ISSN: 0973-7510
E-ISSN: 2581-690X
Proteases are widespread in nature and execute a large variety of functions and have important biotechnological applications. A novel protease producing bacterial stain was isolated from the local soil sample. The bacterium was gram positive and forms spore during adverse condition in the growth medium. After different tests it was suggested and the features agreed with the description of Bacillus subtilis in Bergey’s Manual of Systematic Bacteriology. It was also characterized and identified by using a bioinformatics tool PIB that suggests the organism was B. subtilis (ID=0.9760). The experimentally determined isoelectric point was 5.3 and the optimal enzyme activity was at 60°C and at pH 8.5. We found that proteolytic activity of enzyme was 21.13 units and extracellular protease activity was higher than intracellular protease activity of the bacterium. Native enzyme preparations typically showed a Michaelis constant (Km) and Vmax of 0.43mM and 12,300 U mg)-1, respectively. Enzyme purity was tested by SDS-PAGE. The molecular weight of the protease was found to be ~30 KDa. The enzyme was slightly modulated by MG++ ion, and significantly by Hg++ ion, while Zn++ ion slightly decrease the proteolytic activity. The enzyme was also seen to be able to remove blood and curry stain from clothes; making it a very promising candidate to be used in a leather, poultry and detergent industry. A number of companies such as NOVO chemicals started to produce NOVOzymes for tannery and poultry industries.
Bacillus subtilis, Proteases, Enzymatic dehairing, Detergent industry, SDS PAGE.
Proteases produced by enzymatic method are more environment friendly than chemical process and they have tremendous potential in the leather and in other several industries. The global market for industrial enzymes in 2015 and 2016 was nearly $4.5 and $4.8 billion21. They find application in a number of biotechnological processes, food processing, Pharmaceuticals, leather industry, silk, bakery, soy processing, meat tendering, detergent and brewery industries10,7. However, its application in the production of peptide synthesis in organic media is limited by the presence of organic solvents but microbial proteases are more environments friendly when compared with the chemical process6, 17.
Microbial proteases are extracellular in nature and directly secreted into the fermentation broth, thus simplifying downstream processing of the enzyme as compared to proteases obtained from plants and animals. In this regard, the Bacillus species specially (B. subtilis) were exploited for their ability to produce extracellular enzymes in submerged fermentation18,19. Use of microbial enzymes for industrial processing has received considerable attention in recent years owing mainly environmental and health concerns7.
Microbial proteases are among the most important hydrolytic enzymes and have been studied extensively since the advent of Enzymology. It is essential to develop a cost effective and eco-friendly technology by screening for efficient enzymes from microbial sources and producing them in large quantities by applying rDNA technology13. Enzymes found in nature are quite often not readily available in quantities sufficient for industrial use, so use of gene expression methods to express recombinant proteins in the suitable heterologous expression systems is required1. Genetic engineering could be used to increase the gene copy number as an effective method for improving enzyme productivity9.
Most of the tannery and poultry industries in Bangladesh use chemicals for processing that led great environmental hazards and health problem. However, leather industries are one of the most promising fields for export to earn foreign currency in Bangladesh. Recently government of People’s Republic of Bangladesh has taken initiative to develop the industry from outside the city and modernize it. Enzymatic dehairing is suggested as an environment friendly alternative to the conventional chemical process. Enzymes have been pursued as one of the promising alternates to lime and sodium sulfide3. The aim of the study were to culture dehairing protease producing Bacillus sp. in large scale for production and purification of thermotolerant extracellular protease from it, than evaluating practical application of the enzyme in leather, poultry and detergent industry.
Sample collection and culture conditions: The experiment was conducted in the Department of Biochemistry and Microbiology of the Gono University, Savar, Dhaka, Bangladesh. The soil sample was collected from the poultry wastes in Savar. After serial dilution, culture was given in LB broth media from the sample for 16 h at 37°C. Each colony was inoculated into screw capped test tubes containing autoclaved feather with liquid broth media and incubated overnight at 37°C with shaking at 160 rpm. One media was used as negative control. Chemicals used in the experiment were from Oxoid Ltd (Basingstoke, UK), Merck AG (Darmstadt, Germany) and Sigma (USA).
Identification of Protease producing bacterial strains: A rapid bacterial identification test kit for Bacillus, API 50 CHB (BioMerieux, France), was used to identify species of bacteria. Morphological studies, physiological and biochemical characteristics of the isolate were investigated according to Bergey’s Manuals.22 For correct interpretation of the results in every test Escherichia coli was taken as control. Some Microbiological tests that were performed are the Gram staining, Spore staining, colony morphology and growth curve determination of the organism.
Determination of extracellular and intracellular protease activity: The organism was grown overnight in a psychrotherm incubated shaker incubator for overnight at 37°C and 180 rpm. Two fractions were collected of the bacterial culture. For one fraction the culture was centrifuged at 5000g for 25 minutes and supernatant was taken and normal azocasein assay was carried out. For the other fraction the after centrifugation supernatant was discarded and pellet (cells) was washed in saline water and then they were disrupted by ultrasonic treatment (Sonifier Branson 250) by using a 20% duty cycle at output 3 for 5 min and centrifuged at 6,000g for 15 minutes. Then azocasein assay was carried out.
Determination of protein content and proteolytic activity: The bacterium was cultivated in sterile nutrient broth medium. The culture was grown overnight on a rotary shaker at 150 rpm and incubated at 37°C for 15-20 hours. The culture was then centrifuged at 10000 rpm for 10 minutes at 4°C. The supernatant was collected and used as crude enzyme sample. Proteolytic activities were assayed by Azocasien test, described by Kreger and Lockwood (1981) was done. Here azocasein is used as a substrate. Optical density was measured at 440 nm.
Determination of co-relation between bacterial growth and extracellular protease synthesis at different time interval at 37°C: The bacterial culture was grown in nutrient broth at various temperatures (25ºC, 30ºC, 35ºC, 40ºC, 50ºC, 60ºC) and was incubated for 48 hours to measure its growth profile. For the determination of the effect of temperature, the culture medium was incubated at temperature ranging from 25-60°C and the protease activity was determined at 37°C using the usual methods.
Observation of dehairing capability of the enzyme: For the dehairing studies, the organism was grown in TSB broth at 37ºC for around 20 hours. Then it was centrifuged at 4000 rpm for 8 minutes. The cell free supernatant was added on washed skin to observed enzymatic dehairing capability of the organism. Sodium azide was used at 1% so that no organism can grow. Nutrient broth was used as control.
Growth of the organism and feather degradation: The organisms were allowed to grow at different temperature and its father degradation capabilities were also observed up to 72 hours. The growth pH profile was seen for each organism. Effect of divalent ions on the growth of these bacteria was tested.
Estimation of kinetic parameters and isoelectric point of protease: The kinetic parameters Km and Vmax were determined in 30 mM Tris–HCl, pH 9.0, at 25ºC over the substrate concentration range from 0.01 to 5 mM p-nitrophenyl acetate. Analytical isoelectric focusing of the purified enzyme was performed with an Ampholine PAG plate precast polyacrylamide gel, with pH values ranging from 3 to 10 and the broad pI calibration kit (Amersham Biosciences) as pI marker.
Effect of various reagents and effectors on protease activity: The activity of the isolated protease was tested in the presence of various known protease effectors. The azocasein assay was used with the addition of these effectors solution to achieve a final desired effectors concentration of 5mM. Control was taken where azocasein assay without these effectors was carried out. The protease activity was measured with adding different salts like ZnSO4, MgSO4, CuSO4, NaCl and HCl at different concentration and then azocasein assay was performed.
Enzyme Purification: All subsequent enzyme purification steps were carried out at 0–4 °C. For enzyme purification crude enzyme was re-dissolved in buffer and ammonium sulfate was removed by dialysis. After that centricon (ultra-filtration device) is used for collecting enzyme larger than 30-KDa. These enzymes were further purified by DEAE cellulose column ion-exchange chromatography, where 1.0 M Tris-HCl used as buffer and 0.1-3.0 M NaCl solution as gradient. Enzyme purification was confirmed by SDS-PAGE.
Evaluation of detergent activity of the protease enzyme: To evaluate the ability of the enzyme to remove stain (blood, curry) from clothes crude enzyme supernatant was used along with/without commercial detergent powder.
Detection of storage stability of protease: The Storage stability of protease was determined at 40C, room temperature and room temperature with Sodium benzoate 0.60%. The residual enzyme activity of each line measured under standard assay condition at every 7 days of interval.
Identification and characterization of the isolated bacterial stains: The work was to isolate, identify and characterize thermophilic protease enzyme producing bacterium which could specifically be used for poultry processing industry, detergent and tannery industries. This organism was characterized and identified as a member of gram positive Bacillus sp. by several test. The features agreed with the description of Bacillus subtilis in Bergey’s Manual of Systematic Bacteriology.22 It was also identified as B. subtilis with 99.9% identity by API 50 CHB. So this bacteria is named here as a Bacillus subtilis.
Extracellular and Intracellular protease activities at 37°C: The organism was grown in nutrient broth at 37°C with continuous shaking, two samples were taken.
One was assayed for extracellular protease activity by Kreger and Lockwood method’s after removing the cells by centrifugation while the other was assayed for intracellular protease activity after cell disruption by sonication. We found that extracellular protease activity was higher than intracellular protease activity of the bacterium (Fig 1).
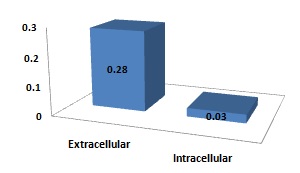
Fig. 1. Graphical presentation of extracellular and intracellular protease activity.
Proteolytic activity of the enzyme: Proteolytic activities were assayed by Azocasien test. The proteolytic activity was found as 21.13 units for the sample. The enzyme hydrolyses a number of proteins including Azocasien which suggest that it is an extracellular protease. Bacillus species have been reported to produce proteases24. Therefore, it may be called a very good method for the large scale screening of bacterial protease8. One unit of proteolytic activity is defined as the amount of enzyme that produces an increase in the absorbance of 0.01 at 440nm.
Evaluation of effect of temperature on bacterial growth and enzyme synthesis: The aim of this experiment was to monitor the effect of temperature on the bacterial growth. For this purpose this organism was grown in nutrient agar medium at various temperatures (25ºC, 30ºC, 35ºC, 40ºC, 45ºC, 50ºC, 60ºC) for 48 hours and observed the growth profile of the bacteria
The presented fig 2 shows that the optimum growth temperature is 37°C. Above this temperature the organism grows very slowly. The growth rate also determined by taking absorbance of the bacterial growth culture at 600nm. The absorbance was plotted against temperature. Enzyme activity of each culture was measured after 24 hours of growth. No substantial change in enzyme activity could be shown after 24 hours. This showed that the enzyme remain stable at 60°C in growth media at least up to 10-12 hours.
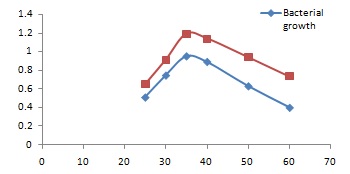
Fig. 2. Graphical presentation of bacterial growth and protease activity at different temperature.
Effect of salts and other effectors on the protease activity: The effect of different salts (MgSO4, ZnSO4, CuSO4, NaCl, and KCl) and other effectors (EDTA, 2-mercaptoethanol, sodium thiosulfate) at different concentration was measured. MgSO4 increased the activity and β-Mercaptoethanol decreased the activity of the enzyme. NaCl didn’t change the activity. Others had little deactivating effect. The effect of a number of ions on the activity of the enzyme was observed.
The result in Fig 3 shows that 5mM Mg++ ion slightly increased the activity of the enzyme while Zn++ showed slightly decrease. Other elements Na+, K+ and EDTA showed no effect on the protease activity which suggested that the enzyme might not be metallo protease. The enzyme activity was significantly reduced by β-Merceptoethanol. β-Mercaptoethanol has been reported to stabilize cystein proteases by protecting the oxidation of sufhydral group in proteins. But β-Mercaptoethanol will cause alteration of structure of enzyme by reducing disulfide bonds of the enzyme. Thus the protease could contain disulfide bonds. No effect of EDTA was detected on enzyme activity suggesting that the metal might not be involved in enzyme activity14.
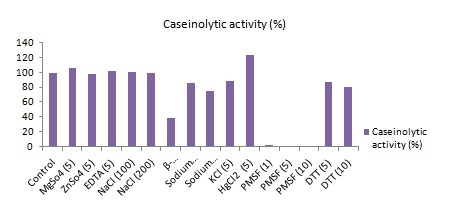
Fig. 3. Graphical presentation of the effect of different metal ions and effectors on protease activity
Effect of pH and temperature on protease activity from the organism: The pH of the reaction media can affect the protease activity. For this purpose the enzyme activity over a pH range between 4 and 11 was studied. The enzyme shows its maximum activity at pH 8.5. The activity declined at pH 8.0 or above 8.5. Therefore pH 8.5 might be the optimum pH for enzyme activity. Additionally, its optimum pH was similar to that of previous reports20. Most proteases are active in neutral to alkali conditions, from pH 7.0 to pH 9.5. For example, the activity optimum of protease from Mycobacterium kr10 is pH 7.0, B. pumilus FH9 of pH 8.0 4, Fervido bacterium islandicum AW-1 of pH 9.0 15.
The activity of the enzyme was measured over a range of temperature (0°C, 4°C, 20°C, 30ºC, 37ºC, 40ºC, 50ºC, 60ºC, 65ºC, 80°C). The enzyme activity is increased with the increase of temperature. The protease was active over a temperature range of 4°C~80°C, with an optimum at 60°C. Most proteases possess an activity optimum in the range of 30~80°C, for example, protease from B. pseudofirmusAL-89 is of 60~70°C 5 and a few have exceptionally high temperature optimum of 100 °C 15. Studies on growth temperature and pH suggest that the organism might be alkaline and thermophilic Bacillus.
Purification of Protease Enzyme: To remove unwanted proteins from the crude enzyme solution, 40–80% saturation of (NH4)2SO4 had the best effect on enzyme purification. The most active enzyme protein preparation could be obtained at the ammonium sulphate level of 60%. This result was in complete accordance with other workers.12
The overall purification factor was about 22.6 fold and the final yield was 51%. The final product had a specific activity of about 839.41 U/mg. The desired enzyme was found in 53-55 numbers tube by Azocasein test. The result is presented in table 1 and fig. 3
Table (1):
Protein purification and different enzymetic properties of the protease
Protein purification status |
Protein concentration (mg/mL) |
Protease activity |
Enzyme unites/mL |
Specific activity |
Protein purification fold |
|---|---|---|---|---|---|
Crude culture supernatant |
0.82 |
1.159 |
289.75 |
350 |
1 |
75% saturation with ammonium sulfate |
3.52 |
1.79 |
447.5 |
127 |
2.3 |
Removal of salt by dialysis |
1.96 |
0.967 |
242 |
123 |
2.9 |
Ultrafiltration by centricon |
1.36 |
1.01 |
252.5 |
185 |
4.9 |
Gell filtration chromatography |
135µg/mL |
0.217 |
54.25 |
54.41 |
11.5 |
Specific activity = Unites of enzyme /mg of protein per mL.
Fig 4 shows that the desired enzyme was found in 53-55 numbers of tubes/fractions and it was also found that 54 numbers of tube/fraction contains large amount of desired enzyme. The protease precipitated by the ammonium sulphate had been reported in many previous studies.24 The precipitates were found to be very active after the dialysis. This gave 2.9 folds purification of the proteins. After ultra filtration protein was further purified by gel filtration chromatography using DEAE cellulose. Ultra filtration is another method for the separation of proteins of different molecular weight.23 Proteins having molecular weight higher than or equal to 100kDa were used. In this process the protein were purified to 4.9 fold. Three different protein picks of different molecular weight was found and one of the pick showed considerable enzyme activity.2 In this process the protein was purified to 11.5 fold.
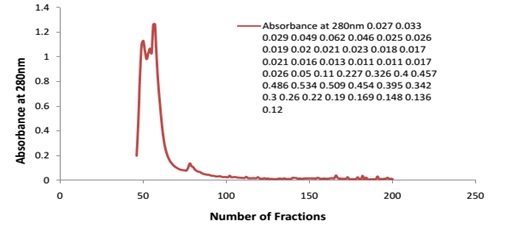
Fig. 4. Graphical presentation of OD of collected fractions from DEAE cellulose column chroma-tography.
Enzyme purity was tested by SDS-PAGE according to Laemmli (1970) and operated at 4°C. It was found that a clear single band is appeared in the gel. The molecular weight of the protease was found to be ~30-KDa. It proves that the enzyme has purified and separated. The level of purification is higher than those reported in other similar papers12.
Assessment of kinetic parameters and isoelectric point of protease
The kcat kinetic parameter was determined using some common acetylated substrates and the values. The Michaelis constant Km for alkaline proteases was 0.43 € 0.02 mM and the maximal velocity nmax was 12,300 € 500 U mg)-1. The pI of the protein was estimated by isoelectric focusing to be approximately 5.3, in agreement with the theoretically predicted pI value of 5.0.
Dehairing Capability of the Isolated Protease Enzyme: The cell-free supernatants were used as sources of crude enzyme.
The treated skins and controls showed visible differences after 9 h incubation. No color alteration was observed, although the presence of depilated areas was noticed in the skins treated with enzymes.
After 9 h incubation intact hairs could be taken out of the skins easily by simple scraping shows in table 2. In controls, hair loosening was not observed, even by the mechanical action of a forceps in fig 5. This result was much better than other different bacteria that also caused dehairing. Proteases have been used in the hide dehairing process, where dehairing is carried out at pH values between 8-10.11 In most cases the enzymes work and bring about efficient dehairing within 6-20h.
Table (2):
Comparison of dehairing ability of B. subtilis with other bacteria
Time of incubation for dehairing |
Change of color of leather |
|
|---|---|---|
Bacillus subtilis |
9h |
no change |
Vibrio sp. kr2 |
24h |
no change |
Flavobacterium sp. kr6 |
24h |
no change |
Bacillus sp. kr10 |
24h |
no change |
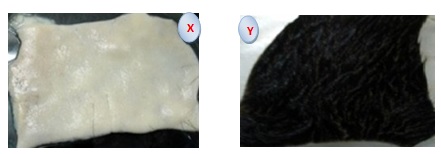
Fig. 5. Enzymatic dehairing by B. subtilis protease on left (X), control (without protease)is on the right (Y).
Screening of Storage Stability of Protease from Bacillus subtilis: The crude extracellular protease was stored under three different condition namely 4°C, room temperature and room temperature with Sodium Benzoate 0.60% for 4 weeks. Enzyme activity was determined from each sample at seven days intervals and the process was continued for 4 weeks. There was no significant decrease in enzyme activity when stored at 4°C within the above mentioned period of investigation. After fourth week the enzyme activity was found for 562U/ml (100%) at 4°C, room temperature 492U/ml (87.5%) and room temperature with chemical 542 U/ml (96.4%) respectively.
Detergent activity of the protease enzyme: The enzyme was seen to be able to remove blood and curry stain from clothes, making it a very promising candidate to be used in leather and detergent industry. Apart from protease the bacterium was also seen to have lipase and collagenase activity shows in fig 6. So, the bacteria are potentially good candidate for industrial application
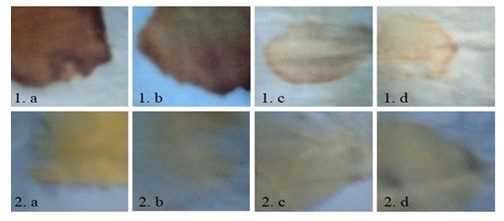
Fig. 6. Evaluation of different commercial detergent and the enzyme to remove blood stain from cloth. (1.a) Control, (1.b) Wheel powder slightly removed the blood stain, (1.c) Wheel powder + enzyme nearly remove all blood stain, (1.d) Enzyme alone is sufficient to remove blood stain. And for curry (2.a) Control, (2.b) Wheel powder, (2.c) Wheel powder + enzyme (2.d) Enzyme alone, all of them were shown to be very negligibly removing curry stain.
All of them were shown to be very negligibly removing curry stain. The above two results indicates that it was the protease enzyme from bacterial culture supernatant that remove the blood Stain as curry stain was not removed significantly. Protease could have degraded the proteins present in blood stain, thereby the removal of the stain.
Growth of the organisms and feather degradation: The organism grew almost at similar rate at 60ºC but feather degrading capabilities are quite high at 53ºC (Fig. 4). This result also indicates that the bacteria secrets an enzyme which is proteolytic and responsible for feather degradation. Visible degradation of feather could be observed after 24 hours shows in fig. 7. It is apparent from the figures that the feather degradation activity of the organism was reasonably high.
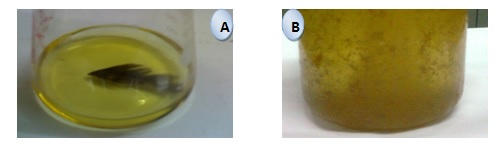
Fig. 7. Feather degrading activity of the sample A (Control) and sample B with enzyme (100% Feather degraded)
In this research the results presented the bacterial isolates might belong to Bacillus subtilis. Bacterial alkaline protease has got its particular ecofriendly technical applications in leather processing, detergent and feathers digestion to feed in Bangladesh. The culture characteristics and biochemical tests of the organism suggest that it is a thermophilic, Gram positive, spore forming and aerobic bacteria. Circumstantial evidences are there to suggest that the enzymes might be proteases. As the bacterial protease showed that high activity in poultry processing industry and dehairing of animal skin and our next target is to introduce it to the poultry and tannery industries, so that they can use it instead of hazardous chemicals for better quality and most importantly for a better environment. The results showed that the B. subtilis proteases enzyme can be utilized in poultry processing industry and enzymatic dehairing of skin in tannery industry to control the environment from pollution, which is a prerequisite for biotechnological applications. The isolation, identification, purification procedure set up and the characterization study of the protease were important to foresee potential production and uses of this enzyme. The sequencing of the protein and identification of the gene is the future plan of the research work. Finally, it plans to clone and over-express the genes encoding enzymes for large scale industrial production and commercial use for leather, detergent and poultry processing industry.
ACKNOWLEDGMENTS
This research was financially supported with proper guidance and helping for data analysis in the Department of Biochemistry and Microbiology of the Gono Bishwabidyalay, Savar, Dhaka, Bangladesh. Heartiest thanks to the authority of Gono Bishwabidyalay for helping us to conduct the research work.
- Araujo R, Casal M and Cavaco Paulo A. Application of enzymes for textile fibers processing, Biocatal Biotransfor., 2008; 26: 332-349
- Cappuccino J.G. and Sherman N. Microbiology: A Laboratory Manual. 6th Edition. Benjamin Cummings. CA 2001.
- Dettmer A, Cavalli E and Gutterres M. Environmentally friendly hide unhairing: enzymatic hide processing for the replacement of sodium sulfide and delimig, Journal of Cleaner Production, 2013; 47: 11-18,
- Gessesse, A., Rajni, H.K., Gashe, B.A. Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enzyme Microb. Technol., 2003; 32 (5): 519-524
- Ghorbel-Frikha B. and Nasri M. Production and purification of a calcium-dependent protease from Bacillus cereus BG1. J. Ind. Microbiol Biotechnol., 2005; 32(5):186-94
- Gupta R, Beg Q, Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Applied microbiology and biotechnology, 2002; 59(1):15-32
- Ibrahim ASS and al-Salamah AA. Optimization of media and cultivation conditions for alkaline protease production by alkaliphilic Bacillus halodurans, Res J Microbiol., 2009;4:251-59
- Ishikawa and Fujiwara, N. Kinetics and mechanism of enzymatic hydrolysis of gelatin layers of X-ray film and release of silver particles. J. Ferm. Bioeng., 1993; 76: 300-305
- Jorgensen PL, Tangney M and Jorgensen ST. Cloning and sequencing of an alkaline protease gene from Bacillus lentus and amplification of the gene on the B. lentus chromosome by an improved technique, Appl. Environ. Microbiol., 2000; 66: 825-827
- Kamath P, Subrahmanyam V, Rao JV, Raj PV. Optimization of cultural conditions for protease production by a fungal species. Indian journal of pharmaceutical sciences, 2010; 72(2):161.
- Kembhavi, A.A.; Kulkarni, A. and Pant, A. Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM no 64. Appl. Biochem. Biotechnol., 1993; 38: 10-22
- Kim J.D. Purification and Characterization of a Keratinase from a Feather-Degrading Fungus, Aspergillus flavus Strain K-03. Microbiology, 2004; 35 (4): 219-225
- Md. Ekhlas Uddin, Dr. M. Rahman*, H. M. Faruquee, Md. R. I. Khan, M. Feroz Mortuza, M. H. Rahman & Pulak Maitra. Isolation, Identification and Partial Characterization of Protease Producing Bacteria that Exhibiting Remarkable Dehairing Capabilities. Global J. of Science Frontier Research, 2015; 15(1): 09-18
- Madern, D., Ebel, C., and Zaccai, G. Halophilic adaptation of enzymes. Extremophiles, 2000; 4: 91-98.
- Nam and Y. R. Pyun. Native-feather degradation by Fervido bacterium islandicum, a newly isolated keratinase-producing thermophilic anaerobe, Arch. microbiol., 2002; 178:538–547
- Nigam PS. Microbial enzymes with special characteristics for biotechnological applications. Biomolecules, 2013; 3(3):597-611
- Ogawa J, Shimizu S. Microbial enzymes: new industrial applications from traditional screening methods. Trends in Biotechnology, 1999; 17(1):13-20
- Reddy MN, Kumar C, Swathi K, Nagamani B, Venkateshwar S, Rao L. Extracellular alkaline protease production from isolated Bacillus subtilis svr-07 by using submerged fermentation. International journal of pharma Research and development, 2011 3:216-223
- Rifaat HM, El-Said OH, Hassanein SM, Selim MS. Protease activity of some Mesophilic streptomycetes isolated from Egyptian habitats. Journal of Culture Collections, 2007; 5(1):16-24
- Rozs M, Manczinger L, Vágvölgyi C, Kevei F. Secretion of a trypsin- like thiol protease by a new keratinolytic strain of Bacillus licheniformis. Indian Journal of Biotechnology, 2001; 205: 221-224
- Singhal P, Nigam V, Vidyarthi A. Studies on production, characterization and applications of microbial alkaline proteases. International Journal of Advanced Biotechnology and Research, 2012; 3(3):653-669
- Sneath, P.H.A., Mair, N.S., Sharpe, M.E. & Holt, J.G. Bergey’s Manual of Systematic Bacteriology, Vol. 2 Baltimore: Williams and Wilkins. 1986; ISBN 0-683-07893-3
- Song J, Tao W and Chen W. Kinetics of enzymatic unhairing by protease in leather industry, Journal of Cleaner Production, 2011; 19: 325-331
- Tatineni, R. and L.N. Mangamoori. Optimization of keratinase production and enzyme activity with Streptomyces sp7. Appl. Biochem. Biotechnol., 2007; 141: 187-201.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


