ISSN: 0973-7510
E-ISSN: 2581-690X
There is an alarming rise in carbapenem-resistant Enterobacteriaceae (CRE) causing nosocomial infections such as ventilator-associated respiratory infections (VARIs). The use of rapid phenotypic methods for the detection and differentiation of carbapenemases elaborated by these CRE would be helpful in providing timely empirical therapeutic options for management of these infections and preventing spread of these CRE strains in hospital settings. Hence, this study aimed to detect CRE among pathogens isolated from the endotracheal secretions recieved from suspected cases of VARIs and differentiate carbapenemases elaborated by these CRE using combined phenotypic methods, such as the modified carbapenem inactivation method (mCIM) and EDTA modified CIM (eCIM). This observational study was conducted over a period of 1 year in the Department of Microbiology and the intensive care unit of a tertiary care center. Carbapenem resistance was found in 75% of Klebsiella pneumoniae isolates and 50% of Escherichia coli isolates, of which 58.4% were metallo-β-lactamases and 41.6% were serine carbapenemase producers. In conclusion, the combination of the mCIM and eCIM could be useful as an epidemiological tool and be considered essential in deciding the initial antibiotic therapy, help reduce morbidity and mortality associated with VARIs, and guide hospital infection control practices.
Modified carbapenem inactivation method, EDTA-modified CIM, carbapenemase-producing Enterobacteriaceae, ventilator-associated respiratory infections
Multidrug-resistant (MDR) gram-negative bacteria such as carbapenem-resistant Enterobacteriaceae (CRE) have increasingly been reported as etiopathogens of most common nosocomial infections such as Ventilator-associated respiratory infections (VARIs).¹ This condition includes both ventilator-associated tracheobronchitis (VAT) and ventilator-associated pneumonia (VAP).¹ An increase in MDR Enterobacteriaceae causing invasive and nosocomial infections, including VARI, poses a global therapeutic and diagnostic challenge.2,3 Carbapenems such as ertapenem, meropenem, and imipenem have provided the best therapeutic responses for infections caused by robust MDR in mechanically ventilated patients.4,5 Therefore, the development of resistance to carbapenems has emerged as a global threat to the management of this spectrum of respiratory infections.6
The emergence of carbapenem-resistant Enterobacteriaceae (CRE) is associated with the expression of carbapenemase-encoding genes in conjugative plasmids.7 These enzymes belong to the class A, B, and D beta-lactamases and hydrolyze carbapenems, rendering them ineffective against these bacteria.8 Carbapenemases are broadly classified into two groups: metallo-β-lactamases (MBLs) and non-metallo-β-lactamases (serine carbapenemases).9 MBLs are zinc-dependent; thus, EDTA can block their activity by binding to zinc and rendering their enzymatic action ineffective.10 Although in routine laboratory practices, detection of the various drug resistance mechanisms among these CRE is not conducted, it would be helpful in periodic monitoring of MDR strains in hospital and in effectively guiding the emperical management of these nosocomial infections.
According to the Clinical and Laboratory Standards Institute (CLSI) recommendations, the Modified Hodge Test (MHT) was the first laboratory test recommended to detect carbapenemases.11 In recent years, carbapenem inactivation methods have been added to the list of substantially less expensive phenotypic tests. These methods assess the growth of carbapenem-susceptible bacteria around a carbapenem drug disc that is incubated prior to the introduction of a test strain that is carbapenem-resistant and a suspected carbapenemase producer. If the test organism is found to express the carbapenemase enzyme, the drug in the disc will be hydrolyzed and the organism will grow to the edge of the drug disc.12 The disadvantage of this method is that it cannot differentiate between MBLs and non-metallo-β-lactamases (serine carbapenemases). Hence, it was further modified by adding EDTA to help identify and differentiate between the two carbapenemases.9,12
Detection and differentiation between the two classes of carbapenemases is important as the newer classes of β-lactam-β-lactamase inhibitors, such as ceftazidime-avibactam and meropenem-vaborbactam, are found to be more active against serine carbapenemase producers, and not MBL producers, in addition to the fact that MBLs are widely prevalent in hospital microbiota.13 Combination therapies with ceftazidime-avibactam and aztreonam, tigecycline, aminoglycosides, and colistin were more effective against MBL producers. With this background, our study aimed to detect CRE causing VARI and differentiate the classes of carbapenemases expressed using rapid and economically feasible phenotypic methods, such as combined mCIM and eCIM.
Study setup
This observational study was conducted in the Department of Microbiology at Himalayan Institute of Medical Sciences, Jolly Grant, on endotracheal secretions received in the bacteriology laboratory from suspected cases of VARI admitted in the hospital’s intensive care unit, for a period of one year from December 2018 to November 2019. Ethical approval from the institutes ethics committee was obtained and patient’s informed and written consent was also taken.
Case definition of VARI
VARIs were suspected when respiratory tract infections developed after 48 h after mechanical ventilation in critically ill patients, as these infectious respiratory complications are associated with artificial ventilation. This spectrum of respiratory diseases includes VAT and VAP1. Both VAP and VAT overlap in terms of clinical symptoms and signs the patient presents with and microbiology, but differ in the fact that for the diagnosis of VAP, there must be radiological evidence of persistent infiltration on chest X-rays.
Methodology
Endotracheal secretions from suspected
cases of VARI were received in the microbiological laboratory. The samples were subjected to Gram staining and aerobic culture on blood and MacConkey agar, and incubated at 37° C overnight. Gram-stained smears were screened under an oil immersion lens for the presence of predominant bacterial morphology, namely cocci and bacilli, gram reaction was noted and were reported. All samples were subjected to qualitative assessment during screening and specimens with more than 25 polymorphonuclear cells were considered for further processing. Isolates recovered from overnight cultures were identified with the VITEK-2 automated system. This automated system uses growth-based technology for identification and provides the minimum inhibitory concentration (MIC) for the antimicrobial agents tested, which was used to identify and report CRE.
Phenotypic detection of carbapenemase producing Enterobacteriaceae
Both mCIM and eCIM were performed on CRE isolates according to CLSI guidelines.12 For each isolate to be tested, a 1 μL loopful of bacteria from an overnight blood agar plate was emulsified in 2 mL of trypticase soy broth (TSB). For each isolate, a second 2 mL TSB tube was labelled for the eCIM test. To this tube, 20 μL of 0.5 M EDTA was added. A 10 μg meropenem disk was placed in each tube using sterile forceps. The tubes were incubated at 35°C for 4 ± 15 min. The drug discs were removed and applied onto a Mueller Hinton agar (MHA) plate with a 0.5 McFarland suspension of carbapenem-susceptible Escherichia coli ATCC 25922 strain. The MHA plates were subsequently incubated at 35°C for 18-24 h and the mCIM and eCIM results were interpreted. The mCIM test was considered positive if the zone size was between 6 and 15 mm and negative if the zone size was ≥19 mm. An isolate was considered positive for metallo-carbapenemase production when the eCIM zone size increased by ≥5 mm compared with the mCIM zone size observed. Isolates were considered negative for metallo-carbapenemase if a zone size increase of <4 mm was observed.
Statistical methods
Interpretation and analysis of the obtained results were performed using version 20 of Statistical Package for Social Sciences (SPSS) and Microsoft Excel. Qualitative data were expressed in terms of frequency and percentage, and quantitative data were expressed as means and standard deviations.
A total of 141 gram negative isolates were obtained from endotracheal secretions of consecutive clinically suspected cases of VARIs included in our study. Of these 141 isolates, 118 (83.69%) were resistant to one or more carbapenem drugs. The predominant carbapenem-resistant Enterobacteriaceae were Klebsiella pneumoniae (27.96%) and Escherichia coli (7%).
Phenotypic detection of carbapenemase producing Enterobacteriaceae
Of the 44 isolates of K. pneumoniae, 33 (75%) were carbapenem resistant. Of the 16 E. coli isolates, eight (50%) were carbapenem resistant. The carbapenem resistance patterns of each carbapenem-resistant gram-negative isolate are shown in Table 1.
Table (1):
Carbapenem-resistant pattern for carbapenem-resistant Enterobacteriaceae.
IPM |
MER |
ETP |
|
|---|---|---|---|
Klebsiella pneumoniae (33) |
32 (96.9%) |
33 (100%) |
33 (100%) |
E. coli (8) |
6 (75%) |
6 (75%) |
8 (100%) |
Abbreviations: IPM, imipenem; MER, meropenem; ETP, ertapenem.
A total of 41 carbapenem resistant Enterobacteriaceae, i.e., 33 K. pneumoniae and 8 E. coli isolates were subjected to the mCIM test. Of the 41 isolates, 24 (58.5%) were mCIM test-positive, implying that they were carbapenemase producers, including E. coli (100 %) and K. pneumoniae (48.48%). The results of the mCIM test are shown in Table 2.
Table (2):
Outcome of combined modified carbapenem inactivation method (mCIM) and EDTA-modified carbapenem inactivation method (eCIM).
mCIM Positive |
mCIM Negative |
eCIM Positive |
eCIM Negative |
|
|---|---|---|---|---|
Klebsiella pneumoniae (33) |
16 (48.48%) |
17 (51.52%) |
12 (75%) |
4 (25%) |
E. coli (8) |
8 (100%) |
0 |
2 (25%) |
6 (75%) |
Abbreviations: mCIM, modified carbapenem inactivation method; eCIM, EDTA-modified carbapenem inactivation method.
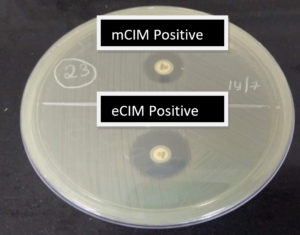
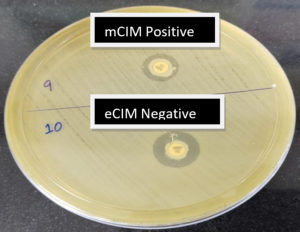
A total of 24 mCIM test-positive isolates were subjected to the EDTA-modified CIM test. Of the 24 mCIM-positive isolates, 14 (58.4%) were eCIM test-positive, implying that these were metallo-β-lactamase producers. These included 75% of K. pneumoniae and 25% of E.coli isolates which were carbapenemase producers Fig. 1 shows the outcomes of the combined mCIM and eCIM methods. Both mCIM and eCIM positive results indicate that the isolate in Fig. 1 is a metallo-β-lactamase producer. Of the 24 mCIM-positive isolates, 10 (41.6%) were eCIM test-negative, implying that these were serine carbapenemase producers, 75% of which were E. coli and 25% of which were K. pneumoniae isolates. Table 2 shows the results of the eCIM test. Fig. 2 shows the mCIM-positive and eCIM-negative results, which imply that the isolate is a serine carbapenemase producer.
Fig. 1. Outcome of the combined modified carbapenem inactivation method (mCIM) and EDTA modified carbapenem inactivation method (eCIM). mCIM-positive and eCIM positive result implies the isolate is a metallo-β-lactamase producer
In our study, we found that out of 141 gram-negative isolates, 118 (83.69%) were resistant to one or more carbapenem drugs. All isolates were found to be MDR. CRE was identified
after interpreting the breakpoints according to CLSI guidelines.12 Of the carbapenem-resistant isolates, 41 (34.7%) were CRE. Of these CRE, carbapenem resistance was found in 75% of K. pneumoniae isolates and 50% of E. coli isolates. In a similar study, Phu et al. reported carbapenem resistance in K. pneumoniae (23.1%) and P. aeruginosa (45.8%).1 Carbapenemase-producing Enterobacteriaceae (CPE) was predicted using the advanced expert system of the automated VITEK-2 system.
The first carbapenemase detection test to be used, according to CLSI recommendations, is the MHT test. The biggest limitation of this method is the inability to differentiate between the different classes of carbapenemases expressed by CRE and the insensitivity of MBLs.14 Previous studies have reported that the mCIM test has high sensitivity and specificity for identifying carbapenemase producers, and that a combined mCIM and eCIM test would help in differentiating between the different types of carbapenemases efficiently.5,15 Thus, the 41 CRE isolates were subjected to the mCIM test. Out of 41 isolates, 24 (58.5%) were mCIM test-positive, implying that they were carbapenemase producers. As differentiation between the two classes of carbapenemases is important in deciding the effective β-lactam β-lactamase drug combination to be started in managing cases of CRE-caused VARIs, the mCIM-positive isolates were subjected to the eCIM test according to CLSI guidelines.12 Of the 24 mCIM-positive isolates, 14 (58.4%) were eCIM test-positive, implying that these are metallo-β-lactamase producers, which included 75% of K. pneumoniae and 25% of E. coli isolates. Of the 24 mCIM-positive isolates, 10 (41.6%) were eCIM test-negative, implying that these are serine carbapenemase producers, which included 75% of E. coli and 25% of K. pneumoniae isolates. Gao et al. also reported metallo-β-lactamase production among 80% of K. pneumoniae isolates.16
There has been an alarming increase in the infection burden of MDR pathogens causing VARIs, thus challenging the effective management of these nosocomial infections. In this study, we found that gram-negative MDR and carbapenem-resistant pathogens were the predominant etiological causes of VARIs.
The available and substantially less expensive phenotypic tests, such as MHT, do not differentiate between MBLs and non-metallo-β-lactamases (serine carbapenemases), the former being more prevalent strains in hospital microbiota, as was the finding in our study.
Newer classes of β-lactam-β-lactamase inhibitors, such as ceftazidime-avibactam and meropenem-vaborbactam, have been found to be more active against serine carbapenemase producers. Combination therapies with ceftazidime-avibactam and aztreonam, tigecycline, aminoglycosides, and colistin were more effective against MBL producers and could be used in the therapeutic management of VARIs.
Hence, implementation of phenotypic methods such as mCIM and eCIM for detection and differentiation of CPE would prove beneficial for guiding effective empirical antibiotic therapy, especially in resource-poor laboratories where molecular methods are not readily available. Knowledge of the prevalent strains in hospital settings would further serve epidemiological interests. It would also help and guide the hospital infection control practices in taking adequate measures to reduce the spread of robust CPE strains causing common nosocomial infections.
ACKNOWLEDGMENTS
The authors would sincerely like to thank the Himalayan Institute of Medical Sciences at Swami Rama Himalayan University, Jolly Grant, Dehradun, Uttarakhand, India for providing us a platform for the research work. We would also wish to thank the technical staff of bacteriology laboratory for their constant support throughout the experimental process.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NK collected the data. NK, BK and SA analysed the data and wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Himalayan Institute of Medical Sciences, Dehradun, India with reference number SRHU/HIMS/ETHICS/2020/104.
AVAILABILITY OF DATA
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
- Phu VD, Nadjim B, Duy NHA, et al. Ventilator-associated respiratory infection in a resource-restricted setting: impact and etiology. Journal of Intensive Care. 2017;5:69.
Crossref - Wang Q, Zhang Y, Yao X, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Infect Dis. 2016;35(10):1679-1689.
Crossref - Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ. 2016;352.
Crossref - Barry AL, Jones RN, Thornsberry C, Ayers LW, Kundargi R. Imipenem (Nformimidoylthienamycin): in vitro antimicrobial activity and beta-lactamase stability. Diagn Microbiol Infect Dis. 1985;3(2):93-104.
Crossref - Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections caused by Carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol. 2019;10:80.
Crossref - Suay-Garcia B, Perez-Gracia MT. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics (Basel). 2019;8(3):122.
Crossref - Kopotsa K, Osei Sekyere J, Mbelle NM. Plasmid evolution in carbapenemase producing Enterobacteriaceae: a review. Ann N Y Acad Sci. 2019;1457(1):61-91.
Crossref - Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440-458.
Crossref - Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54(3):969-976.
Crossref - Cohen Stuart J, Leverstein-Van Hall MA. Dutch working party on the detection of highly resistant Microorganisms. Guideline for phenotypic screening and confirmation of carbapenemases in Enterobacteriaceae. Int J Antimicrob Agents. 2010;36(3):205-210.
Crossref - Bialvaei AZ, Kafil HS, Asgharzadeh M, Yousef Memar M, Yousefi M. Current methods for the identification of carbapenemases. J Chemother. 2016;28(1):1-19.
Crossref - Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 28th ed; 2018. CLSI supplement M100-S28.
- Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis. 218;66(8):1290-1297.
Crossref - Hoang CQ, Nguyen HD, Vu HQ, et al. Emergence of New Delhi Metallo-Beta-lactamase (NDM) and Klebsiella pneumoniae Carbapenemase (KPC) production by Escherichia coli and Klebsiella pneumoniae in southern Vietnam and appropriate methods of detection: a cross-sectional study. Biomed Res Int. 2019;2019:9757625.
Crossref - Tsai Y-M, Wang S, Chiu H-C, Kao C-Y, Wen L-L. Combination of modified carbapenem inactivation method (mCIM) and EDTA-CIM (eCIM) for phenotypic detection of carbapenemase-producing Enterobacteriaceae. BMC Microbiol. 2020;20:315.
Crossref - Gao B, Li X, Yang F, et al. Molecular Epidemiology and Risk Factors of Ventilator-Associated Pneumonia Infection Caused by Carbapenem-Resistant Enterobacteriaceae. Front Pharmacol. 2019;10:262.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.