ISSN: 0973-7510
E-ISSN: 2581-690X
Wastewater of textile industry is considered as toxic waste containing various dyes and chemicals that makes it recalcitrants. Decolorization of wastewater of the industry was conducted in our laboratorium using fungi isolated from a local forest of North Sumatera. Six potential isolates, previously screened for the ability to grow in plate media containing 100% of wastewater, were used for the study in a liquid culture condition. The results show that all tested isolates grew. The growth response of isolates cultured in a media supplemeted with 1% glucose was about two to three times higher than that of cultures without glucose. In the first culture, the fungi grew on the surface of the media however in the latter experiment, it concentrated on the bottom of culture flask for all of tested isolates. Decolorization analysis shows that all isolates were able to decolorize the wastewater. The cultures supplemented with glucose decolorized wastewater from 20% to 46%, while cultures without glucose did much higher about 56% to 92%. Furthermore, a microscopic analysis shows that dyes molecules in the wastewater were absorbed to fungal mycelia. The findings show that extraceluler molecules existed on the surface of fungal mycelia play an important role in the absorption of dye molecules.
Decolorization, Fungal biomass, Mycelial absorption of dyes, wastewater of textile industry.
The textile industry is one of important sectors in many countries including Indonesia. The industry uses a lot of water and generates a huge volume of highly polluted effluents including metals and color materials. Hessel et al. (2007) states that color is noticeable at a dye concentration of 1 mg/L and an average concentration of 300 mg/L has been reported in effluents from textile manufacturing processes. During the dyeing processes, 2-60% of the initial used dyes are lost in the effluents. Nilsson et al. (1993); Somasiri et al. (2008); Haroun and Idris (2009) prove that coloured wastewater from the textile industries is one of the most obvious indicators of water pollution. The presence of dyes in water bodies even in a small amount reduces the sunlight penetration into deeper layers inhibiting photosynthetic activities of aquatic flora and decreasing the amount of dissolved oxygen in the water which causes detrimental effects on aquatic flora and fauna. Furthermore, dyes are resistant to degradation when discharged into the environment. And, these dyes are mutagenic and carcinogenic to living creatures.
Efforts to remove color in wastewater of textile industry have been done, many encouraging results have been reported and some techniques have been proposed. Robinson et al. (2001) and Pearce et al. (2003) say that three types of treatment techniques of decolorization of wastewaters of textile industry have been intensively studied; chemical, physical and biological approaches. Chemical treatment techniques include ozonation, fenton oxidation and photo catalytic oxidation, and reduction reactions. They convert hazardous pollutant to nonhazardous or less toxic compounds. The physical techniques are absorption, ion exchange, irradiation, membrane filtration, electrolysis, coagulation/flocculation and ultrasonic mineralization methods. The last treatment is through biological technique, which includes bacterial and fungal biodegradation and biosorption in aerobic, anaerobic or combined treatment processes. The latter has attracted much attention from the environmental safety viewpoint compared to others.
Recently, researchers have focused on biological techniques as an alternative. Then potential use of microorganisms to decolorize dyes of textile industry has been obtaining much attention. The white-rot fungi Bjerkandera adusta packed in a fixed-bed bioreactor exhibits decolorization of wastewater of dyeing process from the textile industry. The fungus remains effective during 4 cycles of decolorization for a period of 70 days under non-sterile conditions and with no nutrient addition (Anastasi et al., 2010). Ganoderma lucidum grow under optimal condition (pH 6.6; temperature 26.5ºC; agitation speed 200 rpm; dye wastewater concentration 1:2) exhibits decolorization rate upto 81.4% and reduces chemical oxygen demand by 90.3% (Selvakumar et al., 2012). Under sterile conditions, Bjerkandera sp. enables wastewater decolorization from the textile effluent by 65% in 8 days and its toxicity reduced by 58%, whereas under non-sterile conditions, the decolorization percentage is only 40% (Echevarria et al., 2012).
In recent years, intensive researches have been focused on the decolorization of wastewater by fungi, particularly the white-rot fungi, because they produce extracellular enzymes such as laccases, lignin peroxidases (LiP) and manganese-dependent peroxidases (MnP), which have been related to the degradation of different synthetic chemicals, including dyes (Tang et al., 2011). This ability has opened new prospects for the development of biotechnology processes aiming at the effluents decolorization. The main limitation of this process is that it can require a significant long period of time because the fungal decolorization is usually slow (Faraco et al., 2009). Therefore, the development of new strategies for fungi application in the decolorization of textile effluent, with reduced time of treatment, is needed. Finding fungal strains that able to efficiently decolorize the diversity of dyes presents in the textile effluents, with wide ranges of pH and salt concentration, is of a great interest (Alcalde et al., 2006; Kaushik and Malik 2009). The objective of our study is to evalute the ability of fungal biomass in decolorization of wastewater of the textile industry. This paper describes how selected fungal isolates, newly isolated from local forest, were able to decolorize wastewater upto 90% through an absorption process of dye molecules.
Microbe and wastewater
Microbes used in this study were fungi isolated from Gunung Barus Forest of North Sumatera. Isolation was conducted from soil and fruitbody collected from the forest. The potato dextrose agar (PDA) was used for isolation. The growth colony was transferred to fresh PDA plate until pure culture was obtained. The waste used for this study was wastewater of the textile industry of dyeing process obtained from local batik industry in Medan, Nort Sumatera Indonesia. The waste contained indogosol dye.
Cultivation and measurement of fungal growth
Potential isolates were screened previously by culturing the isolates on a plate agar containing wastewater. The isolates exhibited good growth in the media used. In this study, fungi were cultured in a liquid media containing 25% of wastewater. Two types of media were prepared, one supplemented with 1% of glucose and another without glucose. In addition, minimal salt nutrient containing KH2PO4, K2HPO4, MgSO4, NaCl, FeSO4, ZnSO4, MnSO4 and NH4NO3 was included in both media. Ten plugs (w, 5 mm) of active growing colony were inoculated to 100 ml media in 250 ml Erlenmeyer flask. Cultures were incubated ata room temperature (±27ºC) under stationary condition for one month. For measurement of fungal growth, cultures were filtered on to filter paper and dried in an oven overnight at 60ºC until reaching a constant weight.
Measurement of decolorization activity
In this study, decolorization analyses of the wastewater were focused on absorption ability of fungal mycelia on dyes material instead of degradation, since current results was unable to examine substantial degradation or reduction of dyes. At the end of cultivation period, one month incubation, the culture media was carefully pipetted to spectrophotometer cuvette. The absorbance of media was read at 590 nm using UV-Mini-1240 type spectrophotometer of Shimadzu at a room temperature. The maximum absorbance, at 590 nm, was previously scanned and media containing 25% of wastewater was used as a control and distilled water was used for a comparison. Percentage of decolorization was calculated following the formula proposed by Mahmoodi et al. (2006) with a modification. The value of absorbance was corrected with absorbance of dH2O.
Microscopic observation
Microscopic analyses was considered the important and initial step to elaborate the possible decolorization process. The liquid part of the culture media was pipetted to a clean Erlenmeyer flask for analyses of metals and total suspended solid and the results will be reported latter. The mass of mycelia was carefully transferred to a clean petridish and observed under a microscope of Zeiss type with a low magnification (10 x 10). And pictures were taken with an AxioCam Erc 5s type camera equipped at the microscope.
The growth response of isolates in a media containing wastewater
During the course of this study, all 26 isolates which were obtained from Gunung Barus Forest were screened for their ability to produce ligninolytic enzyme qualitatively in CzapekDox Agar containing 0.02% of guaiacol. The positive ligninolytic isolates were indicated by a brown precipitate forming on the culture plate as an indicator of phenolic compound of guaiacol was oxidized (Coll et al., 1993). Five of those isolates (TB01, TB04, TB06, ZN02, and ZN04) showed strong ligninolytic activity and other isolates exhibited low activity to not detected. The first group was considered as ligninolytic potential isolates and the second was non-ligninolytic. Then all five selected ligninolytic isolates were tested on their potential to grow in MSM plates containing the wastewater. At the same time, the other 21 isolates, non-ligninolytic, were also cultured. Results showed that all ligninolytic potential did not exhibit good growth in the media containing 100% of wastewater supplemented with 1% of glucose. Meanwhile, six isolates which were non-ligninolytic from the previous test, the ligninolytic test, exhibited a good growth and grew faster. They were isolate TB02, TB02K, TB03, TB11, ZN03, and ZN06. These results were considered contradicted with our first concern, in which ligninolytic isolates would have good growth in the wastewater containing media. The profile of ligninolytic enzyme activity including lingin peroxidase, managanese peroxidase and laccase of ligninolytic isolates was studied separately and the results are reported somewhere. Then, all six isolates growing better in the wastewater containing media were used for decolorization analysis in a liquid condition.
Two types of media were prepared for the analysis, the first media was supplemented with 1% of glucose and the second without glucose. Both media contained 25% of wastewater of the textile industry. Surprisingly, all six isolates grew in both conditions, including in the media containing no glucose. The results showed a different growth response. Fungi grew much faster inthe media containing glucose. Table 1 showed biomass (gram dried weight per flask) of four isolates grown in the tested media. The other two isolates (Zn03 and Zn06) were used for adaptation study. The biomass of all isolates were about double when they were cultured in the wastewater media containing 1% of glucose. The glucose in the media contributed to support a good growth of the tested fungi. These results are in common with some reported studies on the untilization of carbon in culture media, in which glucose is the most readily metabolizable molecule.
Table (1):
Dried weight of isolates in the media containing 25% of wastewater of the textile industry (g/flask)
No. |
Code of isolates |
Plus 1% of glucose |
No glucose |
|---|---|---|---|
1. |
TB02 |
0.1570 |
0.0545 |
2. |
TB02K |
0.1565 |
0.0610 |
3. |
TB03 |
0.1755 |
0.0590 |
4. |
TB11 |
0.0840 |
0.0445 |
The most important thing from the results (Table 1); the selected isolates were able to grow in the media containing no glucose even at a low rate. The isolate might have utilized the carbon existed in the waste to support the growth even at a much lower rate compared with their growth in a glucose containing culture. The fungi grew and of covered surface of media. As shown in the figure above, when glucose was not included in the culture, the growth response was relatively much slower and the fungal mycelia were concentrated on the bottom of the culture flask, as shown in the following Figure 1. Furthermore, it was clearly noticed that fungi growth on the bottom (right) absorbed dyes and suspended solid content in the media. Thus, it was creating clean solution media surrounding the mycelial mass. The dark green color of mycelial mass could have been forming as a consequence of dyes and suspended solid absorbed.

Fig. 1. Growth of selected isolate (TB11) in the wastewater containing media (the left supple-mented with 1% of glucose and the right side with no glucose)
Decolorization of the wastewater
The liquid phase of cultures was carefully transferred to a spectrophotometric cuvette and the absorbance was read at 590 nm at a room temperature. The results (Table. 2) showed that all isolates could decolorize waste to a different rate in both conditions. Different with the growth response as described above in which biomass was higher in the glucose containing media, the rate of decolorization was found higher in the culture with no glucose. In cultures supplemented with 1% of glucose, decolorization was ranging from 20 to 46%, while in the culture without glucose was from 59 to 92%. The highest rate was found in the isolate TB11 by 92%. Figure 2 is the photograph of decolorization results as compared to control and distilled water.
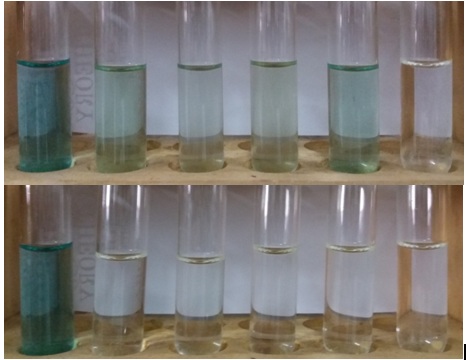
Fig. 2.The liquid phase of the culture media after cultivation of selected isolates in the media containing 25% of wastewater (top figure containing 1% of glucose and the bottom with no glucose). From left to right, control (25% of waste), isolate TB02, TB02K, TB03, TB11, and dH2O.
The results, as shown in Figure 2, clearly showed the intensity of color was still higher in the media containing glucose (top) relative to the culture media without glucose (bottom). In the latter cultures, most of dyes were removed from the liquid, and the highest rate of decolorization was achieved by isolate TB11 at 92%. However, these results did not directly reflect dyes in the wastewater that has been degraded, since dyes was absorbed to mycelia. The microscopic analysis confirmed the absorption of dyes to mycelia as described below. The results of decolorization analyses of the selected isolates in this study were very much alike with many reported works. Bla´nquez et al. (2008) reported that Trametes versicolor enabled to decolorize wastewater of the textile industry by the rate of 60%, and Amaral et al. (2004) found that the same fungus could decolorized by 92%. Phanerochaete chrysosporium, the model ligninolytic fungus, exhibited even higher decolorization activity. Assadi et al. (2001) proved that the fungus decolorized textile wastewater by 98%, while Dayaram and Daspupta (2008) found that Polyporus rubidus degraded dyes greater than 80% of dyes within 5 days under stationary incubation conditions. Furthermore, Moreira-Neto (2013), basidiomycetes fungi from the genera of Trametes, Lentinus, Peniophora, Pycnoporus, Rigidoporus, Hygrocybe and, Psilocybe have an ability to decolorize CBB H-GR and Cibacron Red FN-2BL by more than 70%. Leidig et al. (1999), found that Trametes versicolour grown in a 1.0-L aerated stirred tank bioreactor under non-sterile conditions was capable of decolorizing polyvinylamine sulphonate anthrapyridone (Poly R-478) with the average dye elimination of 80% and it was achieved after 19-day cultivation.
Dyes absorption on mycelia
After liquid phase of the media was removed carefully from the culture flask, the mycelia was transferred to a clean glass plate and observed under a microscope. The results are shown in Figure 3.
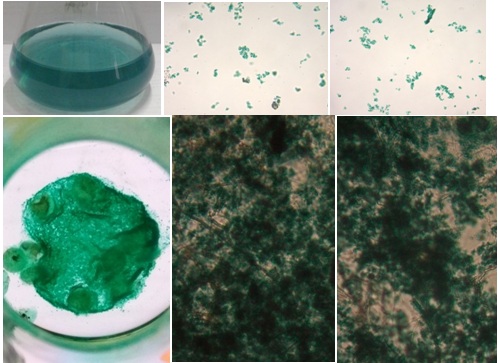
Fig. 3.A photomicrograph of the mycelia absorbing dyes. Top is the control(25% of waste) and its microscopic views, the bottom is the mycelia absorbing dyes. Far right isthe mass of mycelia in the culture flask iluminated with a light from the bottom.
As shown in the results above, the photomicrograph top pictures, the dyes molecule (indogosol) in the wastewater exited as an insoluble molecule. At the bottom left figure, all dyes molecules were almost completely absorbed to mycelia. A bright view was generated since the culture flask was illuminated with a light from the bottom. Importantly, microscope analysis confirmed that dyes molecules were absorbed to fungal mycelia. These results indicated that extra polymer substance (EPS) on existed fungal mycelia might play an important molecule in binding the dyes, as reported by other researchers in different aspects. Among the microbial EPS producers, bacteria and fungi are the most common. The bacterial EPSs have been studied extensively by researchers, and many types of EPS have been reported from different species. Marvasi et al. (2010) described four types of EPS: structural, sorptive, active, and surface-active EPS. The production of the molecules depends on physiological stress encounter in the natural environment (Mahapatra and Banerjee, 2010). In addition to those types, Flemming et al. (2007) previously introduced the redox-active, informative and nutritive EPS. The molecules are released by the microorganisms in their natural environment and in the laboratory conditions. The composition of EPS produced depends on the genetic of the microbes and the phsysiochemical environment in which the molecules develop (Sutherlands, 2001). Initially, most studies of EPS described extracellular polysaccharides. Wigender et al. (1999) and Deco (2000) reported that EPS is considered more complex, including lipopolysacharides, proteins, peptides, nucleic acids, glycolipids, and lipid.
Most studies on EPS have been focused on bacteria. The EPS production from fungi has been studied adequately over the last two decades. Wood rotting fungy including Ganoderma lucidum, Agaricus blazi, Cordycep ssp., Lentinus edodes, and Grifola frondosa through submerged cultures have been reported to produce EPS. All of which have different and interesting biological activities (Yang and He, 2008). Tang et al. (2002) reported that EPSs are generally synthesized intracellularly and secreted to the surroundings. Very little information is available regarding the biosynthesis of EPSs from fungi. Only a few fungal EPS biosynthesis pathways have been studied, such as EPS biosynthesis by G. lucidum. From the results of this study in which dyes of the wastewater absorbed to fungal mycelia as shown at figures above, it is hyothe sized that the fungal hypha were covered by the EPS. Furthermore, since dyes molecules were insoluble, it is indicated that the EPS produced by the fungal myceliamight have a nonpolar molecule. These results were considered very important and under investigation in our laboratory. As far as the literatures have been surveyed, the study of decolorization by fungi has mostly focused on the degradation process involving lignin degrading systems. Based on the results of this study, we have a confident view that this is the first report on the role of EPS of basidiomycetous fungi in the decolorization of dyes of the textile industry.
Table (2):
Decolorization ability of isolates after cultivation for one month (absorbance 590 nm)
| No. | Isolates | Plus 1% of glucose | No glucose | |||
|---|---|---|---|---|---|---|
| Absorbance | Decolorization (%) | Absorbance | Decolorization (%) | |||
| 1. | Control (25% of waste) | 0.221 | – | – | – | – |
| 2. | dH2O | 0.097 | – | – | – | – |
| 3. | TB02 | 0.201 | 20 | 0.139 | 67 | |
| 4. | TB02K | 0.168 | 46 | 0.150 | 59 | |
| 5. | TB03 | 0.170 | 44 | 0.135 | 71 | |
| 6. | TB11 | 0.178 | 38 | 0.107 | 92 | |
ACKNOWLEDGMENTS
This work was supported by grant-in-aid from the Minister of Science and Technology and Higher Education of the Republic of Indonesia through Higher Education Allocation Fund of the University of Sumatera Utara 2016.
- Hessel, C., Allegre, C., Maisseu, M., Charbit, F., Moulin, P. “Guidelines and legislation for dye house effluents, a review”. Journal of Environmental Management, 2007; 83: 171-180.
- Nilsson, R., Nordlinder, R., Wass, U. “Asthma, rhinitis and dermatitis in workers exposed to reactive dyes”, British J. Industrial Medicine, 1993; 50: 65-70.
- Somasiri, W., Li, X., Ruan, W., Jian, C. Evolution of efficacy of upflow anaerobic sludge blanket reactor in removal of colour and reduction of COD in real textile wastewater. Bioresour Technol., 2008; 99: 3692-3699.
- Haroun, M., Idris, A. Treatment of textile dye with an anaerobic fluidized bed reactor. Desalination, 2009; 237: 357-366
- Robinson, T., McMullan, G., Marchant, R. Remediation of dyes in textile effluents: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol., 2001; 77: 247-255.
- Pearce, C.I., Lloyd, J.R., Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigments 2003; 58: 179-196
- Anastasi, A., Spina, F., Prigione, V., Tigini, V., Giansanti, P., Varese, G. C. “Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta”, Bioresource Technology, 2010; 101: 3067- 3075.
- Selvakumar, S., Manivasagan, R., Chinnappan, K. “Biodegradation and decolourization of textile dye wastewater using Ganoderma lucidum”, 3 Biotech, 2012, DOI 10.1007/s13205-012-0073-5.
- Echavarría, J O., Vidal Benavides, A.I.V., Díaz, J. C.Q. “Decolorization of textile wastewater using the white rot fungi anamorph R1 of Bjerkanderasp”, Revista de la Facultad de Ingeniería de la Universidad de Antioquia, 2012; 57: 85-93.
- Tang, W., Jia, R., Zhang, D. Decolorization and degradation of synthetic dyes by Schizophyllum sp. F17 in a novel system. Desalination, 2011; 265: 22-27.
- Faraco, V., Pezzella, C., Miele, A., Giardina, P., Sannia, G. Bio-remediation of colored industrial wastewaters by the white-rot fungi Phanerochaete chrysosporium and and Pleurotus ostreatus and their enzymes. Biodegradation, 2009; 20: 209-220.
- Alcalde, M., Ferrer, M., Plou, F.J., Ballesteros, A. Environmental biocatalysis: from remediation with enzymes to novel green processes. Trends Biotechnol., 2006; 24: 281-287.
- Kaushik, P., Malik, A. Fungal dye decolourization: recent advances and future potential. Environ Int., 2009; 35: 127-141.
- Mahmoodi, N.M., Arami, M., Limaee, Y.N. Photocatalytic degradation of Triazinic ring– containing azo Dye (Reactive Red 198) by using immobilized TiO2 Photoreactor: Bench scale study. J. Hazard. Mater., 2006; 133: 113-118.
- Coll, P. M., Fernandez-Abalos, J. M., Villanueva, J. R., Santamaria, R., Perez, P. Purification and Characterization of a Phenoloxidase(Laccase) from the Lignin-Degrading Basidiomycete PM1 (CECT 2971). Applied And Environ. Microbiol., 1993; 59: 2607-2613.
- Bla´nquez, P., Sarra, M., Vicent, T. Development of a continuous process to adapt the textile wastewater treatment by fungi to industrial conditions. Process Biochem., 2008; 43:1-7.
- Amaral, P.F.F., Fernandes, D.L.A., Tavares, A.P.M., Xavier, A.B.M.R., Cammarota, M.C., Coutinho, J.A.P., Coelho, M.A.Z. Decolorization of dyes from textile wastewater by Trametes versicolor. Environ Technol., 2004; 25:1313-1320
- Assadi, M.M., Jahangiri, M.R. Textile wastewater treatment by Aspergillus niger. Desalination, 2001; 14:1-6
- Dayaram, P., Dasgupta, D. Decolorisation of synthetic dyes and textile wastewater using Polyporus rubidus. J. Environ. Biol., 2008; 29: 831-836.
- Moreira-Neto, S.L., Mussatto, S.I., Machado, K.M.G., Milagres, A.M.F. Decolorization of salt-alkaline effluent with industrial active dyes by laccase-producing basidiomycetes strains. Letters in Applied Microbiol., 2013; 56: 283-290.
- Leidig, E., Prusse, U., Vorlop, K.D., Winter, J. Biotransformation of poly R-478 by continuous cultures of PVAL-encapsulated Trametes versicolor under non-sterile conditions. Bioprocess Engineering, 1999; 21: 5-32.
- Marvasi, M.M., Vissher, P.T., Martinez, L.M. Exopolymeric substances (EPS) rom Bacillus substillis: polymers and genes encoding their sythesis. FEMS Microbial Lett., 2010; 1-9.
- Mahapatra, S., Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiology Insights, 2013; 6: 1-16.
- Flemming, H.C., Neu, T.R., Wozniak, D.J. The EPS matrix: the ‘house of biofilm cells’. J. Bacteriol. 2007; 189: 7945-7947.
- Sutherland, I. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology, 2001; 147: 3-9.
- Wingender, J., Neu, T.R. Flemming, H.C. Microbial Extracellular Polymeric Substances: Characterization, Structure, and Function. Springer, Berlin . 1999.
- Decho, A.W. Exopolymer microdomains as a structuring agent for heterogeneity within microbial biofilm. Microbial Sediments (Riding RE & Awramik SM, eds), 2000; pp. 9-15. Springer, Berlin.
- Yang H., He, G. Influence of nutritional conditions on exopolysaccharide production by submerged cultivation of the medicinal fungus Shiraia bambusicola. World J. Microb. Biot. 2008; 24: 2903-2907.
- Tang, Y.J., Zhong, J.J. Exopolysaccharide biosynthesis and related enzyme activities of the medicinal fungus, Ganodermalucidum, grown on lactose in a bioreactor. Biotechnol. Lett., 2002; 24: 1023-1026.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


