ISSN: 0973-7510
E-ISSN: 2581-690X
Commercial pectic enzymes (CPE) are used in the winemaking process to improve the extraction of aromatic substances and color and for the clarification of juices. These enzymes contain pectinest erase (PE), which reacts with pectin in the process and catalyzesthe de-esterification of pectin by the removal of the C-6 methoxy groups of D-galacturonic acid to release methanol. In this study, cross-linked alcohol insoluble substance (CL-AIS) columns derived from pea pod 50% and 80% degree of esterification (DE) pectin were used to separate PE from other pectinases. Results showed that by using the 80 DE column, PE (PE: 100 unit/mg; polygalacturonase (PG): 6.5 unit/mg; pectinlyase (PL): 82.4 unit/mg; purification fold: 4.8; recovery: 68%) is effectively separated from PL (PL: 445 unit/mg; PG: 8.3 unit/mg; purification fold: 6.7; recovery: 84.3%). Theenzymes were subsequently used to make orange wine to evaluate the effect of different enzymatic treatments on the release of methanol. Lower methanol concentrations throughout fermentation were observed in the enzymatic treatment without PE.
Enzymatic treatment; Fermentation; HM-esterified CL-AIS; Methanol; Winemaking.
Sweet orange (Citrus sinensis Osbeck) is a citrus fruit mainly planted in the southwestern counties of Yunlin, Tainan, and Chiayi in Taiwan, with abundant production from November to January of the following year. Sweet oranges are a natural source of vitamins, flavonoids, and other health-promoting nutrients.
Typically, commercial pectic enzymes(CPE) added in the winemaking process include pectinesterase (PE), polygalacturonase (PG), and pectinlyase (PL)from Aspergillus niger. PE is responsible for removing the methoxylgroup from pectin, and PG and PL cleavethe bonds between galacturonate units (Bai et al. 2004; Jayani et al. 2005; Nedjma et al. 2001; Ortega et al. 2004; Piccoli et al. 2003). CPE play an important role in the winemaking process by improving extraction and filtration, thereby increasing the quality and yield, such as flavor, transmittance, and viscosity. However, CPE are limited as they lead to high methanol content in some wine products (Blanco et al. 1997; Cinar 2005; Jayani et al. 2005; Kashyap et al. 2001).
Methanol is produced as a result of the hydrolysis of methyl ester groups in pectin by PE, which is present in most alcoholic beverages (e.g., wine and beer) and unpackaged juice(Massiot et al. 1994; Revilla and Gonzilez-Sanjos 1998). Methanol is poisonous to the central nervous system, possibly resulting in blindness, coma, and death (Cabaroglu 2005). The legal limit for naturally occurring methanol to prevent danger to public health in Taiwan is2000 mg of methanol/L of ethanol for red wine and 1000 mg of methanol/L of ethanol for other alcoholic beverages in Taiwan, whereas the EU has stipulated a limit of 10 g of methanol/L of ethanol. Hence, the inhibition of methanol production is recommended in the winemaking process.
Alcohol-insoluble substances (AISs) are composed of fiber, hemicellulose, lignin, and pectin. AIS under goes methyl esterification to obtain a highly methoxylated cross-linked alcohol-insoluble solid(HM-CL-AIS), which has been reported to purify PE (Inoue et al. 1984; Klockeman et al. 1991; Mahmood et al. 1998; McKougall et al. 1996; Mudadi and Isbella 1996; Rushing and Huber 1990). Hence, HM-CL-AIS is used to remove PE and isolate PL and PG from CPE for low methanol production. In this study, PE, PG, and PL were separated from CPEusing an HM-CL-AIS affinity column. The separated enzymes were added to orange for comparing the effects of separation on physicochemical properties during the winemaking process and the decrease of methanol content in wine.
CPE from a microbial source, i.e., wine yeast RA-17 (S. cerevisiae), was purchased from Lallemand Australia Pty. Ltd., (North Adelaide, Australia). Sweet orange (Citrussinensis) was purchased from a local supermarket in Pingtung County in Taiwan. Methanol was purchased from Sigma (St. Louis, MO). Polygalacturonic acid was purchased from Fluka (Buchs, Switzerland). Ethanol (95%) was purchased from Taiwan Tobacco and Liquor Corporation, Pingtung, Taiwan.
Preparation of alcohol insoluble solids (AIS)
First, the pea pods were washed with 95% ethanol and ether. The residues thus obtained were dried overnight at room temperature, ground in a mill, and stored in a desiccator.
Preparation of CL-AIS
AIS was mixed with 40% dimethyl sulfoxide (DMSO), 40 mL of epichlorohydrin, and 50mL of 5N NaOH in an Erlenmeyer flask, followed by shaking for 2 h (120 rpm, 40°C) and filtration. According to a previous study (Inoue et al. 1984), to prepare CL-AIS, the solids thus obtained were washed with distilled water, 90% ethanol, and acetone, followed by drying overnight at room temperature. The CL-AIS yield was 30.2%.
Preparation of HM-CL-AIS
First, CL-AIS was slowly mixed by cooling 2L of 2Nmethanol (4°C). Second, the mixture was stirred in a cold room for 6 days for methoxylation, followed by washing several times with methanol and rinsing several times with 80% acetone to remove the free methanol. In a previous study (Monsoor et al. 2001), HM-CL-AIS with degrees of esterification (DE) of 50%and 80%have been investigated. After drying overnight at room temperature, the powder (HM-CL-AIS) obtained was stored in a desiccator until use.
HM-CL-AIS chromatography
First, 10 g of HM-CL-AIS (50DE or 80DE) and 0.2 mL of CPE were applied on an HM-CL-AIS chromatography column (2.5 cm x 20 cm; flow rate, 30 mL/h) for separation. The HM-CL-AIS column was equilibrated with 0.01N of citrate buffer (pH 4) and eluted with the same buffer at a gradient of 0–1 M NaCl for the separation of PG, PL, and PE. Liquid (4 mL) was collected in 128 tubes and assayed for activities of PG, PL, and PE.
Preparation of polyacrylamide gel for electrophoresis
Acrylamide gel was prepared with 12.5% of resolving gel and 4.5% of stacking gel,and a 10% trichloroacetic acid (TCA) solution was used to concentrate and precipitate diluted samples from the phases. Electrophoresis was carried out at 110 V and 36 mA for 75 min. The protein band was observed with the Coomassiebrilliant blue stain (Mehrnoush et al. 2013).
Protein assay
The protein content in the samples was determined using a Bio-Rad Protein Assay kit using bovine serum albumin (BSA) as the standard. The samples were homogeneously mixed with a dyereagent (1:4 v/v) at room temperature for 10 min. The absorbance was measured at 595 nm against a blank (Mehrnoush et al. 2013).
PE activity assay
PE activity was determined according to a previously described method (Wu et al. 2007). Twenty milliliters of 0.1 M NaCl and a 0.5% citrus pectin (DE 60–66%) solution, adjusted to pH 6.5 before assay, were added to 1 mL of the PE solution. PE activity was determined by the titration (PH-Stat Controller PHM-290, Radiometer, Copenhagen, Denmark)of the free protons dissociated from the free carboxyl groups formed as a result of PE activity (PE unit = 1 M COOH/min).
PG activity assay
PG activity (PG unit = 1 mM galacturonic acid/min) was assayed by evaluating the hydrolysis of the reducing group from citrus pectin by the 3,5-dinitrosalicylic acid (DNS) reagent assay according to previously reported studies (Dinu et al. 2007) with some modifications. A total of 0.8 mL of 0.3% DE (60–66%) in 0.2 M acetate buffer (pH4.0) and a 0.2 mL enzyme solution were treated at 37°C for 30 min, and the reaction was stopped in a boiling water bath for 5 min, followed by monitoring the absorbance of the resulting colored mixture at 540 nm.
PL activity assay
PL activity (PL unit = increase in absorbance/min) was determined according to previously reported studies (Dinu et al. 2007) with some modifications. PL activity was measured by investigating the increase in absorbance at 235 nm for the reaction containing 0.2 mL of 10 mM Tris-HCl buffer (pH8), 0.2 mM CaCl2 with 0.5% DE (90–93%), and 0.2 mL of enzyme at 40°C for 1h.
Preparation of wine
Skin was removed from oranges and crushed into musts (7.5kg). Second, sucrose and sodium pyrosulfite were added to reach 24 °Brix and 100 ppm (as SO2). The experiment was divided into five groups: (i) control group: without the addition of external enzyme; (ii) CPE: with CPE per liter of wine (containing PE: 10 IU, PG: 2.4 IU, PL: 32 IU); (iii) PE group: with a partially purified PE solution (containing PE: 20 IU, PG:1.2 IU, PL: 15 IU); (iv) PL-1X group: with a partially purified PL solution (containing PL: 32 IU, PG: 0.56 IU); and (v) PL-3X group: with a partially purified PL solution (containing PL: 96 IU, PG: 1.68 IU). Wine yeast RA-17 (0.25 g) was activated in water at 40–45°C for 10 min, added to orange musts, and fermented at room temperature (25 ± 2 °C) for 15 days. The sample was kept at 0, 3,5,7, 9, 12, and 15 days to determine the change in physicochemical properties and then centrifuged at 13,000´g for 20 min at 4°C.
Determination of specific gravity
Specific gravities of semi-products and wines were determined according to the method described by AOAC (1984), to determine the specific gravity of semi-products and wines at 20 ° C
Determination of the total soluble solids
A hand-held refractometer (N-1E, Atago, Tokyo, Japan) was used to determine the TSS (as ° Brix) of semi-products and wines by AOAC (1984). The refractometer was adjusted with distilled water each time before use.
Determination of pH
Wine during fermentation was separated by centrifugation (13000 xg, 2 min) to determine the pH at room temperature (AOAC 1984).
Determination of citric acidity
Distilled water (20 mL) was added to wine (5 mL) and mixed, and the pH was decreased to 8.1 with 0.1 N NaOH. Citric acid was used as the standard(AOAC 1984).
Determination of color
The CIE color parameters [L (whiteness), a (redness to greenness), and b (yellowness to blueness)] of the sample were determined using a colorimeter (Hunter Lab Color Quest XE, Hunter Lab, Reston, Virginia).
Determination of methanol and ethanol
Changes in the methanol and ethanol concentration in samples were determined according to a previously described method(Wang et al. 2004) with some modifications. First, the wine samples were centrifuged and then filtered using a 0.45-¼m membrane filter (Millipore, Concord, MA) for GC determination. Methanol and ethanol concentrations were analyzed on a Trace 2000 GC (Thermo Quest, Milan, Italy), equipped with a computer integrator software (Chrom-Card version 4.01 for Trace GC, Thermo Quest, Milan, Italy), a flame ionization detector (H2: 30 mL/min and air: 300 mL/min), and a 30 m CP-Wax 52 CB megaborecapillary column (i.d. of 0.53 mm and a film thickness of 1.5 mm; Chrom Pack, Palo Alto, CA). The flow of nitrogen was set at 5 mL/min. The temperatures of the injector port and detector were set at 210 and 280 °C, respectively. The oven temperature was controlled by a temperature elevation program during analysis, which was initially set at 40 °C for 4 min, elevated to 230 °C at a rate of 40 °C/min, and maintained for 1 min.
Statistical analysis
Statistical analysis was carried out using SPSS 13.0 statistical software program (SPSS Inc., IL, USA). Triplicate samples were analyzed twice. The difference between the means was analyzed by Duncan’s multiple range tests.
Isolation of PL on HM-CL-AIS columns
In an HM-CL-AIS column, protein purification is achieved on the basis of the specific affinity between pectin and pectinases. To determine the effect of 50 DE and 80 DE HM-CL-AIS columns on pectinase purification, CPEs were loaded on the columns, and eluted fractions of samples were obtained by washing with 0 to 1 M NaCl. In the 50 DE column, PE activity was observed in fractions 70–88, PG activity was observed in fractions 26–55 and 70–108, and maximum PL activity was observed in fractions 78–108 (Fig. 1a). Thus, the 50 DE column does not completely separate PE, PG, and PL. On the other hand, using the 80 DE column, PE activity (including PG activity) was detected in fractions 25–37 in the absence of NaCl, whereas PL activity (including PG activity) was detected in fractions 75–115 with the addition of 0 to 1 M NaCl (Fig. 1b).
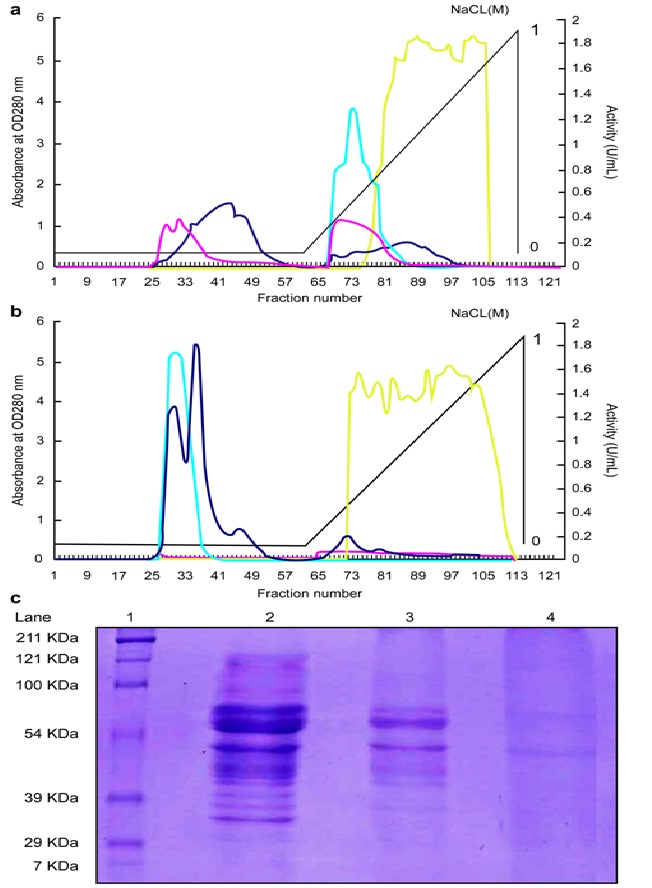
Fig. 1: Elution profiles of CPE (PE, PG, and PL) from 50 DE and 80 DE CL-AIS columns and SDS-PAGE pattern of protein fractions. (a) 50 DE and (b) 80 DE CL-AIS columns (2.5 cm ´ 20 cm) were eluted with 0.01 M citrate buffer, and the absorbances of protein (dark blue), PE (light blue), PG (pink), and PL (yellow) activity was measured at 280 nm. (c) SDS-PAGE protein bands: lane 1: molecular-weight marker; lane 2: CPE; lane 3: 50 DE CL-AIS column fractions (including PE, PG, and PL); and lane 4: 80 DE CL-AIS column fractions (including PG and PL).
Results from SDS-PAGE analysis revealed that the 80 DE column achieves excellent purification with no impurity (Fig. 1c). Table 1 summarizes the calculated data for the purification effect of PE, PG, and PL. PL obtained from the 80 DE column exhibited 6.7 purification fold, with a yield of 84.3% (Table 1c). Hence, by changing the degree of esterification of the CL-AIS affinity columns, CPE comprising PE, PG, and PL is separated and used in the winemaking experiment.
Table (1):
Purification of (a) PG, (b) PE, and (c) PL from commercial pectinolytic enzymes using 50 DE and 80 DE CL-AIS columns.
(a):
Types |
Total activity (U) |
Total protein (mg) |
Specific activity (U/mg) |
Purification fold |
Yield (%) |
|---|---|---|---|---|---|
Commercial pectinolytic enzyme |
50 |
2.4 |
20.8 |
1 |
100 |
50DE-CL-AIS |
34 |
0.34 |
100 |
4.8 |
68 |
80DE-CL-AIS |
– |
– |
– |
– |
– |
(b):
Types |
Total activity (U) |
Total protein (mg) |
Specific activity (U/mg) |
Purification fold |
Yield (%) |
|---|---|---|---|---|---|
Commercial pectinolytic enzyme |
12 |
2.4 |
5 |
1 |
100 |
50DE-CL-AIS |
2.2 |
0.34 |
6.5 |
1.3 |
18.3 |
80DE-CL-AIS |
2.5 |
0.3 |
8.3 |
1.6 |
20.8 |
(c):
Types |
Total activity (U) |
Total protein (mg) |
Specific activity (U/mg) |
Purification fold |
Yield (%) |
|---|---|---|---|---|---|
Commercial pectinolytic enzyme |
160 |
2.4 |
66.7 |
1 |
100 |
50DE-CL-AIS |
28 |
0.34 |
82.4 |
1.2 |
17.5 |
80DE-CL-AIS |
133.5 |
0.3 |
450 |
6.7 |
84.3 |
Changes in the quality parameters of orange wine during fermentation
Purified pectic enzymes were subsequently used in making orange wine to determine whether the methanol content in orange wine is decreased by CPE without PE. Results from the analysis of the physicochemical properties of orange wine indicated no significant difference between the treatment groups with respect to specific gravity, TSS, and ethanol content (Fig. 2). Specific gravity was approximately 1.1 on Day 0, which gradually decreased to 0.996 at the end of fermentation (Fig. 2a). As shown in Fig. 2b, the TSS decreased from ~25 (on Day 0) to 10 at the end of the fermentation process. The ethanol concentration reached 11% on Day 15 (Fig. 2c). In this study, the titratable acidity in orange wine increased sharply at the beginning of fermentation until it stabilized on Day 9, and no significant difference was observed thereafter between the groups (Fig. 2d). The pH levels were similar among the groups: the pH decreased with progress of fermentation, and the final pH values were 3.90–3.95 (Fig. 2e). The color of the groups also changed during fermentation. In Table 2, the whiteness of CPE, PE (including PG and PL activity), PL-1X (including PG activity), and PL-3 (including PG activity) treatment significantly increased on Day 3 and increased continuously with time. On Day 15, the wine samples prepared with the addition of pectic enzymes exhibited significantly higher L values than the control; the highest increase in whiteness was exhibited in wines with added CPE and PL-3X. The b values of wine prepared with pectic enzymes were significantly greater than the control, with the PL-3X (including PG activity) samples exhibiting the highest value.
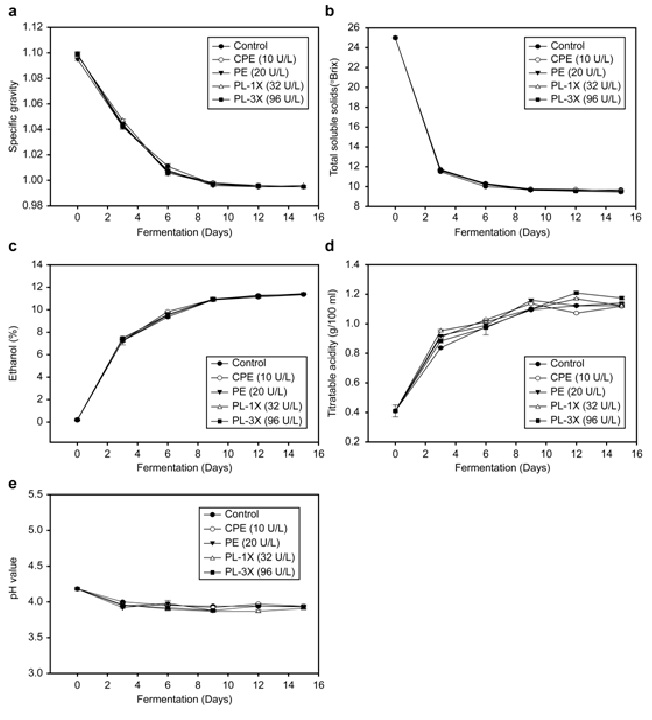
Fig. 2: Effect of control, CPE, PE (including PG and PL), and PL (including PG) treatments on (a) specific gravity, (b) total soluble solids (°Brix), (c) ethanol concentration (%), (d) titratable acidity, and (e) pH during the fermentation of orange wine
(Table 2): Analysis of control, CPE, PE (including PG and PL), and PL (including PG) on Hunter L, a, and b values during the fermentation of orange wine
(a) L Value
Day |
Control |
CPE |
PE |
PL-1X |
PL-3X |
|---|---|---|---|---|---|
0 |
1.53 ± 0.03a |
1.53 ± 0.03a |
1.53 ± 0.03a |
1.53 ± 0.03a |
1.53 ± 0.03a |
3 |
53.21 ± 0.44e |
68.94 ± 0a |
57.29 ± 1.48d |
60.98 ± 0c |
64.11 ± 0.05b |
6 |
68.34 ± 0.08b |
71.93 ± 0.07a |
69.28 ± 2.67b |
64.25 ± 0.85c |
64.68 ± 0.2c |
9 |
67.96 ± 0.01d |
72.74 ± 0.58a |
71.64 ± 0.52b |
71.46 ± 0.27b,c |
70.88 ± 0.2c |
12 |
68.7 ± 0.01b |
72.91 ± 0.17a |
72.68 ± 1.14a |
72.08 ± 0.1a |
73.08 ± 0.18a |
15 |
69.62 ± 0.01c |
76.32 ± 0.63a |
73.62 ± 0.28b |
73.65 ± 0.7b |
75.58 ± 0.1a |
(b) a Value
Day |
Control |
CPE |
PE |
PL-1X |
PL-3X |
|---|---|---|---|---|---|
0 |
1.17 ± 0.05a |
1.17 ± 0.05a |
1.17 ± 0.05a |
1.17 ± 0.05a |
1.17 ± 0.05a |
3 |
4.36 ± 0.59a |
3.993 ± 0.025a,b |
2.98 ± 0.03c |
3.71 ± 0.006b |
3.79 ± 0.01b |
6 |
4.3 ± 0.01e |
4.78 ± 0.02c |
4.86 ± 0.02b |
5.04 ± 0.033a |
4.47 ± 0.01b |
9 |
4.75 ± 0.01c |
5.34 ± 0.02a |
5.02 ± 0.22b |
4.76 ± 0.02c |
4.7 ± 0.02c |
12 |
5.21 ± 0.57a |
4.74 ± 0.02a,b |
4.82 ± 0.01a,b |
4.49 ± 0.01b |
4.71 ± 0.012b |
15 |
4.81 ± 0.01a |
4.73 ± 0.01d |
4.75 ± 0.005c |
4.78 ± 0.006b |
4.55 ± 0.006e |
(c) b Value
Day |
Control |
CPE |
PE |
PL-1X |
PL-3X |
|---|---|---|---|---|---|
0 |
26.81 ± 0.02a |
26.81 ± 0.02a |
26.81 ± 0.02a |
26.81 ± 0.02a |
26.81 ± 0.02a |
3 |
15.92 ± 0.03d |
16.22 ± 0.02c |
21.51 ± 0.06a |
17.73 ± 0.03b |
16.28 ± 0.015c,d |
6 |
16.33 ± 0.01d |
16.26 ± 0.05e |
16.8 ± 0.02b |
17.3 ± 0.02a |
16.57 ± 0.01c,d |
9 |
16.64 ± 0.05d |
16.9 ± 0.26c |
17.41 ± 0.03a |
17.13 ± 0.02b |
16.83 ± 0.02c,d |
12 |
16.91 ± 0.02a |
17.54 ± 1.14a |
17.14 ± 0.02a |
16.61 ± 0.02a |
17.26 ± 0.01a |
15 |
17.00 ± 0.02e |
17.64 ± 0.02c |
17.23 ± 0.01d |
17.83 ± 0.04b |
18.88 ± 0.04a |
a-e Different letters in the same row represent data that are significantly different (p < 0.05).
In Figure 3a, the total pectin content in all the treatment groups containing pectic enzymes was in the range of 0.06–0.07, less than that observed for the control (0.15). However, no significant difference was observed between the enzymatic treatment groups. The total pectin amount decreased with progress of fermentation.
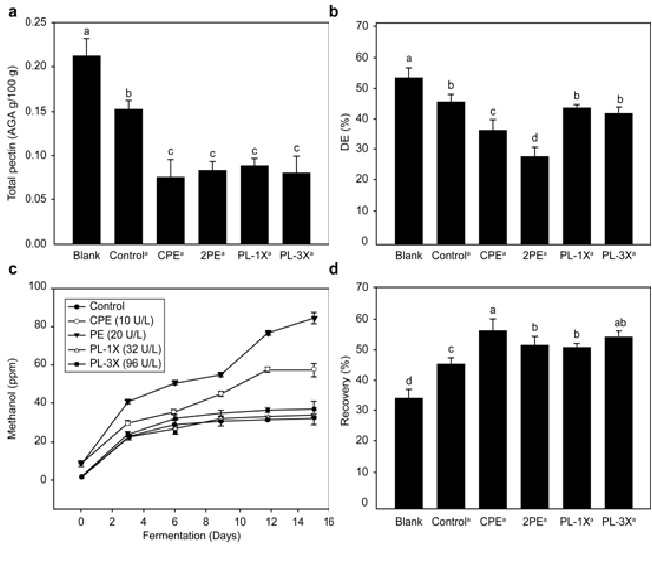
Fig. 3: Effect of control, CPE, PE (including PG and PL), and PL (including PG) on (a) total pectin, (b) DE (%), (c) methanol concentration (%), and (d) recovery during the fermentation of orange wine.
Figure 3b shows the results obtained from the titration-based measurements of DE (%). A significantly lower DE was observed with the CPE 34% and PE 26% groups than the remaining treatment groups, the PE group exhibiting the lowest DE. The methanol concentration was also evaluated to determine the effect of different enzymatic treatments on methanol release during fermentation, where the enzymes used in making orange wine, i.e. PE (including PG activity) and PL (including PG activity), were purified using 50 DE and 80 DE CL-AIS columns, respectively. Figure 3c shows the results. The methanol concentration in wine prepared with PL was similar to that in the control. On the other hand, a methanol concentration of 60 ppm after CPE treatment was observed at the end of fermentation. The methanol concentration was 85 ppm after PE treatment, where the PE activity was twice that obtained after the CPE treatment; hence, a 1.4-fold increase in methanol production (as compared to the CPE treatment) is observed.
Enzymatic treatment recovery ranges from 49% to 54%, which led to a higher recovery percentage than the control (44%), with no significant difference between the CPE and PL-3X treatment groups (Fig. 3d). Pectin is degraded by pectic enzymes, converting the polygalacturonic acid chains to simple sugars because of PG and PL, thereby increasing product recovery (Kashyap et al. 2001).
In the isolation of PL on HM-CL-AIS columns, PE is effectively separated from PL using the 80 DE column. The experimental results are different from previously reported results (Wu et al. 2005). We isolated PE and PL on an HM-CL-AIS column. Although PE was not adsorbed on the 80 DE column, it was adsorbed on the 50 DE column, whereas PL was isolated from the 80 DE column. Both PE and PL would be used in wine making in the latter part of this study. PL obtained from the 80 DE column exhibited 6.7-fold purification, greater than that reported previously (Wu et al. 2005) (5.4 fold).
The quality parameters of orange wine during fermentation, i.e. those obtained from the analysis of the physicochemical properties of orange wine indicated no significant difference between the treatment groups in terms of specific gravity, TSS, and ethanol content. During fermentation, the TSS decreased due to the ability of yeast to produce ethanol during fermentation using reducing sugar from pectin as the carbon source; hence, ethanol concentration increases with time (Nevoigt 2008). According to Faquembergue and Grassin, the use of pectic enzymes in wine preparation facilitated the formation of polygalacturonic acidsand titratable acid. Therefore, the titratable acidity sharply increased at the beginning of fermentation, whilethe pH levels decreased.
Color significantly affectsthe consumers’ perception of product appearance and quality. Hence, the color of each orange wine sample is measured using a HunterLab Color Quest XE colorimeter. Color was measured by detecting the intensity of light passing through a sample, expressed by Hunter values of L, a, and b. The L value refers to the whiteness of the sample, with 0 and 100 representing black and white, respectively. The positive and negative a values indicate the intensities of the red and green colors, respectively. Positive and negative b values represent the intensities of the yellow and blue colors, respectively. During the production of orange wine, the whiteness of the wine increases with progress of fermentation because of the presence of pectic enzymes, which clarify the juices by degrading pectic substances and producing clear orange wine (Blanco et al. 1997; Cinar 2005; Jayani et al. 2005; Kashyap et al. 2001). The intensity of yellowness in the orange wine prepared with pectic enzymes was greater than that exhibited by the control, showing greater resemblance to the color of an orange fruit (Fan et al. 2009).
The total pectin contentsof all the treatment groups containing pectic enzymes were less than that observed for the control. Pectic substances are heterogeneous polysaccharides present in higher plants, which contribute to the firmness of plant tissue (Kapoor et al. 2000). These substances are predominantly composed of polygalacturonic acid chains with a(1–4)-glycosidic linkages of D-galactopyranosyluronic acid units, with neutral sugars (e.g., L-rhamnose, L-arabinose, D-glucose, and D-galactose) connected to the backbone as side chains. PE catalyzes the cleavage of the methoxy group in pectin, releasing methanol and decreasing the DE of pectic substances. On the other hand, PG cleaves the a(1–4)-glycosidic bonds to yield D-galactopyranosyluronic acid (Gummadi and Panda 2003). In HM-esterified pectin, PL cleaves the a(1–4)-glycosidic bonds adjacent to the methyl ester group via a-elimination, forming a double bond (Jayani et al. 2005). This observation indicates that pectin hydrolysis is primarily dependent on PG and PL.
The CPEand PE groups showed lower DE than the remaining treatment groups, the PE group exhibiting the lowest DE. The association was because both groups contain PE, a pectic enzyme that liberates galacturonic acids by the cleavage of methyl ester linkages at C-6 of HM-esterified pectin (Massiot et al. 1994; Revilla and Gonzilez-Sanjos 1998). In contrast, no significant difference was observed among PL-1X, PL-3X, and the control as PL utilizes ±-elimination to catalyze pectin hydrolysis (Jayani et al. 2005).
The CPE and PE groups showed higher methanol concentration than the remaining treatment groups, the PE group exhibiting the highest methanol concentration. The PL-1X and PL-3X groups show lowest methanol concentration, same as that of the control groups. This observation reflects the previously stated actions of pectic enzymes: PE is one of the main factors responsible for producing methanol using CPE, and the release of methanol from orange wine significantly decreases upon removal of PE (Massiot et al. 1994; Revilla and Gonzilez-Sanjos 1998).
The enzymatic treatments show higher recovery rates than the control, with no significant difference between the CPE and PL-3X treatment groups. Pectin is degraded by pectic enzymes, the polygalacturonic acid chains being converted to simple sugars because of PG and PL, thereby enhancing product recovery (Kashyap et al. 2001).
In conclusion, Fruit wines and distilled fruit wines in the wine market contain methanol, which is produced when pectin from the pulp reacts with PE from the added CPE, and the reaction catalyzes the de-esterification of the methyl ester linkages to release methanol. CPE are important in the winemaking process because they improve the extraction of color and aromatic compounds and filtration of musts, as well as enhance the clarity and yield of juice. Hence,winemakers typically add CPE in the winemaking process; however, using CPE, the concentration of methanol in the final product might exceed the acceptable level. Hence, the application of 80 DE CL-AIS column is investigated for the self-separation of PE from CPE. Results suggested that the column removes PE and the remaining pectic enzymes, which are subsequently used in making orange wine, and the release of methanol decreases as fermentation progresses. Moreover, enzymatic treatment without PE activity resulted in brightness, clarity, and product recovery, comparable to those obtained using CPE; hence, 80 DE CL-AIS columns demonstrate potential for use in commercial applicationsofthe winemaking industry.
- Association of official analytical chemists Official methods of analysis, 20th edn. Washington DC: USA, 2016.
- Bai, Z.H., Zhang, H.X., Qi, H.Y., Peng, X.W., Li, B.J. Pectinase production by Aspergillus niger using wastewater in solid state fermentation for eliciting plant disease resistance. Bioresour. Technol., 2004; 95: 49–52.
- Blanco, P., Sieiro, C., Diaz, A., Reboredo, N.M., Villa, TG. Grape juice biodegradation by polygalacturonases from Saccharomyces cerevisiae. Int. Biodeterior. Biodegrad., 1997; 40: 2–4.
- Cabaroglu, T. Methanol contents of Turkish varietal wines and effect of processing. Food Control., 2005; 16: 177–181.
- Cinar, I. Effects of cellulase and pectinase concentrations on the colour yield of enzyme extracted plant cartenoids. Process. Biochem., 2005; 40: 9459.
- Dinu, D., Nechifor, M.T., Stoian, G., Costache, M., Dinischotu. Enzymes with new biochemical properties in the pectinolytic complex produced by Aspergillus niger MIUG 16. J. Biotechnol., 2007; 131: 128–137.
- Fan, G., Xu, C., Qiao, Y., Xu, Y., Zhang, Y., Li, L., Pan, S. Volatiles of orange juice and orange wines using spontaneous and inoculated fermentations. Eur. Food Res. Technol., 2009; 228: 849–856.
- Faquembergue, P., Grassin, C. Enzymatic liquefaction of apples. Fruit Process., 1993; 7: 242–245.
- Gummadi, S.N., Panda, T. Purification and biochemical properties of microbial pectinases-a review. Process Biochem., 2003; 38: 987–996.
- Inoue, S., Nagamatsu, Y., Hatanaka, C. Preparations of cross linked pectate and its application to the purification of endopolygalacturonase of Kuyveromyces fragilis. Agric. Biol. Chem., 1984; 48: 633–640.
- Jayani, R.S., Saxena, S., Gupta, R. Microbial pectinolytic enzymes: A review. Process Biochem., 2005; 40: 2931–2944.
- Kapoor, M., Beg, Q.K., Bhushan, B., Dadhich, K.S., Hoondal, G.S. Production and partial purification and characterization of a thermo-alkali stable polygalacturonase from Bacillus sp. MG-cp-3. Process Biochem., 2005; 36: 476–483.
- Kashyap, D.R., Vohra, P.K., Chopra, S., Tewari, R. Application of pectinases in the commercial sector: a review. Bioresour. Technol., 2001; 77: 215–227.
- Klockeman, D.M., Pressey, R., Jen, J.J. Characterization of cell wall polysaccharides of jicama (Pachyrrhizus erosus) and water chestnut (Eleocharis dulicis). J. Food Biochem., 1991; 15: 317–329.
- Mahmood, A.U., Greenman, J., Scragg, A.H. Orange and potato peel extracts: analysis and use as Bacillus substrates for the production of extracellular enzymes in continuous culture. Enzyme Microb. Technol., 1998; 22: 130–137.
- Massiot, P., Baron, A., Drilleau, J.F. Characterization and enzymatic hydrolysis of cell-wall polysaccharides from different tissue zones of apple. Carbohydr. Polym., 1994; 25: 145–154.
- McKougall G.J., Morrison, I.M., Stewart, D., Hillmam, J.R. Plant cell walls as dietary fiber: range, structure, processing and function. J. Sci. Food Agric., 1996; 70: 133–150.
- Mehrnoush, A., Mohd, Y.A.M., Shuhaimi, M. Purification of pectinase from mango (Mangiferaindica L. cv. Chokanan) waste using an aqueous organic phase system: A potential low cost source of the enzyme. J. ChromatogrB., 2013; 931: 17–22.
- Monsoor, M.A., Kalapathy, U., Proctor, A. Improved method for determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. J. Agric. Food Chem., 2001; 46: 2756–2760.
- Mudadi, A.N.B., Isbella, M. Use of crosslinked mucilage prepared from ruredzo (Dicerocaryum zanguebarium) in the purification of polygalacturonase extracted from tomato. Food Chem., 1996; 56: 433-437.
- Nedjma, M., Hoffmann, N., Belarbi, A. Selective and sensitive detection of pectin lyase activity using a colorimetric test: application to the screening of microorganisms possessing pectin lyase activity. Anal. Biochem., 2001; 291: 290–296.
- Nevoigt, E. Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev., 2008; 72: 379–412.
- Ortega, N., Diego, S.D., Mateos, P.M., Busto, M.D. Kinetic properties and thermal behaviour of polygalacturonase used in fruit juice clarification. Food Chem., 2004 88: 209–217.
- Piccoli, V.R.H., Passos, F.J.V., Brandi, I.V., Peternelli, L.A., Silva, D.O. Influence of different mixing and aeration regimens on pectin lyase production by Penicillium griseoroseum. Process Biochem., 2003; 38: 849–854.
- Revilla, I., Gonzilez-Sanjos, M.L. Methanol release during fermentation of red grapes treated with pectolytic enzymes. J. Food Chem., 1998; 63: 307–312.
- Rushing, J.W., Huber, D.J. Mobility limitations of bound polygalacturonase in isolated cell wall from tomato pericarp tissue. J. Am. SocHorticSci., 1990; 115: 97–101.
- Wang, Y.T., Wu, B.J., Chang, H.M., Wu, J.S.B. A novel method for the determination of pectinesterase inhibitor in banana. J. Food Drug Anal., 2004; 15: 185–190.
- Wu, M.C., Jiang, C.M., Huang, P.H., Wu, M.Y., Wang, Y.T. Separation and utilization of pectin lyase from commercial pectic enzyme via highly methoxylated cross-linked alcohol insoluble solid chromatography for wine methanol reduction. J. Agric. Food Chem., 2007; 55: 1557–1562.
- Wu, M.C., Lin, G.H., Wang, Y.T., Jiang, C.M., Chang, H.M. Novel Cross-linked alcohol-insoluble solid (CL-AIS) affinity gel from pea pod for pecitnesterase. J. of Agric. Food Chem., 2005; 53: 7991–7996.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


