo and enzymatic catalysis for biodegradation of favipiravir. Int J Biol Macromol. 2025;292:139288.
Crossref - Shahbaz A, Hussain N, Saleem MZ, Saeed MU, Bilal M, Iqbal HMN. Nanoparticles as stimulants for efficient generation of biofuels and renewables. Fuel. 2022;319:123724.
Crossref - Fouda A, Alshallash KS, Atta HM, et al. Synthesis, optimization, and characterization of cellulase enzyme obtained from thermotolerant Bacillus subtilis F3:An insight into cotton fabric polishing activity. J Microbiol Biotechnol. 2023;34(1):207.
Crossref - Dehmani Y, Laine J, Daouli A, et al. Unravelling the adsorption mechanism of phenol on zinc oxide at various coverages via statistical physics, artificial neural network modeling and ab initio molecular dynamics. Chem Eng J. 2023;452(Part 2):139171.
Crossref - Elkady MF, Hassan HS, Amer WA, et al. Novel magnetic zinc oxide nanotubes for phenol adsorption:mechanism modeling. Materials. 2017;10(12):1355.
Crossref - Chandrangsu P. Helmann JD, Intracellular Zn (II) intoxication leads to dysregulation of the PerR regulon resulting in heme toxicity in Bacillus subtilis. PLoS Genet. 2016;12(12):p.e1006515.
Crossref - Mirzapour-Kouhdasht A, Garcia-Vaquero M, Huang JY. Algae-derived compounds: Bioactivity, allergenicity and technologies enhancing their values. Bioresour Technol. 2024;406:130963.
Crossref - Nutautaite M, Raceviciute-Stupeliene A, Bliznikas S, et al. Evaluation of phenolic compounds and pigments in freshwater Cladophora glomerata biomass from various Lithuanian rivers as a potential future raw material for biotechnology. Water. 2022;14(7):1138.
Crossref - Garofalo C, Norici A, Mollo L, Osimani A, Aquilanti L. Fermentation of microalgal biomass for innovative food production. Microorganisms. 2022;10(10):2069.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.