Terpenoids are the most diverse and largest class of chemicals of the innumerable plant-based compounds. Plants carry out a number of essential growth and production functions using terpenoid metabolites. In contrast, most terpenoids are used in the abiotic and biotic systems for complex chemical interactions and defense. Terpenoids derived from plants mostly used humans for pharmaceutical, food, and chemical industries in the past. However, recently biofuel products have been developed by terpenoids. The metabolism of high-quality terpenoids in plants and microbes is facilitated in synthetic biology by genomic resources and emerging tools. Further focus has been given to the ecological value of terpenoids for establishing effective pesticide control approaches and abiotic stress protection. The awareness of the diverse metabolic and molecular regulatory networks for terpenoid biosynthesis needs to be increased continuously in all these efforts. This review gives an overview and highlights current improvements in our understanding of the organization, regulation, and diversification of core and specialized terpenoid metabolic pathways and discusses the prominent therapeutic roles of terpenoids. This review provides an overview and highlights recent literature in our understanding about the biomedical and therapeutic importance of terpenoids, regulation as well as the diversion of core and specialized metabolized terpenoid pathways.
Terpenoids, Biomedical, Therapeutic, Pharmaceutical, Bioactivities
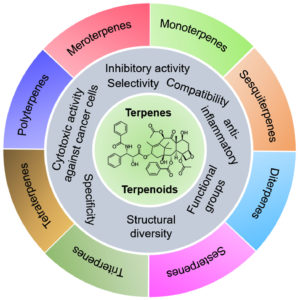
Plants perform a range of different metabolic functions with the help of primary and secondary metabolites1. These metabolites include carbohydrates, proteins, nucleic acids, and lipids molecules, which are essential for metabolic processes. The additional metabolites used for the expression of secondary characteristics result in the feedback of stress, such as plant-show response against plant-eating animals. Several types of secondary metabolites could be produced from the plants, useful for human beings in a wide range of applications2. Sometimes, secondary metabolites are considered natural products of plants when they act against other living things or organisms. This review gives a significant focus on therapeutic terpenoids, which are also studied from organisms other than plants, such as entophytic fungi isolated from Hypericum perforatum, leading to the production of two types of terpenoids name lactones3. The major classifications of terpenoids have been represented in Fig. 1. These compounds exhibit a wide variety, e.g., terpenoids with almost twenty-five thousand types, phenolics with nearly eight thousand types, and alkaloids with nearly twelve thousand types1. Due to this large diversity, terpenoids are used for biomedical and many other industrial and biotechnological purposes.
Terpenoids are a group of natural compounds present in all living organisms, also named terpenes or isoprenoids. Above sixty thousand terpenoids have been discovered, making them a major class of natural compounds4,5. Essential oils are mainly extracted from the terpenoids such as aroma plants. Turpentine, known as tree resins or cholesterol which are present in the plasma membrane of animals. Due to these essential oils’ useful properties, these are used as a critical part of perfumes, fragrance, and flavor purpose6. In lower invertebrates and plants, terpenoids are present in vast amounts as secondary metabolites. These compounds have been used for biomedical and therapeutic applications for a long time. However, the level of research is continuously enhancing the methods of extraction and identifying of novel compounds. These discoveries are also investigating the medicinal and industrial applications of these compounds7. The therapeutic utilization of fragrant plants has been recorded in old natural messages such as “De Materia Medica” composed by the Greek physician Pedanius Dioscorides in the main century of the common era. Pitches, an Ayurvedic and old Chinese medicine, have the characteristics of fragrance plants8. The selection of pharmaceutical herbals indicates that herbs’ taste and smell are essential for old pharmaceutical methods9.
In the eighteenth century, work on isoprenoids was started in an advanced way. The inventors of terpenes are considered the German chemist Otto Wallach, who got Nobel prize in 1910 in discovering the structural form of monoterpenes. He started work under the supervision of August Kekule in Bonn, Germany11. Kekule defined a mixture of carbon, hydrogen, and oxygen extracted from plant essential oil that has a formula (C5H8)2 remain in a liquid state at minimum heat value and identified as terpenoids due to their identification terpenes oils. Mixes with the sub-atomic equations (C5H8O)2 or (C5H7O)2 containing liquor or a ketone individually and could be solidified or hastened as defined them as camphor’s12. Wallach and his colleagues were able to identify oil components with the help of fractional and vacuum distillation. Scientists enhanced their work and discovered their genera related to each other and led to the production of many types of oils from families that are not related to each other but gave the same oils13. In 1939 a scientist named L. Ruzicka was awarded Nobel Prize on discovered the structural form of higher terpenoids. The essential oil extract from the herbaceous plant, lemon, fruit flavor, or many other related plants is the fundamental part of monoterpenoids and sesquiterpenoids. Essential oils have various biological capacities in the kingdom Plantae, for example, going about as allopathic operators, repulsive, or attractive forces in herbs–herbs or herbs–parasite/plant-eating animal’s relations14. Plant such as pine trees or temperature-bearing species has a function of cure from injuries15.
Biosynthesis of terpenoids
Biosynthesis of terpenes follows three basic steps such as (i) synthesis of five-carbon isoprenoid; (ii) further formation of compounds from this five carbons isoprenoid, and (iii) the transformation of compounds into end products16. Isopentyl diphosphate and dimethylallyl diphosphate are part of terpenes and are generated by the isoprenoid unit. A reversibly attached isomers form that change insolently diphosphate to dimethylallyl diphosphate with the help of IDI or IPP isomerase5.
In plants, two main pathways are used for the biosynthesis of terpenoids that produce isopentyl diphosphate and dimethylallyl diphosphate. Mevalonic acid pathway, which is also known as MVA pathway, occurs in the cytosol, and methylerythritol phosphate, also known as MEP pathway, occurs in plastids. The MVA pathway leads to the production of sesquiterpenes, triterpenes, steroids, and terpenes production in mitochondria17. The DMAPP pathway leads to the production of hemiterpenes, diterpenes, monoterpenes, carotene and its derivatives, different plant hormones required for their growth and proper functioning are used in the photosynthesis process18. Table 1 shows the pathways involved in a different organism for the biosynthesis of terpenoids.
Table (1):
Pathways for the biosynthesis of terpenoids present in a different organism.
Organism |
Pathways |
Reference |
|---|---|---|
Plants |
MVA or MEP |
[18] |
Animal |
MVA |
[5] |
Green algae |
MEP |
[18] |
Bacteria |
MVA and MEP |
[19] |
Fungi |
MVA |
[20] |
Archaea |
MVA |
[19] |
MVA: mevalonate pathway. MEP: methylerythritol 4-phosphate pathway.
Mevalonate (MVA) pathway
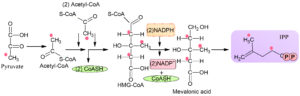
In the mid of twentieth century, a pathway was investigated that form iso-pentyl diphosphate and dimethylallyl diphosphate, which was known as MVA pathway. It was found in unicellular eukaryotes as well as multicellular eukaryotes such as mammals21. This pathway was found in cytosolic parts of archaebacteria, eukaryotic organisms, and gram-positive and negative bacteria5,18. Six steps are involved in this pathway (Fig. 2), and the first step is the condensation step in which two molecules of acetyl-CoA condense to form aceto-acetyl-CoA enzyme AACT (thiolase). This reaction further proceeded by HMG-CoA synthase in which aceto-acetyl-CoA bind with third acetyl-CoA that results in the formation of HMG-CoA. This CoA exhibits their action in different steroid’s metabolism. This initiates MVA pathway with the help of 3-hydroxy-3-methylglutaryl reductase in the endoplasmic reticulum. Due to its multiple functions, it is considered a basic enzyme of MVA pathway22-24. MVA is believed to be originated from archaea bacteria, while 2-Cmethyl-D-erythritol 4 phosphate is derived from prokaryotes20,25. MVA starts from IPP and DMAPP but 2-Cmethyl erythritol phosphate starts from pyruvate and G3P produced in Melvin Calvin cycle26,27. This pyruvate and G3P are combines to form deoxy xylulose 5-phosphate enzyme DXP synthase. In Mevalonic acid, HMGR is the principal enzyme and in MEP pathway, the primary enzymatic reaction starts with DXS that complete all the limited steps28.
Methylerythritol 4-phosphate (MEP) pathway
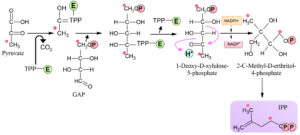
Methylerythritol 4-phosphate (MEP) pathway was discovered in the late twentieth century. This MEP pathway present in the plastid of malarial parasite, kingdom, Plantae, phylum protozoa, algae, and Escherichia coli19. The final product of MEP pathway is IPP and the DMAPP (Fig. 3). These two products are produced from HMBPP by enzyme 4-hydroxy-3-methylbut-2-enyldiphosphate reductase, which converts large amounts of IPP into a reversible reaction DMAPP20. Bacteria, fungi, mammals, archaea used MVA pathway. Some bacteria like cyanobacteria used MEP method for terpenoids biosynthesis. Plants used both of these pathways because they are beneficial for plants in different ways20,24,27. Algae have unique characteristics like green algae and unicellular Chlamydomonas reinhardtii not show response towards MVA pathway. This pathway has been identified to be present in golden and green algae24,27.
The “isoprene rule”
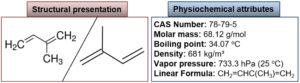
In the 20th century, structural experimentations of most terpenes led Otto Wallach to prepare the “isoprene rule,” which hypothesizes that most terpenes are formed by the combination of isoprene units. This rule gives information about the relationship between terpenes natural products. O. Wallach’s concept was proved in the 1930s when Leopold structured the “biogenetic isoprenoid” theory. This theory overcomes the accurate biological role and supposes that these are “isoprene” in structure. As a working principle for terpenoid biosynthesis, the biogenetic isoprenoid law was accurate and correct. Structural and physicochemical attributes of isoprene are shown in Fig. 4.
Types of terpenoids
The primary classification of terpenoids based on the number of carbon units and their biological activities have been shown in Table 2. Mono-terpenoids comprise two isoprene units (10-C backbone) structure, which can be categorized into monocyclic, acyclic, and bicyclic subgroups. These may exhibit ketone, aldehydes or alcoholic functional groups or a simple unsaturated hydrocarbon chain. Commonly found aliphatic hydrocarbons include linalool, lavandulol, geraniol, citral, and myrcene29. Iridoids consist of a broad category of monoterpenoids, characterized by 6-membered ring, which is fused to iridane skeleton. Sesquiterpenoids are synthesized by three isoprene subunits and consist of a large variety of linear, mono-, bi-, and tricyclic forms. Sesquiterpenoids differ from sesquiterpenes, which contain γ-lactone system, and could be divided into annulations, i.e., matricin, artabsin, santonin, parthenolide, costunolide, and 8,12-olides, i.e., helenalin, thapsigargin, alantolactone, inunolide. Diterpenoids contain a heterogeneous group of compounds with C-20 skeleton. A detailed overview of the classification of terpenoids has been provided in Table 2.
Table (2):
Classification of terpenoids based on the number of carbon units and their activities as reported by recent studies.
| Class | Name | Plant source | Activity | Reference |
|---|---|---|---|---|
| Monoterpenes (Two isoprene units) (C10H16) | 9-OH-isoegomaketone | Perilla frutescens leaves | Show inhibitory activity against nitric oxide production in lipopolysaccharide and with diode array detection and HPLC plant | [30] |
| Sesquiterpenes (Three isoprene units) (C15H24)
|
Artefreynic acid | Artemisia freyniana | Show inhibitory activity against LPS-stimulated nitric oxide | [31] |
| Chrysanthemulide A | Chrysanthemum indicum | The anti-inflammatory action of A tends to be mediated by the elimination of an NF-κB pathway triggered by the LPS | [32] | |
| Diterpenes (Four isoprene units) (C20H32) | Genkwanin P and laurifolioside A | Wikstroemia chamaedaphne buds | Show antihepatitic activity against HBV | [33] |
| Drechmerin B | Endophytic fungus Drechmeria sp. | Excellent antimicrobial against HCV | [34] | |
| Sesterpenes (Five isoprene units) (C25H40)
|
Cybastacines | Nostoc sp. Cyanobacterium | Show antibiotic behavior sesterpenes present in cyanobacteria. It is found rare in alkaloids. This show novel structures of chemicals and show resistance against the bacterial strain | [35] |
| Scalarane sesterpenes | Mushroom species | Micro molar activity shows against parasites P. falciparum and T. cruzi Tulahuen strain. | [36] | |
| Triterpenes (Six isoprene units) (C30H48) | Cyclocariols A, B, and H | Cyclocarya paliurus leaves | Act against colon tumor cell in humans. | [31] |
| Xuedanencins G and H | Hemsleya penxianensis tubers | Show cytotoxic activity in cancer cell of a human | [37] | |
| Tetraterpenes (Seven isoprene units) (C40H64) | Carotenoids (Lycopene) | Tomato | Inhibit transporters that act in cancerous cell | [38] |
| Polyterpenes (Greater than eight isoprene units) (C5H8)n | Naturally occurring rubber | Rubber tree Euphorbiaceae | Polymerization rubber and latex industry | [7] |
| Meroterpenes (Partial terpenoid) (C18H24O) | 6-OH-3-Me-8phenylethylbenzo[b] oxepin-5-one | Liverwort Radula | It shows good activity against a cancerous cell of human and shows apoptosis behavior in cell | [31] |
| Amestolkolide B | Endophytic fungus
|
Show anti-inflammatory activity by stopping nitric oxide production in lipopolysaccharide | [10] |
Extraction of terpenoids
Essential oils can be accumulated by all plant organs (for example, buds, flowers, leaves, stems, seeds, products of the soil). They can be extracted in secretory vesicles, epidermis cells, or trichrome glandular cells. These can be separated from the plant’s organs in different ways; in any case, among the extraction techniques, steam refining, first created in the mid-era by Arabic physicists, is producing for the economical purpose39. The extraction of essential oils can be performed by the processes such as (i) method of steam distillation; (ii) solvent extraction; (iii) maceration; (iv) enfleurage – purified fats adsorption. The terpenoids can be extracted from these essential oils by two methods, i.e., (i) chemical method and (ii) physical methods.
Extraction of essential oils from plant material
Steam distillation
Aromatic plant oils are secreted by specific parts and are considered essential oils. These oils are then obtained by various separation methods such as vaporized water or standard water method or used simultaneously. The distillation procedure is based on separation by vapor pressure in a sample of multiple components. Steam procedure composed of vertical glass chamber with condenser, collector, a hot disk, and other flasks. Boiling water is passed through the mixture, and temperature can be easily handled. It is most suitable for oils showing high sensitivity towards heat. Vaporized water then moves toward the biomass part, and oil and waste material are separated. These vapors then move towards the condenser and receiver, where the oil is collected. The receiver contains both heavy oils and light oils, while waste plant compounds are received in another part. In a dry procedure, water after condensing comes to the boiling chamber. There is no direct link between sample and water. Steam distillation procedure has many drawbacks, such as decomposition of oil constituent, e.g., esters40.
Solvent extraction
The solvent extraction principle is based on the solid material and comes in contact with the solvent. The soluble components that present in solid form rapidly move towards the solvent. In plants, just like that, active ingredients move towards solvent, which all occurs according to the concentration gradient. The solvents hexane and ethanol are used to extract essential oils41-43. A solvent treats the plants; the substance produces a waxy aromatic compound known as concrete. Instead, the oil particles are released mixed with alcohol. If a condenser moves, then it splits. Diverse types of extractions are generated: cold percolation, hot percolation, and concentration. These oils are used for aromatherapy or the perfume business44.
Enfleurage
Fat has a high intensity of retention and, when acquired contact with fragrant flowers, promptly assimilates the aroma transmitted. This rule, deliberately connected on a considerable scale, establishes enfleurage. During the whole time of reap, which goes on for eight to ten weeks, clumps of naturally picked blooms are strewn over the outside of an uncommonly arranged fat base (for 24 h on account of jasmine and longer on account of tuberose), and after that supplanted by crisp blossoms. Toward the finish of the collection, the fat, which is not recharged during the procedure, is soaked with blossom oil. In the wake of evacuating the petals, the fat is treated with ethanol when every one of the oils present in fat is broken down in ethanol. The alcoholic distillate is then partially refined under low pressure to expel the solvent. The alcoholic distillate is then partially refined under low pressure to remove the solvent. From that point, the oil is separated from the fat with ethanol and afterward isolated. The accomplishment of enfleurage depends, as it were, upon the nature of the fat base utilization. As of late, the fat is supplanted by coconut charcoal because of more superior dependability and higher adsorptive limit45,46.
Extraction of terpenoids from essential oils
Specific chemical methods have been applied for the separation of terpenoids. In 1877, Tilden discovered that whenever terpenoid hydrocarbon is treated with nitrosyl chloride in chloroform [Tilden’s reagent], crystalline products having sharp melting points are obtained the adducts were separated and decomposed into their corresponding hydrocarbons. The diesters are extracted with NaHCO3 and then decomposed by alkali to the parent terpenoid alcohol. Terpenoid aldehydes and ketones were separated by reaction with standard carbonyl reagent such asNaHCO3, 2,4 dinitro phenylhydrazine, phenylhydrazine reagent47.
Various terpenoids are present in essential oils, which are separated by the fractional distillation method. The terpenoid hydrocarbon distills over first, followed by the oxygenated derivative. Distillation of the residue under reduced pressure yields sesquiterpenoids, and these are separated by fractional distillation. Fractional distillation is to be carried out under reduced pressure in the presence of inert gas. These conditions are essential because many terpenes are sensitive to heat and atmospheric oxygen48.
In adsorption chromatography, the essential oil is made to flow through a particular adsorbent. Different types of terpenoids are adsorbed at various places on the adsorbent to form different chromatograms. After analysis, almost seven hundred ingredients are found in these volatile oils, and in these are mostly terpenoids. In contrast to this above technique, another technique used for extraction is gas chromatography-mass spectroscopy that is less sensitive for molecule variety. New methods of extraction and recognition helped out in terpenoids analysis. Then various chromatograms are eluted by the different solvent systems to get different elute. Alumina and silica gel are used as adsorbents. Vapor phase partition, counter-current, and gas chromatography have been used49.
Medicinal importance
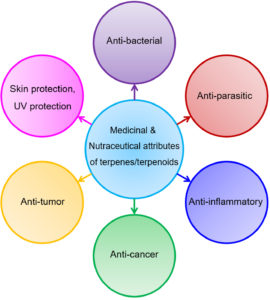
As shown in Fig. 5, Terpenes have numerous applications in medicine and present everywhere in the world, also used in the traditional pharmaceutical world. In all of them, some are available as a drug, and some are used as a dietary medicine. Many medicinal plants like citral (neral and geranial mixture), carvacrol, and others have parts like flowers, stem, roots, branches, bark, fruits, and leaves have terpenoids abundantly50. Terpenoids have much more importance in the pharmaceutical industry. Terpenoids are used in ointments and creams in response to itching and pain. It also showed antimicrobial activity by which they offer resistance towards microorganisms examples are fungi and yeast etc. Clover leaves and eucalyptus oil are used in dentistry. Eucalyptus oil act as an expectorant means help in mucus secretion, and it also helps in the gastrointestinal problem. Menthol is also a type of terpenoid used as tea aids to overcome the alimentary canal issue and digestion problem. Terpenoids act as natural produce agriculture pesticides by preparing resins and rubber. Fontanellar gun, a mechanism in which termites used terpenoids to kill harmful insect termites, belongs to Nasutitermitinae. Although beneficial, terpenoids are beneficial, but it also has harmful effects if overdose like cause respiratory problems, central nervous system effected, and caused vomiting and nausea. Children’s are mostly affected by indigestion of terpenoids51,52.
Anti-bacterial drugs
The studies related to the topical use of essential oils have demonstrated that the remedial method includes Ocimum gratissimum, which is rich in thymol and Melaleuca alternifolia (also known as tea tree oil) has proved entirely curative against Propionibacterium acnes infections. Positive and assured conclusions have also been drawn from various studies regarding the use of different essential oils to treat fungal infections of a topical kind. The remedial method of topical application of essential oils in infectious bacteria related to the oral cavity has been most widely studied on the clinical research level. The functional and active components in Listerine that is used as a mouth rinse on a commercial level include thymol, 1,8-cineole (cineol), and menthol53.
Anti-parasitic drug
Artemisia annua (Asteraceae) consists of artemisinin, a sesquiterpene lactone, and is considered a terpenoid compound with strong activity against microorganisms parasites that cause malaria, i.e., Plasmodium falciparum, for which its endoperoxide, the biological activity is of great importance. Globally, the predominant curative methods used in malaria cases include artemisinin and other such compounds that have an almost similar structure to artemisinin as they can interfere destructively with the majority of stages of the life cycle of this malarial parasite54. The pathway by which artemisinin performs its antimicrobial action has not been completely identified yet. However, the studies have confirmed the cleavage of endoperoxide via an iron source (e.g., Fe2+) after the entry of artemisinin into the parasite, which results in the synthesis of intermediates in the form of radical ions that attach themselves to various proteins, including SERCA (sarco-endoplasmic reticulum Ca2+-ATPase), which ultimately kills the parasite [55]. Activated artemisinin leads the inhibition of PfATP6, damage the parasitic membrane by incorporate into their neutral lipids, damage the mitochondrial membrane, and produce free radical which creates the oxidative stress and ultimately death of the parasite occurs. It also interferes with the heme detoxification pathway, prevents the conversion of heme into hemozoin. It also inhibits the histidine-rich proteins and heme detoxification protein56.
Anti-inflammatory drugs
As far as conventional medicinal treatments are concerned, plants of various kinds have anti-inflammatory characteristics due to terpenoids. A variety of Asteraceae family species, such as parthenolide from Tanacetum parthenium (having the common name of feverfew), act as anti-inflammatory agents yielding sesquiterpene lactones, has been used since ancient times. Clinical experiments have been conducted using essential oils on a small scale, concluding that essential oils exhibit promising impacts against conditions like dysmenorrhea (menstrual pain), infantile colic, traumatic or surgical joint-related pain, headaches, postherpetic neuralgia, and irritable bowel syndrome53. Triptolide that is obtained from the conventional plant of Chinese origin i.e., Tripterygium wilfordii having remedial properties, is considered as one of the significant drugs consisting of diterpenoids that have been used against inflammation. Triptolide has also been found its conventional use in the cure against autoimmune diseases. It can also incur its impacts through the mechanisms that are regulated by NF-KB57.
Tumor necrosis factor α (TNF-α) and interleukin-12 play a crucial role as regulators in preventing pro-inflammation, and it is through this pathway that labdane diterpenoid and andrographolide (obtained from Andrographis panuculata) express their action against inflammation58. Abietane diterpenoids such as carnosol and carnosic acid that can be obtained from Rosmarinus officinalis (commonly called rosemary) and Salvia officinalis (sage) also exhibit anti-inflammatory characteristics. It has been demonstrated by studies that these compounds keep the pro-inflammatory responses in check that has been activated by human leukocytes of a polymorphonuclear kind59, and they are also involved in stimulating peroxisome proliferator-activated receptor gamma (PPARγ)60. The membrane-bound transient receptor potential (TRP) channels, also named capsaicin or vanilloid receptors, are also considered potent protein targets in the case of terpenoids and play a vital role in detecting cold or hot temperatures and other such harmful stimuli. The interaction of TRPs with certain compounds, including camphor and menthol, brings about these compounds’ cooling effects8. In conditions like peripheral nerve injuries and skin inflammation, the TRP vanilloid-3 (TRPV3) has been used significantly.
Anti-cancer drug
Taxol, which is also termed as paclitaxel, is a diterpene obtained from the bark of Taxus species and has found significant use as an anticancer drug. The curative methods against various kinds of cancer, including breast cancer, lung cancer, and ovarian cancer, use Taxol61. The capability of Taxol to enhance the formation of stable microtubules by polymerizing tubulin and ultimately inhibition of mitosis is basically the reason behind its anticancer impacts. The link between structural activity and anticancer mechanism of Taxol has confirmed the significance of aromatic substituents and oxetane ring for its anticancer action62. Specific cytotoxic and antitumor characteristics have also been observed in carnosol and carnosic acid63,64.
Anti-tumor drugs
Parthenolide contains α-methylene-γ-lactone and is one of the antitumor sesquiterpene lactones. Anticonvulsant, antidepressant, anxiolytic, and hypnotic effects are psychopharmacological effects of pure monoterpenoids and essential oils in animals65. Mono-terpenoids like limonene and perillyl alcohol that is the primary metabolite of limonone found in humans possess the antitumor activities. Other alcohols like geraniol and carveol linalool are monoterpenoid alcohols that protect the chemically induced tumor animals by preventing tumor formation. Studies have shown that cytotoxic and antitumor properties of many necessary oils have also been tested so that they can be used as antitumor drugs66. Mazatec traditional healers use Salvia divinorum for divination. To produce hallucinations required for their healing customs, the plant is often smoked or ground. The chief active element present in Salvia divinorum is Salvinorin A, a neoclerodane diterpenoid.
Nutraceutical drugs
The largest class of phytonutrients is terpenoids found in green foods, grains, and soya plants. The terpenoids are essential to plants, as they are necessary to fix the carbon through photosynthesis by using the photosensitizing pigments. The dependence of plants on photoreactive chemistry and plants’ inability to move to protect themselves from irradiation put a firm reliance on oxidative reactions using a spectrum of phytochemical protectants. Terpenoids, particularly carotenoids, when interacting with free radicals, acts as a unique antioxidant. β-Carotene, together with γ-carotene, lutein, and lycopene, seems to protect the body’s different parts like lung, breast, uterine, colorectal, and prostate against cancer67. Carotenes are specific in their role in tissue protection. When all carotenes are taken together, the overall protective effects are more significant. Carotenes also make the immune system more robust as they boost immune response and shield skin cells against UV radiation. Dietary cholesterol is competed by phytosterols for uptake in the intestines. Studies have shown that the uptake of cholesterol can be blocked by phytosterols (to which they are structurally related), and these phytosterols help in the excretion of cholesterol from the body. In terms of nutritional physiology, our nutrition’s value is not only accustomed by calorie and nutrient contents, but suitable meal preparation and presentation is also necessary. The aroma of foodstuff is beneficial in play an essential role in nutrition. It can be related to the terminology “soul food,” as the information of fragrances surpass our entire being, both intellectually and physically contributing as a whole68. According to the Aroma-Vital Cuisine, the addition of spice in essential oils combines with healing power. Essential oils that are the extracts of aromatic plants and spices are not thought to replace fresh herbs but can supplement them. Essential oils extracted from controlled organic cultivation are 100% pure that should be used, especially for cooking. While on the other hand, the oils not having controlled organic sources should be checked and used for pesticide content. Clinical studies reveal the potential of cannabis and cannabinoids in treatment for reducing chronic neuropathic pain, treating multiple sclerosis, and playing a role as appetite stimulants in cancer patients undertaking chemotherapy and AIDS patients69.
In conclusion, numerous terpenoids serve as pigments or phytohormones in preserving cell membrane fluidity. Still, so many of them are specialized metabolites implicated in plant-herbivores resistance and plants interactions with the environment. Terpenoids are an essential part of human nutrition and pharmaceutical, aromatic, and potential biofuels of future economic importance. They are becoming a primary target for metabolic engineering and synthetic biology projects due to their often-low abundance of natural sources and demand for new terpenoid structures with new or improved bioactivities. Terpenoids of large number such as monocyte/macrophages, neutrophils, mastocytes, and leukocytes, have also been analyzed as potential anti-inflammatory molecules in both in vivo animal models and well defined ex-vivo cultures of inflammatory cells. Furthermore, some evidence from the use of herbal extracts rich in these terpenoid medicines indicates that likely candidates exist to act as powerful anti-inflammatory drugs. The kingdom of plants was a possible source of such compounds in this way. However, precisely molecular motives that are widely spread and involved in their anti-inflammatory activity have been difficult to define. Many of the terpenoids serve as plantation hormones that regulate specific physiological functions (e.g., gibberellins). Some of them are secondary metabolites that protect the host against potential pathogens and the plant/animal. The latest developments in the control of innate immunity from insects to mammals indicate previously unexpected conservation in the pathways (receptors, kinases and effector molecules) involved in this process.
ACKNOWLEDGMENTS
Consejo Nacional de Ciencia y Tecnologia (MX) is thankfully acknowledged for partially supporting this work under Sistema Nacional de Investigadores (SNI) program awarded to Hafiz M.N. Iqbal (CVU: 735340). All authors are grateful to their representative institutes and universities for providing literature facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SAQ , MB and HMNI Conceptualize the research. MJ, AB, and SAQ did the literature screening and data curation. MJ, RF, MA, AB and SAQ wrote original draft. MB and HMNI did the reviewing and editing of the manuscript. All the authors have read and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All data sets generated or analyzed during this study are included in the manuscript.
- Croteau R, Kutchan TM, Lewis NG. Natural products (secondary metabolites). Biochemistry and Molecular Biology of Plants. 2000;24:1250-1319.
- Balandrin MF, Klocke JA, Wurtele ES, Bollinger WH. Natural plant chemicals: sources of industrial and medicinal materials. Science. 1985;228(4704):1154-1160.
Crossref - Kusari S, Lamshoft M, Zuhlke S, Spiteller M. An endophytic fungus from Hypericum perforatum that produces hypericin. J Nat Prod. 2008;71(2):159-162.
Crossref - Koksal M, Hu H, Coates RM, Peters RJ, Christianson DW. Structure and mechanism of the diterpene cyclase ent-copalyl diphosphate synthase. Nat Chem Biol. 2011;7(7):431-433.
Crossref - Berthelot K, Estevez Y, Deffieux A, Peruch F. Isopentenyl diphosphate isomerase: a checkpoint to isoprenoid biosynthesis. Biochimie. 2012;94(8):1621-1634.
Crossref - Baser KHC, Buchbauer G. Handbook of essential oils: science, technology, and applications: CRC press; 2015.
Crossref - Ludwiczuk A, Skalicka-Wozniak K, Georgiev M. Terpenoids. Pharmacognosy: Elsevier. 2017:233-266.
Crossref - Maffei ME, Gertsch J, Appendino G. Plant volatiles: production, function and pharmacology. Nat Prod Rep. 2011;28(8):1359-1380.
Crossref - Leonti M, Fernando RR, Sticher O, Heinrich M. Medicinal flora of the Popoluca, Mexico: a botanical systematical perspective. Economic Botany. 2003;57(2):218-230.
Crossref - Wang S, Alseekh S, Fernie AR, Luo J. The structure and function of major plant metabolite modifications. Molecular Plant. 2019;12(7):899-919.
Crossref - Christmann M. Otto Wallach: founder of terpene chemistry and Nobel Laureate 1910. Angew Chem Int Ed Engl. 2010;49(50):9580-9586.
Crossref - Hmaied M, Bouafif H, Magdouli S, Braghiroli FL, Koubaa A. Effect of Forest Biomass Pretreatment on Essential Oil Yield and Properties. Forests. 2019;10(11):1042.
Crossref - Kubeczka K-H. 2 History and Sources of Essential Oil Research. In K. Hüsnü Can Baser and Gerhard Buchbauer (eds.), Handbook of essential oils : science, technology, and applications. CRC Press, Taylor and Francis Group. Boca Raton, FL 33487-2742 USA. 2010:3-38.
- Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135(4):1893-1902.
Crossref - Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997;115(4):1413-1420.
Crossref - Grassmann J. Terpenoids as plant antioxidants. Vitamins & Hormones. 2005;72:505-535.
Crossref - Henry LK, Thomas ST, Widhalm JR, et al. Contribution of isopentenyl phosphate to plant terpenoid metabolism. Nature Plants. 2018;4(9):721-729.
Crossref - Oldfield E, Lin FY. Terpene biosynthesis: modularity rules. Angew Chem Int Ed Engl. 2012;51(5):1124-1137.
Crossref - Phillips MA, Leon P, Boronat A, Rodriguez-Concepcion M. The plastidial MEP pathway: unified nomenclature and resources. Trends in Plant Science. 2008;13(12):619-623.
Crossref - Hemmerlin A, Harwood JL, Bach TJ. A raison d’etre for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog Lipid Res. 2012;51(2):95-148.
Crossref - Rodriguez-Concepcion M, Boronat A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002;130(3):1079-1789.
Crossref - Hemmerlin A. Post-translational events and modifications regulating plant enzymes involved in isoprenoid precursor biosynthesis. Plant Sci. 2013;203:41-54.
Crossref - Pollier J, Moses T, Gonzalez-Guzman M, et al. The protein quality control system manages plant defence compound synthesis. Nature. 2013;504(7478):148-152.
Crossref - Vranova E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol. 2013;64:665-700.
Crossref - Koga Y, Morii H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol Mol Biol Rev. 2007;71(1):97-120.
Crossref - Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc. 1996;118(11):2564-2566.
Crossref - Lichtenthaler HK. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50(1):47-65.
Crossref - Estevez JM, Cantero A, Reindl A, Reichler S, Leon P. 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. Journal of Biological Chemistry. 2001;276(25):22901-22909.
Crossref - Grayson DH. Monoterpenoids. Nat Prod Rep. 2000;17(4):385-419.
Crossref - Nam B, So Y, Kim H-Y, Kim J-B, Jin CH, Han A-R. A new monoterpene from the leaves of a radiation mutant cultivar of Perilla frutescens var. crispa with inhibitory activity on LPS-induced NO production. Molecules. 2017;22(9):1471.
Crossref - Chen Y-j, Na L, Fan J, et al. Seco-dammarane triterpenoids from the leaves of Cyclocarya paliurus. Phytochemistry. 2018;145:85-92.
Crossref - Xue G-M, Li X-Q, Chen C, et al. Highly oxidized guaianolide sesquiterpenoids with potential anti-inflammatory activity from chrysanthemum indicum. J Nat Prod. 2018;81(2):378-386.
Crossref - Li S-F, Jiao Y-Y, Zhang Z-Q, et al. Diterpenes from buds of Wikstroemia chamaedaphne showing anti-hepatitis B virus activities. Phytochemistry. 2018;151:17-25.
Crossref - Yip Delormel T, Boudsocq M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytologist. 2019;224(2):585-604.
Crossref - Cabanillas AH, Perez VT, Corral SM, et al. Cybastacines A and B: Antibiotic Sesterterpenes from a Nostoc sp. Cyanobacterium. J Nat Prod. 2018;81(2):410-413.
Crossref - Annang F, Perez-Victoria I, Appiah T, et al. Antiprotozoan sesterterpenes and triterpenes isolated from two Ghanaian mushrooms. Fitoterapia. 2018;127:341-348.
Crossref - Wang X, Li L, Zhu R, Zhang J, Zhou J, Lou H. Bibenzyl-Based Meroterpenoid Enantiomers from the Chinese Liverwort Radula sumatrana. J Nat Prod. 2017;80(12):3143-3150.
Crossref - Huang T, Zeng Z, Li C, Leung CS. (Eds.). Neural Information Processing: 19th International Conference, ICONIP 2012, Doha, Qatar. 2012;7663.
Crossref - Tongnuanchan P, Benjakul S. Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci. 2014;79(7):R1231-R1249.
Crossref - Masango P. Cleaner production of essential oils by steam distillation. Journal of Cleaner Production. 2005;13(8):833-839.
Crossref - Zhang X, Gao H, Zhang L, Liu D, Ye X. Extraction of essential oil from discarded tobacco leaves by solvent extraction and steam distillation, and identification of its chemical composition. Industrial Crops and Products. 2012;39:162-169.
Crossref - Lago S, Rodriguez H, Arce A, Soto A. Improved concentration of citrus essential oil by solvent extraction with acetate ionic liquids. Fluid Phase Equilibria. 2014;361:37-44.
Crossref - Umar A, Nisar S, Ghnia JB, Wifek M, Rezgui M. Effect of Plant Growth Hormones and Plant Nutrients on different plants: A detailed literature review. IJCBS. 2019;16:35-40.
- Handa S. Traditional and Modern methods of extraction of essential oils from aromatic plants. Presentation at the training course on cultivation, post-harvesting and processing technologies of medicinal and aromatic plants in developing countries. ICS UNIDO organized at Bomako, Mali (West Africa). 2005:25-29.
- Handa S, Rakesh D, Vasisht K. Compendium of medicinal and aromatic plants Asia’. ICS UNIDO Asia. 2006;2:305.
- Soe’eib S, Asri NP, NH ADS. Enfleurage Essential Oil From Jasmine and Rose Using Cold Fat Adsorbent. Widya Teknik. 2017;15(1):58-61.
- Franz C, Baser K, Windisch W. Essential oils and aromatic plants in animal feeding-a European perspective. A review. Flavour Fragr J. 2010;25(5):327-340.
Crossref - Silvestre WP, Agostini F, Muniz LA, Pauletti GF. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. Journal of Food Engineering. 2016;178:90-94.
Crossref - Greenberg J, Friedli H, Guenther A, Hanson D, Harley P, Karl T. Volatile organic emissions from the distillation and pyrolysis of vegetation. Atmospheric Chemistry and Physics. 2006;6(1):81-91.
Crossref - Ortega-Ramirez LA, Rodriguez-Garcia I, Leyva JM, et al. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: a hypothesis. J Food Sci. 2014;79(2):R129-R137.
Crossref - Paduch R, Kandefer-Szerszen M, Trytek M, Fiedurek J. Terpenes: substances useful in human healthcare. Arch Immunol Ther Exp (Warsz). 2007;55(5):315-327.
Crossref - Santos ML, Magalhaes CF, da Rosa MB, et al. Antifungal activity of extracts from Piper aduncum leaves prepared by different solvents and extraction techniques against dermatophytes Trichophyton rubrum and Trichophyton interdigitale. Braz J Microbiol. 2013;44(4):1275-1278.
Crossref - Harris B. 11 Phytotherapeutic Uses of Essential Oils. Essential. 2010:315.
- White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320(5874):330-334.
Crossref - O’neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin-the debate continues. Molecules. 2010;15(3):1705-1721.
Crossref - Sadiq MB, Tharaphan P, Chotivanich K, Tarning J, Anal AK. In vitro antioxidant and antimalarial activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. BMC Complement Altern Med. 2017;17(1):372.
Crossref - Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65(19):2979-2999.
Crossref - Bonito MC, Cicala C, Marcotullio MC, Maione F, Mascolo N. Biological activity of bicyclic and tricyclic diterpenoids from Salvia species of immediate pharmacological and pharmaceutical interest. Nat Prod Commun. 2011;6(8):1205-1215.
Crossref - Poeckel D, Greiner C, Verhoff M, et al. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76(1):91-97.
Crossref - Bauer J, Kuehnl S, Rollinger JM, et al. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J Pharmacol Exp Ther. 2012;342(1):169-176.
Crossref - Ingy IA, Wim JQ. A Glimpse into the Biosynthesis of Terpenoids. KnE Life Sciences. 2017;3(5)81-98.
Crossref - Kingston DG. Taxol: the chemistry and structure-activity relationships of a novel anticancer agent. Trends Biotechnol. 1994;12(6):222-227.
Crossref - Johnson JJ. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011;305(1):1-7.
Crossref - Ngo SN, Williams DB, Head RJ. Rosemary and cancer prevention: preclinical perspectives. Crit Rev Food Sci Nutr. 2011;51(10):946-954.
Crossref - Lage H, Duarte N, Coburger C, Hilgeroth A, Ferreira M. Antitumor activity of terpenoids against classical and atypical multidrug resistant cancer cells. Phytomedicine. 2010;17(6):441-448.
Crossref - PetroviC M, VukosavljeviC P, Durovic S, Antic M, Gorjanovic S. New herbal bitter liqueur with high antioxidant activity and lower sugar content: Innovative approach to liqueurs formulations. J Food Sci Technol. 2019;56(10):4465-4473.
Crossref - Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. Journal of the Science of Food and Agriculture. 2000;80(12):1744-1756.
Crossref - Bendich A. Carotenoids and the immune response. J Nutr. 1989;119(1):112-115.
Crossref - Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2005-2009. Cannabinoids. 2010;5(special issue):1-21.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.