ISSN: 0973-7510
E-ISSN: 2581-690X
Utilization of rice straw (Oryza sativa Linn) as a raw material in the production of poly(3-hydroxybutyrate), P(3HB), biopolymer by fermentation using Pseudomonas aeruginosa has been conducted. Rice straw stew and microcrystalline cellulose obtained from rice straw were used as carbon source for bacterial growth at various concentrations. The content of P(3HB) in the biomass was determined by gas chromatography using the RTX-1 column and the FID detector. Results showed that the best condition in producing P(3HB) was using 1% microcrystalline cellulose as carbon source and duration of fermentation of 48 hours. The biomass and P(3HB) obtained from MCC were 982 mg/100 mL and 17.19% w/w, respectively. While using rice straw stew were 269 mg/100 mL and 1.73%, respectively. It can be concluded that the highest P(3HB) produced was found using microcrystalline cellulose obtained from rice straw waste as a carbon source.
Rice straw, poly(3-hydroxybutyrate), Pseudomonas aeruginosa, Microcrystalline cellulose
The need for plastics has greatly increased, leading to enhance of its production. One type of plastic is synthetic plastics. Synthetic plastics are macromolecules composed of simple molecules produced from simple molecules by polymerization, addition, and elimination reactions1. In this decade 300 million tons of plastics are produced worldwide per year2. Plastics are a major source of waste and have a low degradation ability3. Plastic waste could not be decomposed by soil microorganisms, although it has been exposed to sunlight or rain. Plastic waste has a negative impact and causes serious environmental problems. The process of recycling cannot solve the problem of plastic waste accumulation4
In recent years, because of great problems in using of synthetic plastics has led experts to develop environmentally and friendly biodegradable materials that derived from renewable natural resources such as bioplastics or biopolymers2. Bioplastics can reduce the waste of plastics significantly. Its are materials that can naturally decompose by the activity of microorganisms such as bacteria, fungi, and algae1. Recently, researchers focused on the biosynthesis of plastics by fermentation using bioplastic-producing microorganisms. One of the most method in producing of P(3-hydroxyalkanoate) or P(3HA) is a fermentation method using poly-producing biopolymer bacteria. It is known that certain bacteria produce P(3HA) in their cells as food and energy reserves for their growth under adverse conditions, such as lack of nitrogen, phosphate, oxygen, and magnesium5. The biopolymer produced by the bacteria can be extracted out of its cells and processed further according to the desired requirements. One member of P(3HA) group is poly(3-hydroxybutyrate) or P(3HB) is the most widely studied and produced by various microorganisms6. P(3HB) is easy to be decomposed appropriately over a period of time when discharged into the environment1. Some microorganisms could be produced P(3HB) in their cells. They are Alcaligenes, Bacillus, Pseudomonas, and some species of photosynthetic bacteria in nutritional conditions.
The main limitation in the development of bioplastics from P(3HB) is the expensive price compare to synthetic plastics because of high production costs. One effort that can be conducted to minimize the production cost of P(3HB) using of cheap carbon source7 because the highest production cost of P(3HB) comes from the substrate which is 40% of the total production cost8. Based on these analyzes, alternative steps are needed in the use of cheap and renewable substrate feedstocks so that the cost of bioplastic production can be minimized. Therefore, the researchers tried to explore some carbon sources that could reduce the production cost of P(3HB)9. One of the several alternative carbon sources abundant available in Indonesia is rice straw waste.
Paddy waste in Indonesia is used for fodder or mushroom cultivation media. The potential of straw waste is large enough, in other hands it was not widely utilized. Survey results showed that only 4% of rice straw used for feed and 96% disposed. The chemical composition of rice straw indicates that the cellulose content is quite high at 40% that suitable for microbial growth10.
Previous researchers performed the isolation of bacteria collected from the lake Singkarak, Tanah Datar regency, West Sumatra. A potential isolate of Pseudomonas aeruginosa could produce P(3HB) biopolymer. It produced polyhydroxy butyrate higher than polyhydroxy valerate and polyhydroxy hexanoate11 .
The purposes of this research were to produce bioplastic P(3HB) by fermentation of P. aeruginosa bacteria using straw rice stew at various concentrations compared to MCC cellulose and to evaluate the influence of the different type of carbon source of straw compared to MCC obtained from rice straw.
Equipment used were Gas Chromatography (Shimadzu), IR Spectrophotometer (Shimadzu), Digital Scales (Metler PM 200), Rotary Shaker Incubator (Bigger Digital), laminar air flow (ESCO), incubator (Gallenkamp), aseptic cabinet, refrigerator, Water Suction (Memmert), centrifuge, tools and glassware usual used in laboratory.
Materials used were rice straw waste (Oryza sativa Linn), Pseudomonas aeruginosa bacteria isolates, P(3HB) standard (Biopol), Sodium Hypochlorite, Nutrient Agar (Merck), Calcium Chloride (Merck), Copper Chloride (Merck), Zinc Sulphate (Merck), Ammonium Phosphate, Dipotassium hydrogen phosphate (Merck), Diammonium hydrogen phosphate (Merck), Magnesium Sulphate Heptahydrate, Chloroform (Bratachem), Zinc Chloride, Iodine, Sodium Chloride 0.9% and and other organic solvents.
Equipment Sterilization
Equipment used were washed and dried. Flasks were closed with cotton wrapped in gauze. All of the glassware were wrapped with parchment paper, then sterilized in an autoclave at a temperature of 121ºC, at a pressure of 15 lbs, for 15 minutes. The micropipette tips were placed in a beaker glass, covered with aluminum foil, then sterilized in an autoclave at the same condition above. Spatula and Ose needles were sterilized by flame sterilization method over the flame of alcohol lamp for about 20 seconds. The aseptic cabinet was cleaned of dust and sprayed using 70% alcohol into all parts of the cabinet. All works were performed aseptically12.
Preparation of Nutrient Agar Media (NA)
20 g Nutrient Agar (NA) medium was dissolved into 1 liter of boiled distilled water, heated over the heater, then stir until fully dissolved and cleared in color. It was covered with cotton plugs wrapped with sterilized gauze, sterilized with an autoclave13.
Preparation of Slant Agar
Four mL of sterile NA medium was poured into a test tube. Test tubes were put onto a slanting stage to let the medium solidify in the test tubes. The procedure was performed aseptically in Laminar Air Flow or aseptic cabinet 14.
Bacteria Insemination
The isolated and purified P. aeruginosa bacteria were transferred using an Ose needle on to solidified slant agar aseptically in laminar air flow. It was then incubated for 24 hours at a temperature of 37ºC, then stored in a refrigerator at 4 ºC.
Preparation of Bacteria Growth Medium
Preparation of Microelement Solutions: Microelement solution was prepared by dissolving 27.8 mg FeSO4.7H2O; 19.8 mg MnCl2.4H2O; 28.1 mg CoSO4.7H2O; 16.7 mg CaCl2.2H2O; 1.7 mg CuCl2.2H2O; and 2.9 mg ZnSO4.7H2O into 10 mL of 0.1 N HCl. Microelement solution was sterilized using an autoclave aseptically added into the sterilized medium1. Preparation of Carbon Resources: Boiling of Rice Straw: 80 g of cleaned straw was cut to about 1 cm in length and boiled in 800 mL of distilled water for ±30 minutes. It was squeezed the water to separate water-soluble compounds from straw, and then filtered. As nitrogen resources used 1.1 g (NH4) 2HPO4 /L substrate.
The P(3HB) biopolymer producing bacteria used in this research was Pseudomonas aeruginosa isolated and purified by the previous researcher. The water sample was collected from lake Singkarak in Tanah Datar regency, West Sumatra. P. aeruginosa can produce P(3HB) notified based on the test using Nile Blue A 1% reagent and incubated for 30 minutes, then observed under UV light at a wavelength of 365 nm. The orange color of bacterial colonies showed the microorganism could produce P(3HB) 14.
Table (1):
Biomass weight (dry cells) and pH of P. aeruginosa bacterial fermentation medium using rice straw stew and MCC obtained from rice straw as the carbon source.
| No | Carbon Source |
Time (hours) |
Concentrations (%) |
Biomass (mg/100 mL) |
pH |
|---|---|---|---|---|---|
| 1 | Rice straw stew | 48 | 20 | 286 | 6.6 |
| 40 | 275 | 6.7 | |||
| 60 | 263 | 6.6 | |||
| 80 | 269 | 6.7 | |||
| 2 | MCC | 48 | 0.1 | 155 | 6.7 |
| 0.5 | 569 | 6.6 | |||
| 1 | 982 | 6.6 | |||
| 1.5 | 1607 | 6.6 |
Fermentation was carried out using rice straw stew, and MCC obtained from rice straw as the carbon source. The raw materials used were rice straw taken from paddy fields around Limau Manis Padang. From 638 mL of rice staw stew was obtained from 80 g of rice straw that has been boiled using 800 mL of distilled water. 123.2 g or 24.64% of MCC was produced from 220 g ±-cellulose obtained from 500 g of rice straw.
The fermentation process is strongly influenced by the inoculum inserted in the inoculation process. Inoculum as a starter is a seed culture of microorganisms that will be used in a fermentation process. The bacterial inoculum used should fulfill the criteria such as should be active and healthy to minimize the lag phase in the fermentation process. The amount should be available in sufficient quantities to achieve the optimal proportion of inoculum and fermentation medium, free from contaminants and able to form stable products1,6.
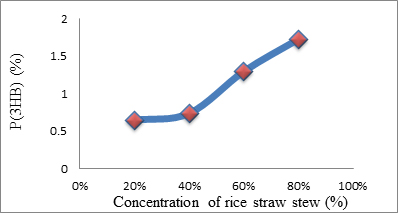
Fig. 1. The relationship between the percentage of P(3HB) accumulated in Psedomonas aeroginasa cells versus concentration rice straw stew used
The fermentation of rice straw stew and MCC obtained from rice straw were performed using P. aeruginosa bacteria. Concentrations of rice straw stew used were 20, 40, 60, and 80% while MCC were 0.1, 0.5, 1, and 1.5%. Fermentation was conducted at pH 7, at a temperature of 30°C, agitation at a rate of 200 rpm, and the addition of microelement and nitrogen source will optimize the bacteria growth. Temperature 30ºC is a good temperature for the growth of P. aeruginosa because this bacterium is mesophilic bacteria.
Table (2):
P(3HB) content accumulated in P. aeruginosa cells after fermentation for 48 hours using rice straw stew and MCC obtained from rice straw waste as the carbon source.
| Carbon Sources |
Time (hours) |
Concentration (g/100 mL) |
AUC | P(3HB) amount (mg/20mg) |
Percentage of P(3HB) (%) |
|
|---|---|---|---|---|---|---|
| 1. | Rice Straw Stew | 48 | 20 | 5.012 | 0.129 | 0.645 |
| 40 | 5.706 | 0.147 | 0.735 | |||
| 60 | 10.065 | 0.259 | 1.295 | |||
| 80 | 13.380 | 0.345 | 1.725 | |||
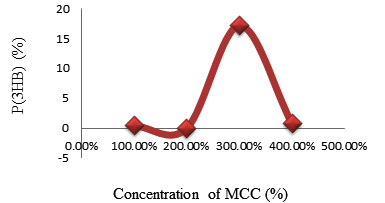
| 2. | MCC | 48 | 0.1 | 12.570 | 0.324 | 1.620 |
| 0.5 | 14.919 | 0.384 | 1.920 | |||
| 1 | 133.398 | 3.437 | 17.185 | |||
| 1.5 | 5.620 | 0.144 | 0.72 |
The effect of agitation is also very important to achieve optimum conditions for bacterial growth. The rate of Rotary Shaker Incubator is one of the parameters that can influence bacteria growth. The appropriate shaking during fermentation causes the mixture in the bacteria growth medium to become homogeneous, and nutrients available in the medium could be consumed by the bacteria sufficiently6. The fermentation time has an effect on the biomass produced. It was performed for 48 hours since the optimum bacteria growth in their replication to produce biomass. The higher biomass results because of the bacteria ability to adapt to the growth time and the nutrients available in the fermentation medium. Enzyme activities were also influenced by substrate concentration. At low enzyme substrate concentration does not reach maximum conversion rate because of the difficulty of the enzyme to find substrate for reaction. Increasing of substrate concentration would also increase the rate of reaction as the enzyme consistently binds to the substrates.
Fig. 2. The relationship between the percentage of P(3HB) accumulated in Psedomonas aeroginasa cell content versus concentration of MCC obtained from rice straw
The formation of P(3HB) occurs related to the availability of nutrients in the growth medium. Changing the number of nutrients will affect the growth phase of the cell. Reducing nutrients would inhibite the growth process over a certain time range. At this stationary phase, the number of living cells is equal to the number of dead cells, and cell concentration is maximum14. Nitrogen was set in limited amount but in contrary with the excess of carbon source. In this condition, bacteria will produce P(3HB) The abundant of carbon will be used by bacteria to produce P(3HB) which can then be converted into food reserves when worse environmental conditions occur1,6.
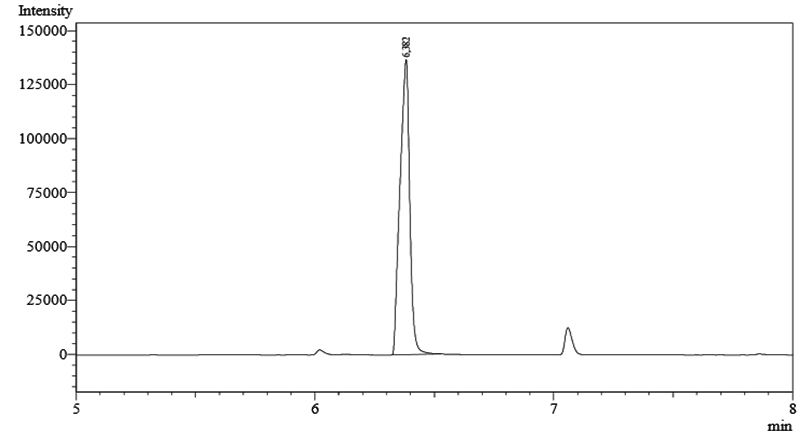
Fig. 3. Chromatogram of P(3HB) after methanolized into 3-hydroxybutyrate methyl ester at retention time 6.382 minute
After completed fermentation process, the biomass was separated from the supernatant by centrifugation at a rate of 3000 rpm for 20 minutes. The biomass layer was separated, dried in an oven at a temperature below 70 °C to constant weight. The dried biomass produced was 286 mg / 100 mL, the highest amount that achieved using 20% rice straw stew as a carbon source after 48 hours of fermentation by P. aeruginosa bacteria. While using MCC obtained from rice straw produced 1607mg/100 mL dried biomass, the highest amount found at MCC concentration of 1.5% at a fermentation time of 48 hours.
There was no changing on medium acidity during P. aeruginosa fermentation significantly. The pH was in the range of 6.6 to 6.8. Changing in pH during the fermentation process greatly affects the production of P(3HB) in the bacteria cell. The decrease in pH during the fermentation process due to the formation of carboxylic acids as a result of the fermentation. Decreasing of pH would reduce the concentration of P(3HB) produced. pH 7 phosphate buffer solution was added to the fermentation medium to avoid a significant decrease in pH.
The amount of P(3HB) produced was determined using gas chromatography. The dried biomass was first methanolized as follows. 20 mg of dry biomass was added with 1.7 mL of methanol, a solvent required in the esterification process, 0.3 mL of H2SO4 98% as catalyst and 2 mL CHCl3 to dissolve P(3HB) heated at temperature 100°C for 4 hours in the tightly closed tube to allow the reaction occurs, and to avoid evaporation during heating. Methanolysis was performed to convert P(3HB) into esteric form, i.e 3 hydroxymethyl ester group. After completing the reaction, the mixture was added with 1 mL of distilled water to the solution, shaken strongly to form two layers. The chloroform layer was pinched and 5 µL of the solution was injected into a gas chromatography.
The highest amount P(3HB) obtained analyzed using gas chromatography was 0.345 mg or 1.73% at a concentration of rice straw stew as a carbon source of 80% and duration of fermentation for 48 hours. While using MCC obtained from rice straw, the highest P(3HB) found was 3.437 mg/20 mg or 17.2% at a concentration of MCC of 1% and duration of fermentation of 48 hours. This suggests that the optimal concentration required by P. aeruginosa bacteria in producing P (3HB) using MCC obtained from rice straw as carbon source occurs at a concentration of 1%. At 1.5% concentrations, the P(3HB) obtained decreased significantly, this was due to depression of nutrients in microbial growth. Depressed microbial growth due to large external nutrients causes decreasing bacteria growth. Since to fulfill energy needed, the bacteria would use the stored P(3HB). While using the rice straw stew, the greater the concentration, the bigger P(3HB) produced. It was caused by not pure rice straw water and still, contains compounds such as lignin, holocellulose which inhibits P(3HB) formation. Judging from the comparison between biomass and the percentage of P(3HB) content resulting from the fermentation process using rice straw stew as a carbon source decreased biomass while the percentage of P(3HB) levels increased. However, using MCC obtained from rice straw as a carbon source has increased in the amount of biomass. So it could be concluded that the increasing amount of biomass did not affect to the percentage of P(3HB) produced.
The best source of carbon was MCC obtained from rice straw because in the fermentation process produced higher biomass amount and greater P(3HB) compared with using rice straw stew. The composition of rice straw stew was not analyzed. Compounds contained in rice straw stew may be water-soluble substance, and boiled water-soluble substance i.e hemicellulose, lignin, and others. It was suspected that there were still contain impurities and no nutritional available that needed for bacterial growth while using MCC even still contain cellulose its could be broken down by microorganisms.
The highest amount of P(3HB) obtained from the fermentation process of Pseudomonas aeruginosa bacteria was 0.3448 mg / 20 mg or 1.73% using the concentration of rice straw stew of 80% as a carbon source. While using MCC obtained from rice straw as carbon source produced the highest P(3HB) amount using MCC at a concentration of 1% was 3.437 mg/20 mg or 17,19%. Concentrations of rice straw stew and MCC produced from rice straw affected the biomass and P(3HB) levels. Fermentation using rice MCC as carbon source produced higher P(3HB) compare to rice straw stew.
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Research and Higher Education, Republic of Indonesia. This research is funded through the Grant Research Scheme Hibah Berbasis Kompetensi, Fiscal Year 2018, under Contract Number: 050/SP2H/LT/DPRM/2018, University of Andalas, Padang, Indonesia.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Akmal, D., Asiska, P. D, Wangi, Q A, Rivai, H, Agustien A., Biosynthesis of copolymer poly(3-hydroxybutyrate-co-3hydroxyvalerate from palm oil and N pentanol, Rasayan Journal of Chemistry, 2015; 8(3): 389-395.
- Arikan, E. B., & Ozsoy H. D. 2015, A Review: Investigation of Bioplastics, Civil Engineering and Architecture 9. Turkey, 188-192.
- Rhim J. W. 2007. Natural Biopolymer-Based nanocomposite films for packaging applications. Critical Reviews in Food Science and Nutrition. Vol 47. 411.
- Suardi M, Wangi Q A, Salman, Zaini E, and Akmal D., Microencapsulation of Verapamil Hydrochloride using Poly (3-hidroxybutyrate) as Coating Materials by Solvent Evaporation Method Research, Journal of Pharmaceutical, Biological and Chemical Sciences, 2016; 7(1): 1725-1732.
- Anderson, A. J., & Dawes, E. A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial of polyhydroxyalkanoates. Microbial Rev, 1990; 54: 450-472.
- Majid, M. I. A., Akmal, D., Few, L. L., Agustien, A., Toh, M. S., Samian, M. R., Najimudin, N. & Azizan, M. N. Production of Poly(3-hydroxybutyrate) and its copolymer Poly(3-hydroxybutyrate-co-3-hydroxy valerate) by Erwinia sp. USMI-20. Int. J. Biol.Macromol, 1999; 25: 95-104.
- Kim, BS. Production of poly (3-hydroxybutyrate) from the inexpensive substrate. Enzym Microb Technol. 2000; 27: 774-777.
- Lee, S. Y. Bacterial polyhydroxyalkanoates. Biotechnology and Bioengineering. 1996; 49: 1-14.
- Wang F & SY Lee. Poly 3-hydroxybutyrates production with high polymer content by a fed-batch culture of alcaligenes latus under nitrogen limitation. Appl Environment Microbiol, 1997; 63: 3703-3706.
- Halim A. Production of Microcrystaline Cellulosa from Rice Straw. J. Sains Tek. Far. 2002; 7(2): 80-87.
- Thirumala M, SV Reddy & K Mahmood. Production and characterization of PHB from two novel strains of Bacillus spp. isolated from soil and activated sludge. J Ind Microbiol Biotechnol. 2010; 37: 271–27814.
- Djamaan A, Noviza D, Septianingsih D, Suardi M, The use of purple sweet potato (Ipomoea batatas) starch as binder in manggosteen peel extract lozenges formulation, Der Pharma Chemica, 2016; 82: 410-414.
- Alen Y, Nufika Y., Suharti N, Nakajima S, Djamaan A., The determination of profenofos insecticide residue cabbage (Brassica oleracea), Der Pharmacia Lettre,, 2016; 8(8): 137-140.
- Sayuti I, Siregar Y I, Amin B, Agustien A, Djamaan A, 2017, Identification of Bacterial Hydrocarbonoclastic in Waste Tanks, Petapahan, Riau, Indonesia, using 16sr RNA, Journal of Pure and Applied Microbiology, 12(2): 671-677.
- Djamaan, A, Mhd Riza Marjoni, and Friardi Ismed, The Influence of Pretreatment Time, Type and the Concentration of Yeast on Ethanol Production from Rice Straw. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2015: 6(3): 583-591.
- Artasastra M A, Yanwirasti, Djamaan A, 2017, Cytotoxic activity screening of ethyl acetate fungal extract derived from the marine sponge Neopetrosia chaliniformis AR-01. Journal of Applied Pharmaceutical Science, 7(12): 174-178.
- Rivai, H, Asia A, Rina W, Alen Y, Handayani D, Aldi Y, Marlina, and Djamaan A., 2016, Isolation of Endophytic Bacteria from Bark, Leaf, and Pericarp of Mangosteen (Garcinia mangostana L.) and Testing of The Antimicrobial Activity, Research Journal of Pharmaceutical,Biological and ChemicalSciences,7(1): 1910-1920.
- Yamane, T., Chen, X & Ueda, S. Polyhydroxyalkanoates Syntesis from Alcohol During The Growth of Paracoccus denitrifications. FEMS Microbiol. Reff, 1996; 135: 207-211.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.