Plasmodium parasites, transmitted to human blood via the bite of the Anopheles mosquito, cause malaria, an acute and severe disease that can potentially be fatal. These parasites and their mosquito vectors proliferate in warmer climates and, therefore, are more prevalent in certain regions. In 2021, fifty percent of the global population was at risk of malaria. Although this disease can affect any individual, specific demographic groups, including young children, pregnant women, neonates, and immunocompromised individuals, are more susceptible to infection and are at higher risk of mortality. Among Plasmodium species, only P. falciparum causes cerebral malaria and is behind the most severe symptoms and fatalities. The pathogenesis of Plasmodium malaria is associated with the downstream signaling pathways and Toll-like receptors (TLRs) of innate immunity. Owing to the potential role of TLRs in the pathophysiology of malaria, TLR gene polymorphisms may be subject to selection pressure in communities where the disease is endemic. This review paper summarizes the prevailing knowledge of the fundamental characteristics of TLRs and their role in malaria disease. In addition, it throws light on the potential role of the TLR signaling system in malaria pathogenesis.
Innate Immunity, Malaria, Plasmodium species, TLRs, Ligands, Nucleic Acid Motifs
Malaria poses a significant threat to approximately half of the global human population. An estimated 247 million individuals in 85 countries contracted malaria in 2021. In the same year, 619,000 people succumbed to the illness.1 Flu-like symptoms, such as cyclic fever, chills, sweating, headache, muscular pains, exhaustion, and joint pain, are indicative of a malaria infection.2 Additionally, there may be gastrointestinal problems such as diarrhea, vomiting, and nausea.3 Complications include anemia, jaundice, and hepatosplenomegaly, which may arise as the infection worsens.4 Severe malaria can result in respiratory distress, kidney and liver failure, cardiovascular collapse, and brain malaria (seizures, confusion, coma) if treatment is not received.5 Immunocompromised people, children, and pregnant women are high-risk categories.6 The most harmful species is Plasmodium falciparum, which frequently causes life-threatening complications. In order to avoid serious consequences, early identification and treatment are essential.7 The Anopheles mosquito and humans are the two hosts in the complicated life cycle of Plasmodium, the parasite that causes malaria.8 Sporozoites are injected into the circulation by an infected mosquito bite and go to the liver.9 Sporozoites develop into schizonts within liver cells, which burst to release merozoites into the bloodstream.10 These merozoites replicate asexually by invading red blood cells.11 Malarial fever and cold cycles are caused by the bursting of infected red blood cells, which release more merozoites.10 In order to continue the cycle of transmission, some merozoites undergo differentiation into sexual forms known as gametocytes, which may be ingested by another mosquito during a blood meal.12 In human evolutionary history, malaria mortality has disproportionately affected children under the age of five, which aligns with the hypothesis that malaria has exerted a substantial influence on the selection of the human genome.13
A potential candidate of malaria vaccine, RTS,S/AS01, was authorized for a 2015 pilot deployment program in three African nations. This development was promising for addressing malignant malaria. But, RTS, S/AS01 has been associated with various limitations, of which, the most notable are ineffectiveness in specific age categories, insufficient immunity, and the requirement of nearly three boosters to achieve satisfactory performance. Therefore, a more thorough comprehension of naturally developed immune responses to the different phases of the parasite, including the accessible stages, may be essential for the development of a potent malaria vaccine.14
TLRs were first discovered in 1997 as proteins crucial for antifungal defense and dorsoventral fetal development of the fruit fly, Drosophila melanogaster.15 The TLR relatives can be divided into two groups based on where they are found within the cell: TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located externally on the cell surface, whereas TLR3, TLR7, TLR8, and TLR9 are found internally either in the endoplasmic reticulum membrane or in the endosomal/lysosomal membranes. Exterior components of pathogens are recognized by cell-surface TLRs, but the nucleic acids of these infectious agents are mostly recognized by internal membrane-surface TLRs.16 TLRs are the biggest family of pattern recognition receptors that can directly identify both extracellular and intracellular microbial antigens, including bacterial, fungal, viral, and protozoal antigens, with resulting activation of innate immune-signaling transduction-mediating inflammatory mediator production.17
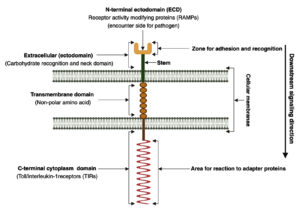
Cell-surface TLRs consist of external, transmembrane, and cytoplasmic domains. They are classified as type I transmembrane glycoproteins. The basement domain (the first domain) is oriented toward either the cytoplasm or the outside of the cell, depending on the location of the binding site. It contains multiple (16-28) leucine-rich repeats (LRRs) comprising 24-9 amino acids and may exhibit either the typical (T) motif (LxxLxLxxNxLxxLxxxxF/LxxLxx) or the bacterial (S) motif (LxxLxLxxNxLxxLPx(x)LPxx).18 The ectodomain structure accommodates the hydrophobic residues of LRRs, thus generating a hydrophobic area of attachment to the linker.19 While the cytoplasmic membrane domain, commonly referred to as the Toll/interleukin-1 receptor (TIR) domain, interacts with signal transduction adaptors and initiates signaling, the LRR motif is responsible for pathogen recognition.20 Although diverse synthetic ligands bind to various TLRs, their sensitivity to microbial substances coincides.21 The second domain of TLRs is the single-spanning transmembrane domain, which is analogous to the interleukin-1 receptor counterpart and secures the receptor in the appropriate orientation on the cell membrane.18 The TIR domain is the third domain of the TLRs.17,22 This domain is shared by almost all TLRs and typically consists of 150 amino acid residues.23 TLRs exhibit a structure characteristic of type I membrane proteins: a cytoplasmic domain that protrudes from the inside of the cell and an extracellular domain projecting outside of the cell, which are connected by a transmembrane domain that traverses the membrane as shown in Figure 1.24,25
Figure 1. Schematic representation illustrating a transmembrane adhesion molecule comprising an extracellular domain, transmembrane segment, and cytoplasmic domain
TLRs, which constitute the most extensively studied family of pattern recognition receptors that directly recognize protozoal antigens, function as the primary receptors of the innate immune response and are absolutely vital for the adaptive immune response, as observed in malaria.26
During a malaria infection, toll-like receptors (TLRs) are essential for bridging adaptive immunity.27 When TLRs-specifically TLR2, TLR4, and TLR9-identify Plasmodium-derived compounds, including hemozoin and glycosylphosphatidylinositols (GPIs), they activate innate immunity cells, which results in the generation of type I interferons and pro-inflammatory cytokines.28,29 This early signaling affects the adaptive immune response by influencing dendritic cell maturation, improving antigen presentation, and encouraging T-cell activation and differentiation.30 Therefore, the establishment of efficient humoral and cellular defense against Plasmodium species depends on TLR-mediated pathways.26
With a focus on their signaling pathways and their role in malaria infection, this review explores the critical function of TLRs in the innate immune response. By elucidating the mechanisms through which TLRs recognize malarial components and modulate immune responses, we aim to highlight their influence on the pathophysiology and progression of malaria. A deeper understanding of these pathways is essential for identifying potential therapeutic targets and improving disease management. Through a comprehensive analysis of recent advancements, this study underscores key aspects of TLR-mediated immunity in malaria and its broader implications for host-pathogen interactions.
Ligands (antigens) of TLR and malaria
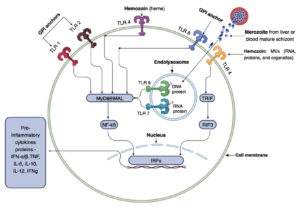
In relation to the protozoan parasite Plasmodium and malaria, three primary TLR ligands have been identified: hemozoin, immunostimulatory nucleic acid motifs (schizonts and merozoites), and glycosylphosphatidylinositol (GPI) anchors as shown in Figure 2.26 In addition, certain endogenous substances produced during malarial infection, including heme groups and microvesicles, function as damage-associated molecular patterns (DAMPs) and activate TLRs in conjunction with these pathogen-associated molecular pattern (PAMP) ligands.31
Figure 2. Three primary ligands can activate malarial Toll-like receptor (TLR) signaling pathways: glycosylphosphatidylinositol (GPI) anchors, immunostimulatory nucleic acid (DNA and RNA) motifs, and hemozoin (heme or microvesicles). TLR1, 2, and 6 are activated in the basement membrane (ectodomain) by parasite GPI, whereas heme or microvesicles containing RNA, proteins, and organelles can stimulate TLR4. Upon phagocytosis, infected erythrocytes release parasite DNA and RNA into phagolysosomes, where innate immunity is activated. TLR7 (an RNA ligand) and TLR9 (a DNA ligand) serve as receptors for these nucleic acid patterns. While TLR4 activation via TRIF (TIR domain-containing adaptor inducing interferon-β) induces the production of IFN-a/b, all other TLRs stimulated by malarial ligands promote the production of proinflammatory proteins, such as TNF, IL-6, IL-10, IL-12, and IFNg, via the Myeloid Differentiation Primary Response Gene 88 (MyD88)/NF-κB pathway. The exception is the TLR4 pathway, which can use TRIF or MyD88 adapter proteins, also known as MyD88-adapter-like (MAL), with subsequent activation of interferon regulatory factors (IRFs)
Hemozoin
During the heme detoxification process, all four species of Plasmodium that infect people synthesize an inorganic crystal termed hemozoin. This crystalline structure represents a significant byproduct of Plasmodium’s hemoglobin metabolism and performs a crucial function in the immune system’s response to malaria infection.32 During erythrocyte breakdown, hemozoin is discharged from the feeding vacuole into the bloodstream, and the liberated parasites invade additional uninfected red blood cells (RBCs). Hemozoin is released into the blood following merozoite egress when innate immune cells phagocytose it.32 The hemozoin concentration in neutrophils and monocytes during phagocytosis serves as a reliable indicator of disease severity and parasite burden.31 The precise mechanisms for its persistence remain unclear; however, one hypothesis suggests that hemozoin fails to induce lysosomal heme oxygenase, an enzyme necessary for catalyzing heme degradation.33 To survive within erythrocytes, P. falciparum modifies the heme group and turns it into an insoluble crystal inside the digestive vacuole.34
Hemagglutinin itself possesses the capacity to activate natural immunity through direct interaction with TLR9 and the inflammasome.35 Hemozoin, a parasite-derived pigment, has been conclusively demonstrated to facilitate immunosuppression by preventing dendritic cells from modulating the host’s immune responses against the parasite and other antigens.36 Upon erythrocyte rupture, merozoites, hemozoin, free heme, and other components of the parasite’s cytoplasm and digestive vacuole are liberated, resulting in hemozoin accumulation. Numerous immune cells, including monocytes, neutrophils, dendritic cells, macrophages, and endothelial cells, engage with and internalize hemozoin, and malaria-infected RBCs (iRBCs) release microvesicles (Figure 2). Of these, monocytes and macrophages are the most extensively studied hemozoin-internalizing cells. Human monocytes have been observed to rapidly absorb hemozoin, which can occupy up to 30% of their whole cellular volume. Moreover, the ingested hemozoin can remain unaltered within monocytes for extended durations.37
Nucleic acid motifs (DNA)
Parasitic DNA activates natural immune system cells through detection by DNA sensors in the cytoplasmic matrix and TLR9 in endolysosomes.38 One hypothesis suggests that TLR9 is activated by parasite DNA, thereby generating an initial signal necessary for hemozoin to activate the inflammasome and produce proinflammatory cytokines.39 After being taken up by merozoites, entire diseased erythrocytes, hemozoin, or DNA-protein structures, parasite DNA infiltrates the endolysosomes of natural immune cells.40 This leads to the generation of proinflammatory cytokines and the activation of the MyD88-NF-κB signaling pathway, as shown in Figure 2. Notably, TLR9 is mainly expressed in human B cells and plasmacytoid dendritic cells; consequently, monocytes are unlikely to contribute significantly in the detection of parasite DNA via this route.31
The cytoplasmic matrix DNA-sensing AIM2 and cGAS detect the presence of parasite DNA as it is released from endolysosomes into the cytoplasmic matrix.41 This may result in the generation of recombinant IL-1b and stimulation of the AIM2 inflammatory process.42 In addition, the cGAS-stimulator of IFN genes (STING) process induces a type I interferon (IFN) response.43 Recent research demonstrated that cGAS acts as a sensor that detects P. falciparum genomic DNA in the cytoplasm downstream of STING.44 Type I IFNs are produced when activated cGAS synthesizes 2’3′-cGAMP, which functions as a secondary messenger to activate STING and phosphorylate TBK1 and IRF3-IRF7 (interferon regulatory factors).45
Nucleic acid motifs (RNA)
The natural immune response detects the RNA of parasites throughout hepatocytes and the bloodstream stages.46 The melanoma differentiation-associated protein 5 (MDA5) exclusively detects RNA in the cytoplasmic matrix during the liver stage via MAVS and IRF3 and IRF7, two transcription factors, while TLR3, TLR7, and TLR8 may identify microbial RNA in endosomes.47 Conversely, during the bloodstream stage of infection, TLR7 in dendritic cell phagolysosomes recognizes mouse parasite RNA, which results in the synthesis of type I IFN.26 RNA sequencing in a liver-stage infection with rodent malaria elicits a robust innate immune response, involving type I IFN and IFNg pathways.48 P. falciparum RNA is recognized by endosomal TLR8 in human monocytes, which induces the production of IL-12p70 and IL-18, subsequently causing natural killer cells to produce IFN-ɣ. Protozoan RNA-induced TLR8 activation demonstrates the distinct function of TLR8 in human immunity as well as its crucial involvement in human blood-stage malaria.49 The notable differences between the innate immune systems of humans and mice in the detection of nucleic acids within endosomes may partially elucidate this phenomenon.31
Direct infusion of the plasma membrane produces microvesicles (Figure 2), which are small vesicles 0.1-1 µm in size. Microvesicles function as intercellular communicators and can contain RNA, proteins, and even organelles.50 Individuals with malaria who are infected with P. falciparum or the related human pathogen P. vivax exhibit higher than normal concentrations of microvesicles derived from RBCs and platelets.51 Peripheral blood parasitemia is associated with an increase in microvesicles in individuals with severe illness.52 As the human malaria parasite, P. falciparum, develops, microvesicles derived from iRBCs (RMVs) are quantitatively released from the iRBCs. The majority of RMVs contain proteins originating from both P. falciparum and humans, and they are produced in the later stages of the asexual cycle. Mantel et al. demonstrated that RMVs function as messengers between iRBCs and are immunostimulatory. RMVs are transferred from one iRBC to another and influence the number of transmitting stages produced in infected individuals.53
Glycosylphosphatidylinositol
Glycosylphosphatidylinositol serves as a lipid anchor for numerous cell-surface proteins. The GPI anchor, which is extensively used in eukaryotes and potentially in certain Archaea but is absent in Eubacteria, is a posttranslational modification of proteins with a glycolipid.8 GPI-anchored proteins constitute the predominant kind of cell-surface proteins in protozoa.8 Many GPI-anchored proteins are incorporated into the cell wall of fungi.54 At minimum of 150 GPI-anchored proteins are found in humans and perform a wide range of functions, for example as protease inhibitors, enzymes, adhesion molecules, receptors, and transcytotic receptors and transporters.55 One of the first factors identified as a PAMP of the parasites associated with malaria was GPI of P. falciparum.56 Parasite GPI consists of a varied array of molecules that are connected to the glucosamine moiety of glycan via triacylated phosphatidylinositol. These molecules have four mannose residues and one glucosamine residue.57
The compositional variability of malaria GPI is attributed to the acyl residues located at various locations on the phosphatidylinositol moiety, which vary in length and degree of unsaturation. This compositional variability does not affect GPI’s capacity to elicit an immunological response.58 Evidence for this is provided by the ability of sn-2 lyso GPI, generated by removing the acyl from the parasite GPI at the sn-2 position, to effectively elicit cytokine responses comparable to those of the original parasite GPI.59 GPI is crucial for the survival of parasite as it binds many merozoite proteins that contribute to erythrocyte invasion of the plasma membrane.60 Without GPI anchoring, the surface expression of these proteins does not take place, thus preventing merozoites from invading RBCs.61 Malaria parasites produce GPI in quantities significantly exceeding those required for attaching proteins to the exterior of merozoites, resulting in substantial amounts of unlinked GPI.60 The unattached GPI particles, visible on the cell exterior, are presumably attacked by the innate immune system.26
The main proteins linked to the GPI particles throughout the Plasmodium circulation period of invasion are members of the merozoite surface protein family (MSP-1 and MSP-2) and the rhoptry-associated membrane antigen (RAMA).62 The main GPI-attached protein in the pre-erythrocyte phase is circumsporozoite protein (CSP).63 PF34, Cys6, and apical sushi protein are other Plasmodium proteins that include GPIs.64 GPIs, which are PAMPs, have been demonstrated to be able to activate TLR4 homodimers or the TLR1-TLR2 and TLR2-TLR6 heterodimers to cause the generation of proinflammatory mediators such as tumor necrosis factor (TNF) and nitric oxide, through the MyD88 pathway.65,66 Consequently, these compounds have been investigated as potential malaria vaccine components.67
Toll-like receptors
Humans have been demonstrated to possess 10 TLRs (TLR1-TLR10).68 TLRs are located both on the cell surface (TLR1, 2, 4, 5, 6, and 10) or within the endosomal membrane (TLR3, TLR7, TLR8, and TLR9), enabling them to identify genetic material from pathogens, including nucleic acids found within intracellular pathogenic microorganisms.
In this context, TLRs recognize proteins, lipids, and carbohydrates present in the pathogen’s exterior membrane.69 TLR2 and TLR4 are surface receptor variants that are internally produced in epithelial, dendritic, and endothelial cells.70 TLR1, 2, 4, 6, and 9 were identified in P. falciparum infections, whereas TLR1, 2, 4, 5, 6, and 9 were observed in P. vivax infections. In addition, TLR2 and 4 were detected in both species.71 The immune response profile elucidated by TLR gene polymorphisms does not seem to be universally applicable to all malaria infection patterns globally. This variability may be influenced by both Plasmodium species and human genetic diversity.72 Primary immunodeficiency diseases (PIDs), which are typified by an increased vulnerability to infections, can result from mutations in the genes encoding TLRs and associated signaling components.73 For example, faulty innate immune responses caused by mutations in the MyD88 and IRAK-4 genes disrupt TLR signaling pathways, leading to repeated pyogenic bacterial infections.74 Similarly, TLR3 signaling is disrupted by mutations in the UNC93B1 gene, which puts people at risk for herpes simplex virus encephalitis.75 These genetic changes highlight how important TLRs and the pathways they are linked to are for preserving the integrity of the immune system (Table).
Table:
Malaria ligands of Toll-like receptors (TLRs)
TLRs |
CD – Name |
Other Name |
TLR coreceptor |
TLR-active version |
Chromosome |
Station |
Binder |
Signaling pathway |
References |
|---|---|---|---|---|---|---|---|---|---|
TLR1 |
CD281 |
TIL; rsc786; KIAA001; DKFZp547I0610; DKFZp564I0682 |
TLR2 |
TLR1-TLR2 |
4p14 |
Cell membrane |
Glycosylphosphatidylinositol |
MyD88/MAL, NF-κB, IRFs |
17,26,76-78 |
TLR2 |
CD282 |
TIL4 |
TLR1, 2, 6 and 10 CD14, CD36, integrin, RP105, MBL, LBP |
TLR1-TLR2 TLR2-TLR2 TLR2-TLR6 |
4q31.3 |
Cell membrane |
Glycosylphosphatidylinositol |
MyD88/MAL, NF-κB, IRFs |
17,26,76-78 |
TLR4 |
CD284 |
TOLL; hToll |
MD2, LY96, CD14, CD36, LBP, RP105 |
TLR4-TLR6 |
9q33.1 |
Cell membrane |
Hemozoin, heme microvesicles |
MyD88/MAL, NF-κB, IRFs Or TRIF, RIF3, IRFs |
17,26,76-78 |
TLR6 |
CD286 |
CD286 Antigen Q9Y2C9 |
TLR2, CD36, LBP |
TLR2-TLR6 TLR4-TLR6 |
4p14 |
Cell membrane |
Glycosylphosphatidylinositol |
MyD88/MAL, NF-κB, IRFs |
17,26,76-78 |
TLR7 |
CD287 |
IMD74; SLEB17; TLR7-like |
CD14 |
TLR7-TLR7 |
Xp22.2 |
Endolysosome |
RNA ligand |
MyD88/MAL, NF-κB, IRFs |
17,26,76-78 |
TLR9 |
CD289 |
CD289 Antigen Q9NR96 |
CD14 |
TLR9-TLR9 |
3p21.2 |
Endolysosome |
DNA ligand |
MyD88/MAL, NF-κB, IRFs |
17,26,76-78 |
TLRs cell surface receptors
TLR1 (CD281)
TLR1 is a gene that encodes the Toll-like receptor 1 protein. It is an essential component of the innate immune system that facilitates the detection and response to pathogens like the parasite responsible for malaria.79 Research on a Southeast Asian population affected by P. falciparum malaria demonstrated an association between elevated parasitemia and a common TLR1 variant. Parasitemia, the presence of parasites in the blood, is a critical indicator of malaria severity. The findings suggested that mutations in TLR1 influenced the host response to P. falciparum malaria in Asian people.80
The results for TLR1 and TLR6 are inconsistent. Two studies examining the impact of these TLRs on susceptibility reported statistical associations, albeit with differing outcomes.71 However, another study suggested an increase in vulnerability.81 The majority of studies found no effect on severity.82 Only one investigation identified a significant association, indicating that the presence of this receptor exacerbated the severity of disease. This investigation included P. falciparum-infected adolescents and adults from Asia (Myanmar).80 Research conducted in west-central Africa identified a link between TLR1 and higher levels of parasitemia in childhood.83 Zhu et al. were able to detect TLR2-TLR6 with marginally higher discrimination than the dimeric pair TLR2-TLR1 due to their findings on sn-2 lyso GPIs, a malarial GPI variant with two fatty acid substituents. Nevertheless, the TLR2-TLR1 dimeric pair is more frequently engaged by malarial GPIs (Figure 2), which possess three fatty acid substituents, than TLR2-TLR6.84
TLR2 (CD282)
TLR2 is a protein-coding gene crucial to the innate immune system. It is expressed on multiple immune cells, such as dendritic cells, monocytes, neutrophils, macrophages, B lymphocytes, Th1, Th2, and Treg lymphocytes.85 Host cellular responses are primarily elicited by GPI through TLR2/MyD88-mediated signaling.65 As PAMPs, GPIs have been demonstrated to activate TLR4 homodimers or the TLR1-2 and TLR2-6 heterodimers, thereby inducing the synthesis of proinflammatory mediators, including TNF and nitric oxide, through the MyD88 pathway.86
According to the results of a meta-analysis on TLR2, the majority of studies assessing this TLR concluded that it does not affect susceptibility.71 However, research conducted in Ghana, Africa, involving P. falciparum-infected individuals of various ages, found that this TLR played a role in protection with regard to malaria susceptibility.87 Two studies have demonstrated that the CD282 receptor confers a protective effect against severe types of malaria.87,88 These investigations, conducted in Ghana and Uganda, Africa, involved P. falciparum-infected individuals. In persons heterozygous for the TLR2D22 mutation, reduced inducible production of TLR2 may result in diminished pro-inflammatory responses and potentially serve as an effective defense against cerebral malaria (Table).88
TLR4 (CD284)
The gene TLR4 is among the most extensively studied TLRs as it encodes the primary receptor for lipopolysaccharides, TLR4, a component of certain microorganisms such as malaria parasites.89 TLR4 may be crucial to the clinical pathophysiology of malaria. This is supported by existing data and suggests that further investigation of TLR4 and associated genes in clinical malaria could lead to novel therapeutic approaches and pharmaceutical discoveries.90 A previous study of patients from Ghana showed that this receptor exacerbated the condition.87 A sequence of feedback events may be a potential explanation for the mechanism by which heme activates the TLR4 signaling pathway.91 Elevated TLR2, 4, and 8 levels were noted in a case study of severe malaria.92
Heme, a byproduct of RBCs, is suggested as a DAMP that influences inflammatory responses in various pathophysiological contexts and has been associated with TLR4 signaling.91,93 Moreover, heme can facilitate the recruitment of WBCs, RBCs, and leukocytes to the vascular endothelium. As initially demonstrated in macrophages, numerous pro-inflammatory actions of heme are linked to TLR4 signaling stimulation.94 Heme-mediated TLR4 signaling seems to involve complex regulatory processes that vary depending on the experimental models and conditions.91,95 The MyD88-dependent or MyD88-independent pathway is responsible for the production of pro-inflammatory cytokines after TLR4 activation by lipopolysaccharides or DAMPs.96 In addition, DAMPs generated during malaria infection can activate TLR4. Macrophages have the capacity to internalize RMVs produced by Plasmodium species and trigger TLR4.53,97 Couper et al.97 showed that the macrophage activation pathway mediated by microvesicles is dependent on TLR-4 and MyD88. This represents a novel and significant mechanism of fundamental inflammation during malaria infection, and it associates the onset of severe malarial illness with microvesicles originating from parasitized RBCs (Table).97
TLR6 (CD286)
The human TLR6 gene encodes TLR6, also known as CD286.98 TLR6 has been shown to increase the prevalence of acute malaria cases in African (Cameroonian) children with P. falciparum infections.83 TLR6 and TLR2 form heterodimers, which enhance the ligand’s potency against certain infections.99 In conjunction with TLR1 and TLR6, TLR2 detects GPI in a heterodimeric form.65 When host immune cells recognize GPIs on parasite surfaces, TLR2, in combination with TLR1 or TLR6 activates NF-κB, leading to the secretion of pro-inflammatory cytokines.100 Murine TLR1 and TLR6 exhibit reduced selectivity compared to individual TLRs in identifying malarial GPIs; furthermore, TLR6 and TLR1 macrophages produce substantial quantities of nitric oxide and TNF-α (Table).65
TLR endosomal membrane receptors
TLR7 (CD287)
The endosomal natural immunity detector, TLR7, has the capacity to identify single-stranded ribonucleic acid.101 Its mechanism of endosomal TLR placement is complex and tightly regulated and shares certain elements with other TLRs.18 Research conducted on P. chabaudi clarified TLR7’s function in IFN-1, IL-12, and IFN-g production. TLR9 was previously identified as the primary indicator of infection. However, studies carried out without TLR7 and MyD88 demonstrated a significant decrease in pro-inflammatory cytokine-like IFN-1. On the other hand, animals lacking TLR2, 4, 9, interleukin-1 receptor, or IL18R had no effect on IFN production.102
This discrepancy is attributed to the difference in accessible ligands and the stimulation of TLR9 or TLR7 based on the duration or phase of infection.103 Despite the lack of evidence for the parasite ligand that activates TLR7, it has been established that single-stranded RNA is necessary for TLR7-mediated IFN-I release in viral infection.104 It has been postulated that the parasite’s RNA might bind to the receptor and function as a ligand.103 In addition, TLR7 responds to pathogens identified in the endosome by initiating an immune response to purine-rich single-stranded ribonucleic acid.101 Notwithstanding this hypothesis, the precise receptor-ligand interaction remains to be elucidated (Table).100
TLR9 (CD289)
A key component of the natural immunological response to malaria is the TLR9 protein. The TLR9 receptor facilitates an effective immunological response against malaria in a manner that is dependent on MyD88.105 The innate immune system has been demonstrated to be activated by the murine malaria pathogens P. berghei, P. chabaudi chabaudi AS, and P. yoelii, as well as TLR2 and TLR9.106,107 Dendritic cells are activated by parasite DNA through the TLR9 receptor.108,109
Parroche et al.110 observed that TLR9 may be induced by natural hemozoin but not by purified heme. Experiments showed that stimulation was inhibited because nuclease prevented TLR9 from binding. Subsequent research confirmed that hemozoin’s exterior contains DNA that interacts with TLR9.110 Through direct interactions with TLR9 and the inflammatory response, in vitro experiments using synthetic versions have demonstrated that hemozoin per se may stimulate the innate immune response.111 The initial signal necessary for hemozoin to activate inflammation and produce pro-inflammatory cytokines is likely caused by parasite DNA activating TLR9 as shown in Figure 2.112
The function of TLRs in malaria remains unclear and is potentially complex, although evidence suggests that these receptors may contribute to both immunological pathogenesis and prevention. Despite numerous research publications on the subject, there is a lack of consensus on whether TLR variations have a detrimental or beneficial effect on the clinical manifestations of malaria infections. Indeed, multiple variables may confound the findings and impede accurate comparison of studies. These variables include subject age, study population size, quality control measures employed during specimen processing, genetic diversity of Plasmodium species distributed across study locations, variation in malaria endemicities, and hereditary traits of the hosts. Nevertheless, the abundant published data strongly suggest that genetic variations of TLRs affect the incidence and progression of malaria. Future research on TLRs as mediators of the innate immune response may enhance comprehension of the immune system’s balanced operation, which is crucial for developing novel treatments for a wide range of immune-related disorders, including malaria.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made substantial, direct and intellectual contributions to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
Not applicable.
ETHICS STATEMENT
Not applicable.
- World Health Organization (WHO). Malaria. 2023. https://www.who.int/news-room/questions-and-answers/item/malaria?gclid= CjwKCAjwpJWoBhA8EiwAHZFzfkonvrfcOUvfK6VSrF8 NCq7OqlaF7jJ_YFj4gLjUyTSr8PGgtRjxjBoCAv4QAvD_BwE Accessed 16 september 2023
- Clark VL, Kruse JA. Clinical methods: the history, physical, and laboratory examinations. JAMA. 1990;264(21):2808-2809.
Crossref - Sey ICM, Ehimiyein AM, Bottomley C, Riley EM, Mooney JP. Does Malaria Cause Diarrhoea? A Systematic Review. Front Med. 2020;7:589379.
Crossref - White NJ. Anaemia and malaria. Malar J. 2018;17(1):371.
Crossref - Balaji SN, Deshmukh R, Trivedi V. Severe malaria: Biology, clinical manifestation, pathogenesis and consequences. J Vector Borne Dis. 2020;57(1):1-13.
Crossref - Mirzohreh ST, Safarpour H, Pagheh AS, Bangoura B, Barac A, Ahmadpour E. Malaria prevalence in HIV-positive children, pregnant women, and adults: a systematic review and meta-analysis. Parasit Vectors. 2022;15(1):324.
Crossref - Zekar L, Sharman T. Plasmodium falciparum malaria. StatPearls [Internet]. StatPearls Publishing. 2023.
- Eichler J, Adams MWW. Posttranslational protein modification in Archaea. Microbiol Mol Biol Rev. 2005;69(3):393-425.
Crossref - Flores-Garcia Y, Nasir G, Hopp CS, et al. Antibody-Mediated Protection against Plasmodium Sporozoites Begins at the Dermal Inoculation Site. mBio. 2018;9(6).
Crossref - Kori LD, Valecha N, Anvikar AR. Insights into the early liver stage biology of Plasmodium. J Vector Borne Dis. 2018;55(1):9-13.
Crossref - Akter J, Khoury DS, Aogo R, et al. Plasmodium-specific antibodies block in vivo parasite growth without clearing infected red blood cells. PLoS Pathog. 2019;15(2):e1007599.
Crossref - Eksi S, Haile Y, Furuya T, Ma L, Su X, Williamson KC. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol. 2005;143(1):90-99.
Crossref - Trivedi S, Chakravarty A. Neurological Complications of Malaria. Curr Neurol Neurosci Rep. 2022;22(8):499-513.
Crossref - Mandala WL, Harawa V, Dzinjalamala F, Tembo D. The role of different components of the immune system against Plasmodium falciparum malaria: Possible contribution towards malaria vaccine development. Mol Biochem Parasitol. 2021;246:111425.
Crossref - Kielian T. Overview of Toll-Like Receptors in the CNS. In: Kielian, T. (eds) Toll-like Receptors: Roles in Infection and Neuropathology. Current Topics in Microbiology and Immunology, vol 336. Springer, Berlin, Heidelberg.
Crossref - Liu G, Zhang H, Zhao C, Zhang H. Evolutionary History of the Toll-Like Receptor Gene Family across Vertebrates. Genome Biol Evol. 2020;12(1):3615-3634.
Crossref - Sameer AS, Nissar S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. Biomed Res Int. 2021;2021(1):1157023.
Crossref - Mokhtari Y, Pourbagheri-Sigaroodi A, Zafari P, Bagheri N, Ghaffari SH, Bashash D. Toll-like receptors (TLRs): An old family of immune receptors with a new face in cancer pathogenesis. J Cell Mol Med. 2021;25(2):639-651.
Crossref - Hinck AP, Mueller TD, Springer TA. Structural Biology and Evolution of the TGF-b Family. Cold Spring Harb Perspect Biol. 2016;8(12)
Crossref - Nie L, Cai SY, Shao JZ, Chen J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front Immunol. 2018;9:1523.
Crossref - Kawasaki T, Kawai T. Discrimination Between Self and Non-Self-Nucleic Acids by the Innate Immune System. Int Rev Cell Mol Biol. 2019;344:1-30.
Crossref - Khan JA, Brint EK, O’Neill LAJ, Tong L. Crystal structure of the Toll/interleukin-1 receptor domain of human IL-1RAPL. J Biol Chem. 2004;279(30):31664-31670.
Crossref - Wicherska-Pawlowska K, Wrobel T, Rybka J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int J Mol Sci. 2021;22(24):13397
Crossref - Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12(3):168-179.
Crossref - Bzowka M, Bagrowska W, Gora A. Recent Advances in Studying Toll-like Receptors with the Use of Computational Methods. J Chem Inf Model. 2023;63(12):3669-3687.
Crossref - Gowda DC, Wu X. Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria. Front Immunol. 2018;9:3006.
Crossref - Gustifante BN, Khairani S, Fauziah N, Riswari SF, Berbudi A. Targeting T-Cell Activation for Malaria Immunotherapy: Scoping Review. Pathogens. 2025;14(1):71.
Crossref - Nebl T, De Veer MJ, Schofield L. Stimulation of innate immune responses by malarial glycosylphosphatidylinositol via pattern recognition receptors. Parasitology. 2005;130(S1):S45-S62.
Crossref - Barboza R, Lima FA, Reis AS, et al. TLR4-mediated placental pathology and pregnancy outcome in experimental malaria. Sci Rep. 2017;7(1):8623.
Crossref - Yap XZ, Lundie RJ, Beeson JG, O’Keeffe M. Dendritic Cell Responses and Function in Malaria. Front Immunol. 2019;10:357.
Crossref - Dobbs KR, Crabtree JN, Dent AE. Innate immunity to malaria-The role of monocytes. Immunol Rev. 2020;293(1):8-24.
Crossref - Olivier M, Van Den Ham K, Shio MT, Kassa FA, Fougeray S. Malarial pigment hemozoin and the innate inflammatory response. Front Immunol. 2014;5:25.
Crossref - Pek RH, Yuan X, Rietzschel N, et al. Hemozoin produced by mammals confers heme tolerance. Elife. 2019;8:49503.
Crossref - Coronado LM, Nadovich CT, Spadafora C. Malarial hemozoin: from target to tool. Biochim Biophys Acta. 2014;1840(6):2032-2041.
Crossref - Hayward JA, Mathur A, Ngo C, Man SM. Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol Mol Biol Rev. 2018;82(4):00015-18.
Crossref - Urban BC, Todryk S. Malaria pigment paralyzes dendritic cells. J Biol. 2006;5(2):4.
Crossref - Shio MT, Kassa FA, Bellemare MJ, Olivier M. Innate inflammatory response to the malarial pigment hemozoin. Microbes Infect. 2010;12(12-13):889-899.
Crossref - Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373-384.
Crossref - Kalantari P. The Emerging Role of Pattern Recognition Receptors in the Pathogenesis of Malaria. Vaccines. 2018;6(1):6010013.
Crossref - Molina-Franky J, Patarroyo ME, Kalkum M, Patarroyo MA. The Cellular and Molecular Interaction Between Erythrocytes and Plasmodium falciparum Merozoites. Front Cell Infect Microbiol. 2022;12:816574.
Crossref - Wan D, Jiang W, Hao J. Research Advances in How the cGAS-STING Pathway Controls the Cellular Inflammatory Response. Front Immunol. 2020;11:615.
Crossref - Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1b secretion. Cytokine Growth Factor Rev. 2011;22(4):189-195.
Crossref - Li F, Wang N, Zheng Y, Luo Y, Zhang Y. cGAS- Stimulator of Interferon Genes Signaling in Central Nervous System Disorders. Aging Dis. 2021;12(7):1658-1674.
Crossref - Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21(9):501-521.
Crossref - Luo J, Cao Q, Zhang J, et al. Porcine IKKe is involved in the STING-induced type I IFN antiviral response of the cytosolic DNA signaling pathway. J Biol Chem. 2023;299(10):105213.
Crossref - Minkah NK, Kappe SHI. Malaria Immunity: The Education of an Unnatural Response. Cell Host Microbe. 2019;25(4):479-481.
Crossref - Hu H, Yang H, Liu Y, Yan B. Pathogenesis of Anti-melanoma Differentiation-Associated Gene 5 Antibody-Positive Dermatomyositis: A Concise Review With an Emphasis on Type I Interferon System. Front Med. 2021;8:833114.
Crossref - Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SHI. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014;7(2):436-447.
Crossref - Coch C, Hommertgen B, Zillinger T, et al. Human TLR8 Senses RNA From Plasmodium falciparum-Infected Red Blood Cells Which Is Uniquely Required for the IFN-g Response in NK Cells. Front Immunol. 2019;10:371.
Crossref - Stahl AL, Johansson K, Mossberg M, Kahn R, Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol. 2019;34(1):11-30.
Crossref - Madkhali AM, Mobarki AA, Ghzwani AH, et al. Elevated Levels of Procoagulant Microvesicles and Tissue-Factor Bearing Microvesicles in Malaria Patients. Int J Gen Med. 2023;16:1205-1215.
Crossref - Ye W, Chew M, Hou J, et al. Microvesicles from malaria-infected red blood cells activate natural killer cells via MDA5 pathway. PLoS Pathog. 2018;14(10):e1007298.
Crossref - Mantel PY, Hoang AN, Goldowitz I, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13(5):521-534.
Crossref - Vogt MS, Schmitz GF, Varon Silva DV, Mosch HU, Essen LO. Structural base for the transfer of GPI-anchored glycoproteins into fungal cell walls. Proc Natl Acad Sci U S A. 2020;117(36):22061-22067.
Crossref - Kinoshita T. Glycosylphosphatidylinositol (GPI) Anchors: Biochemistry and Cell Biology: Introduction to a Thematic Review Series. J Lipid Res. 2016;57(1):4-5.
Crossref - Tachado SD, Mazhari-Tabrizi R, Schofield L. Specificity in signal transduction among glycosylphosphatidylinositols of Plasmodium falciparum, Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. Parasite Immunol. 1999;21(12):609-617.
Crossref - Malik A, Steinbeis F, Carillo MA, Seeberger PH, Lepenies B, Varon Silva D. Immunological Evaluation of Synthetic Glycosylphosphatidylinositol Glycoconjugates as Vaccine Candidates against Malaria. ACS Chem Biol. 2020;15(1):171-178.
Crossref - Naik RS, Branch OH, Woods AS, et al. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J Exp Med. 2000;192(11):1563-1576.
Crossref - Perraut R, Diatta B, Marrama L, et al. Differential antibody responses to Plasmodium falciparum glycosylphosphatidylinositol anchors in patients with cerebral and mild malaria. Microbes Infect. 2005;7(4):682-687.
Crossref - Boyle MJ, Langer C, Chan JA, et al. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun. 2014;82(3):924-936.
Crossref - Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJI, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev. 2016;40(3):343-372.
Crossref - Dijkman PM, Marzluf T, Zhang Y, et al. Structure of the merozoite surface protein 1 from Plasmodium falciparum. Sci Adv. 2021;7(23):eabg0465.
Crossref - Zhang M, Kaneko I, Tsao T, et al. A highly infectious Plasmodium yoelii parasite, bearing Plasmodium falciparum circumsporozoite protein. Malar J. 2016;15:201.
Crossref - Shears MJ, Sekhar Nirujogi RS, Swearingen KE, et al. Proteomic Analysis of Plasmodium Merosomes: The Link between Liver and Blood Stages in Malaria. J Proteome Res. 2019;18(9):3404-3418.
Crossref - Gowda DC. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 2007;23(12):596-604.
Crossref - Goerdeler F, Seeberger PH, Moscovitz O. Unveiling the Sugary Secrets of Plasmodium Parasites. Front Microbiol. 2021;12:712538.
Crossref - de Oliveira Guimarדes L, Barboza R, Wunderlich G, Kirchgatter K. TLRs in Malaria. In: Kremsner PG, Krishna S, eds. Encyclopedia of Malaria. Springer New York; 2018:1-11.
Crossref - Oosting M, Cheng SC, Bolscher JM, et al. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc Natl Acad Sci U S A. 2014;111(42):E4478-84.
Crossref - Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24-32.
Crossref - Uronen-Hansson H, Allen J, Osman M, Squires G, Klein N, Callard RE. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology. 2004;111(2):173-178.
Crossref - Ramirez Ramirez AD, de Jesus MCS, Rossit J, et al. Association of toll-like receptors in malaria susceptibility and immunopathogenesis: A meta-analysis. Heliyon. 2022;8(4):e09318.
Crossref - Ramirez ADR, de Jesus MCS, Menezes RAO, et al. Polymorphisms in Toll-Like receptors genes and their associations with immunological parameters in Plasmodium vivax malaria in the Brazil-French Guiana Border. Cytokine. 2023;169:156278.
Crossref - Maglione PJ, Simchoni N, Cunningham-Rundles C. Toll-like receptor signaling in primary immune deficiencies. Ann N Y Acad Sci. 2015;1356(1):1-21.
Crossref - Deghmane AE, Taha MK. Invasive Bacterial Infections in Subjects with Genetic and Acquired Susceptibility and Impacts on Recommendations for Vaccination: A Narrative Review. Microorganisms. 2021;9(3)
Crossref - Cleaver J, Jeffery K, Klenerman P, et al. The immunobiology of herpes simplex virus encephalitis and post-viral autoimmunity. Brain. 2024;147(4):1130-1148.
Crossref - Database GTHG. TLR. Weizmann Institute of Science. 2024. https://www.genecards.org/Search/Keyword?queryString=TLR. Accessed 10 March 2024.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805-20.
Crossref - Molecules HHCD. Molecule Information. Updated Sunday, 10 March 2024. Accessed Sunday, 10 March 2024, 2024. https://www.hcdm.org/index.php?option=com_molecule&cdnumber=CD281
- Pohl K, Cockburn IA. Innate immunity to malaria: The good, the bad and the unknown. Front Immunol. 2022;13:914598.
Crossref - Hahn WO, Harju-Baker S, Erdman LK, et al. A common TLR1 polymorphism is associated with higher parasitaemia in a Southeast Asian population with Plasmodium falciparum malaria. Malar J. 2016;15:12.
Crossref - Costa AG, Ramasawmy R, Ibiapina HNS, et al. Association of TLR variants with susceptibility to Plasmodium vivax malaria and parasitemia in the Amazon region of Brazil. PLoS One. 2017;12(8):e0183840.
Crossref - Kirtland ME, Tsitoura DC, Durham SR, Shamji MH. Toll-Like Receptor Agonists as Adjuvants for Allergen Immunotherapy. Front Immunol. 2020;11:599083.
Crossref - Apinjoh TO, Anchang-Kimbi JK, Njua-Yafi C, et al. Association of cytokine and Toll-like receptor gene polymorphisms with severe malaria in three regions of Cameroon. PLoS One. 2013;8(11):e81071.
Crossref - Zhu J, Krishnegowda G, Li G, Gowda DC. Proinflammatory responses by glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum are mainly mediated through the recognition of TLR2/TLR1. Exp Parasitol. 2011;128(3):205-211.
Crossref - Colleselli K, Stierschneider A, Wiesner C. An Update on Toll-like Receptor 2, Its Function and Dimerization in Pro- and Anti-Inflammatory Processes. Int J Mol Sci. 2023;24(15):24152464.
Crossref - Durai P, Govindaraj RG, Choi S. Structure and dynamic behavior of Toll-like receptor 2 subfamily triggered by malarial glycosylphosphatidylinositols of Plasmodium falciparum. Febs J. 2013;280(23):6196-6212.
Crossref - May L, van Bodegom D, Frolich M, et al. Polymorphisms in TLR4 and TLR2 genes, cytokine production and survival in rural Ghana. Eur J Hum Genet. 2010;18(4):490-495.
Crossref - Greene JA, Sam-Agudu N, John CC, Opoka RO, Zimmerman PA, Kazura JW. Toll-like receptor polymorphisms and cerebral malaria: TLR2 D22 polymorphism is associated with protection from cerebral malaria in a case control study. Malar J. 2012;11:47.
Crossref - Ferwerda B, McCall MB, Alonso S, et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci U S A. 2007;104(42):16645-16650.
Crossref - Ariff A, Song Y, Aguilar R, et al. Genetic variants of TLR4, including the novel variant, rs5030719, and related genes are associated with susceptibility to clinical malaria in African children. Malar J. 2023;22(1):177.
Crossref - Humayun F, Domingo-Fernandez D, Paul George AA, et al. A Computational Approach for Mapping Heme Biology in the Context of Hemolytic Disorders. Front Bioeng Biotechnol. 2020;8:74.
Crossref - Sobota RS, Dara A, Manning JE, et al. Expression of complement and toll-like receptor pathway genes is associated with malaria severity in Mali: a pilot case control study. Malar J. 2016;15:150.
Crossref - Wagener FA, Eggert A, Boerman OC, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98(6):1802-1811.
Crossref - Figueiredo RT, Fernandez PL, Mourao-Sa DS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282(28):20221-20229.
Crossref - Vallelian F, Schaer CA, Deuel JW, et al. Revisiting the putative role of heme as a trigger of inflammation. Pharmacol Res Perspect. 2018;6(2):e00392.
Crossref - Firmal P, Shah VK, Chattopadhyay S. Insight Into TLR4-Mediated Immunomodulation in Normal Pregnancy and Related Disorders. Front Immunol. 2020;11:807.
Crossref - Couper KN, Barnes T, Hafalla JCR, et al. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010;6(1):e1000744.
Crossref - Mustafa G, Mahrosh HS, Arif R. Sequence and Structural Characterization of Toll-Like Receptor 6 from Human and Related Species. Biomed Res Int. 2021;2021:5545183.
Crossref - Choteau L, Vancraeyneste H, Le Roy D, et al. Role of TLR1, TLR2 and TLR6 in the modulation of intestinal inflammation and Candida albicans elimination. Gut Pathog. 2017;9:9.
Crossref - Soni R, Sharma D, Rai P, Sharma B, Bhatt TK. Signaling Strategies of Malaria Parasite for Its Survival, Proliferation, and Infection during Erythrocytic Stage. Front Immunol. 2017;8:349.
Crossref - Petes C, Odoardi N, Gee K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front Immunol. 2017;8:1075.
Crossref - Fekonja O, Avbelj M, Jerala R. Suppression of TLR signaling by targeting TIR domain-containing proteins. Curr Protein Pept Sci. 2012;13(8):776-88.
Crossref - Baccarella A, Fontana MF, Chen EC, Kim CC. Toll-like receptor 7 mediates early innate immune responses to malaria. Infect Immun. 2013;81(12):4431-42.
Crossref - Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529-31.
Crossref - Gowda NM, Wu X, Gowda DC. TLR9 and MyD88 are crucial for the development of protective immunity to malaria. J Immunol. 2012;188(10):5073-5085.
Crossref - Franklin BS, Rodrigues SO, Antonelli LR, et al. MyD88-dependent activation of dendritic cells and CD4+ T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect. 2007;9(7):881-890.
Crossref - Seixas E, Moura Nunes JF, Matos I, Coutinho A. The interaction between DC and Plasmodium berghei/chabaudi-infected erythrocytes in mice involves direct cell-to-cell contact, internalization and TLR. Eur J Immunol. 2009;39(7):1850-1863.
Crossref - Pichyangkul S, Yongvanitchit K, Kum-arb U, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol. 2004;172(8):4926-4933.
Crossref - Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol. 2010;184(8):4338-4348.
Crossref - Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104(6):1919-1924.
Crossref - Sharma RK, Sharma J, Kumar R, et al. TLR9 signalling activation via direct ligation and its functional consequences in CD4 + T cells. Scand J Immunol. 2022;96(5):13214.
Crossref - Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT. Innate sensing of malaria parasites. Nat Rev Immunol. 2014;14(11):744-757.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.