ISSN: 0973-7510
E-ISSN: 2581-690X
The activity of TLRs as non-specific immunity in defense against T. gondii infections was observed particularly for TLR4 molecules. Several single-nucleotide polymorphisms (SNPs) residing in tow genes encoding these receptors were reported as significant genetic modifications of TLRs and associated with different pregnancy disorders. This study aimed to investigate the association between two single nucleotide polymorphisms (SNPs) in the toll-like receptor 4 gene (Asp299Gly and Thr399Ile) and susceptibility for toxoplasmosis. A total of 50 aborted women with IgM seropositive for Toxoplasma gondii and 50 aborted women with IgM seronegative for Toxoplasma gondii as controls were included in this study. DNA was extracted from the blood samples taken from these participants. TLR4 gene was amplified with polymerase chain reaction (PCR) using specific primers. Genotyping of the SNPs of interest were subjected for direct sequencing by (Macrogen /Korea), and the resultant sequences were compared with reference sequences in NCBI by using Bio edit software. The frequency of the heterozygous genotype (AG) was higher in patients than controls (20% versus 6%) with a significant difference (OR=3.92, 95%CI=1.01-15.22, p=0.037). Among the haplotype blocks, only ACC was significantly less frequent among patients than controls (85% versus 94%, p=0.038), while frequencies of other haplotypes were very close between patients and controls. The mutant allele (G) of the SNP Asp299Gly may be considered as a risk factor for toxoplasmosis and stimulate abortion in pregnant women.
Toxoplasmosis, single nucleotide polymorphism, toll-like receptor 4.
Toxoplasmosis, an infectious disease caused by Toxoplasma gondii, is one of the most prevalent causes of abortion and congenital aberrations in infected women, it has the ability to infect and replicate in any nucleated cells lead to the production of various inflammatory markers by innate and adaptive immune system1. In immunocompromised pregnant women, T. gondii can cause severe encephalitis, myocarditis, pneumonitis, or hepatitis via acute infection or reactivation of a latent infection2. Toll-like receptors (TLRs) are receptors of transmembrane signals that play a key role in innate and adaptive immune response. They are involved in the regulation of inflammatory reactions and activation of immune cells to remove infectious pathogens and cancer cells3.
To date, ten different types of (TLRs) have been described in humans and are able to identify different pathogens and / or molecules4. TLR4 gene is highly polymorphic, fifteen poly-morphisms in its coding sequence have been detected5. Among many SNPs, this gene has two co-segregated SNPs, Asp299Gly and Thr399Ile. The association of these SNPs with susceptibility of toxoplasmosis in pregnant women as risk that has been widely investigated6.
Toll-like receptor (TLR) /MyD88 signaling has been reported as the key pathway in a non-specific antimicrobial response against T. gondii7. The glycosylphosphatidylinositol (GPI) of T. gondii was demonstrated to trigger TLR4 signaling pathways8. In inflammatory monocytes, T. gondii infection induced the production of interferon (IFNg ) through TLR4 and MyD88 signaling that facilitates formation of Th1 type response and activates lymphocyte cytotoxicity that cause abortion9.
The current study included a total of 50 aborted women with IgM seropositive for Toxoplasma gondii and 50 aborted women with IgM seronegative for Toxoplasma gondii as controls were included in this study (Mean age of patients and controls were 30.7±7.2 years and 29.56±6.19 years respectively with no significant difference) at Consulting Clinic of Al-Emamain Al-Khadhumain Teaching hospital, Baghdad, over the period from May 2018 to January 2019. Samples. From each participant, venous blood (2 ml) was collected in EDTA tube for extraction of DNA and the extracted DNA was stored at -20°C until used.
Inclusion criteria
Aborted women with IgM seropositive for Toxoplasma gondii
Exclusion criteria
Aborted women with IgM seronegative for Toxoplasma gondii
Patients under chemo and radio therapy.
Patients with chronic illness such as malignant disease and DM.
DNA extraction and genotyping of TLR4 gene
DNA was extracted from blood samples using ready kit (ReliaPrep™) (DNA Mini Kit Whole Blood Protocol/ promega/ Korea) according to the manufacturer’s instructions. The primers used for amplification of TLR4 gene (Bioneer/Korea) are shown in Table 1.
Table (1):
Primer sets used in the present study
Primer |
Sequence (5’→3’) |
|---|---|
TLR4 |
F: 5’‑TCTGGCTGGTTTAGAAGTCCA‑3′ R: 5’‑AATTGCCAGCCATTTTCAAG‑3′ |
Polymerase chain reaction was used for molecular detection of TLR4 in blood samples. A ready master mix (25ul Bioneer /Korea) was used for mixture preparation. Three microliter of template DNA and 1 micro of each primer (foreword and reverse) were added to the master mix tube. The final volume was adjusted to 25 ul with free nuclease distal water. The mixture was then vortexed for 10 seconds and put in thermo-cycler (My cycler/ U.S.A) which was previously programmed with the following conditions shown in Table 2.
Table (2):
PCR Program
| Temperature | Time | Cycles |
|---|---|---|
| 95°C | 5 minutes | 1X |
| 94°C | 45second | 30X |
| 58°C | 30second | |
| 72°C | 30second | |
| 72°C | 7 minutes | 1X |
Gel Electrophoresis
Effective PCR amplification was confirmed by agarose gel electrophoresis. Agarose gel was set up by dissolving 1gm of agars’ powder in 100 ml of TBE buffer (ph:8) previously prepared (975 ml D.W. were added to 25 ml TBE buffer) in boiling water bath, allowed to cool to 50oC and ethidium bromide at the concentration of 1µg/ml was added. The comb was fixed at one end of the tray for making wells used for loading DNA sample. The agarose was poured gently into the tray, and allowed to solidify at room temperature for 30 min. The comb was then removed gently from the tray.
The tray was fixed in an electrophoresis chamber which was filled with TBE buffer that just covered the surface of the gel. 5µl of DNA sample was transferred into the assigned wells in agarose gel, and in one well 5µl DNA ladder was mixed with 1µl of loading buffer. The electric current was allowed at 100 volts for 74min. UV transilluminater was used for the observation of DNA bands, and the gel was photographed by using a digital camera, as shown in Fig. 1.
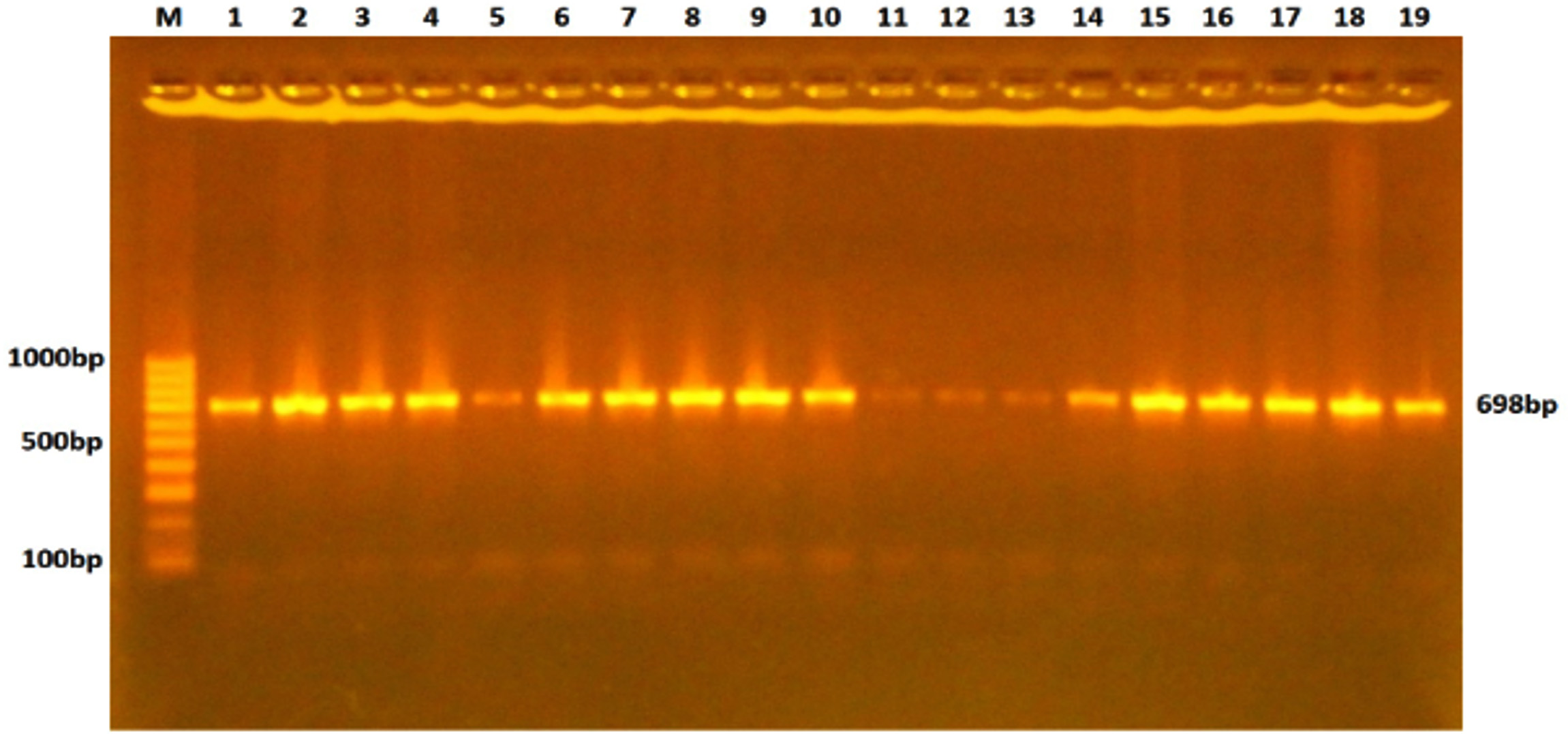 Fig. 1. Gel electrophoresis of TLR-4 gene PCR products stained with ethidium bromide and visualized under the UV light. The fragment length was 698bp.
Fig. 1. Gel electrophoresis of TLR-4 gene PCR products stained with ethidium bromide and visualized under the UV light. The fragment length was 698bp.DNA Sequencing
The polymerase chain reaction products of TLR4 gene were directly sequenced by using (Macrogen /Korea). The obtained sequences were aligned (by using Bio edit software) with normal sequence from Gen Bank.
Statistical analysis
The Statistical Package for the Social sciences version 14.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. The poly-morphisms were tested for deviation from Hardy Weinberg Equilibrium (HWE) by comparing the observed and expected frequencies (Chi-square test). The association between genotype and risk of toxoplasmosis was estimated by calculation of Odds ratio (OR) with 95% confidence interval (95%CI) using logistic regression analysis. Statistical significance was set at a p value < 0.05.
TLR-4 rs4986790
This polymorphism appeared in only two genotypes in both patients and controls. These were AA and AG (Fig. 2). The frequency of the heterozygous genotype (AG) was higher in patients than controls (20% versus 6%) with a significant difference (OR=3.92, 95%CI=1.01-15.22, p=0.037). At allelic level, the frequency of mutant allele (allele G) was higher in patients and controls (10% versus 3%) with a significant difference also (OR=3.59, 95%=0.96-13.47, p=0.045) as shown in table 3.
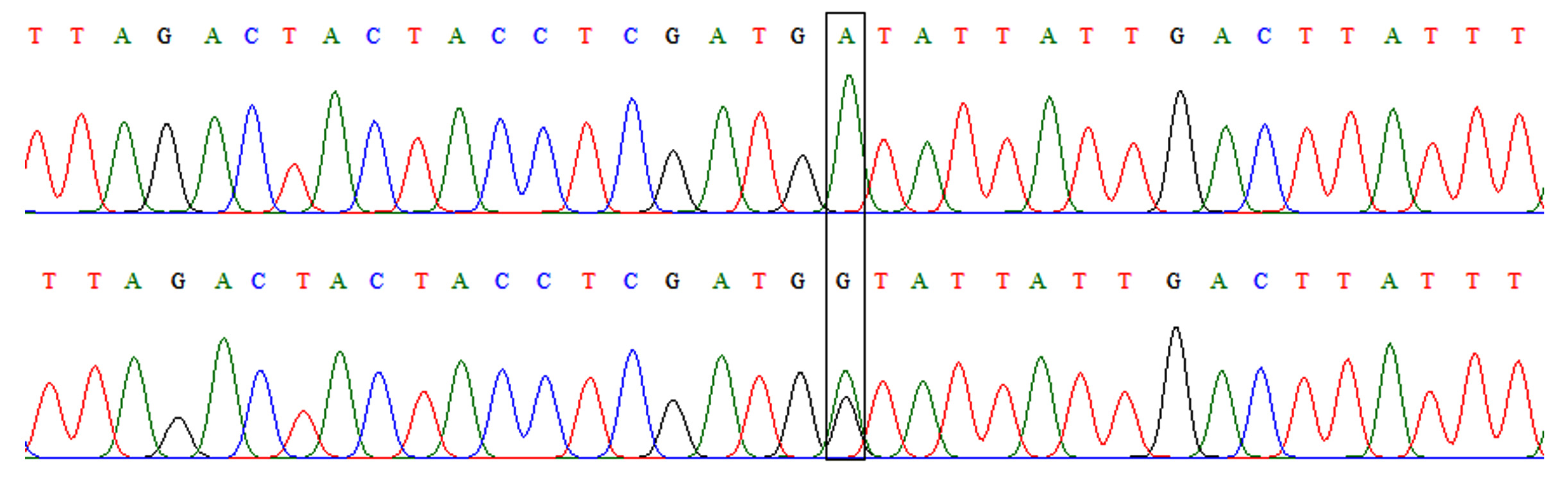 Fig. 2.Chromatograph of DNA sequencing for TLR-4 rs4986790 A/G polymorphism. Upper: wild-type homozygous genotype (AA), lower: heterozygous genotype (AG).
Fig. 2.Chromatograph of DNA sequencing for TLR-4 rs4986790 A/G polymorphism. Upper: wild-type homozygous genotype (AA), lower: heterozygous genotype (AG).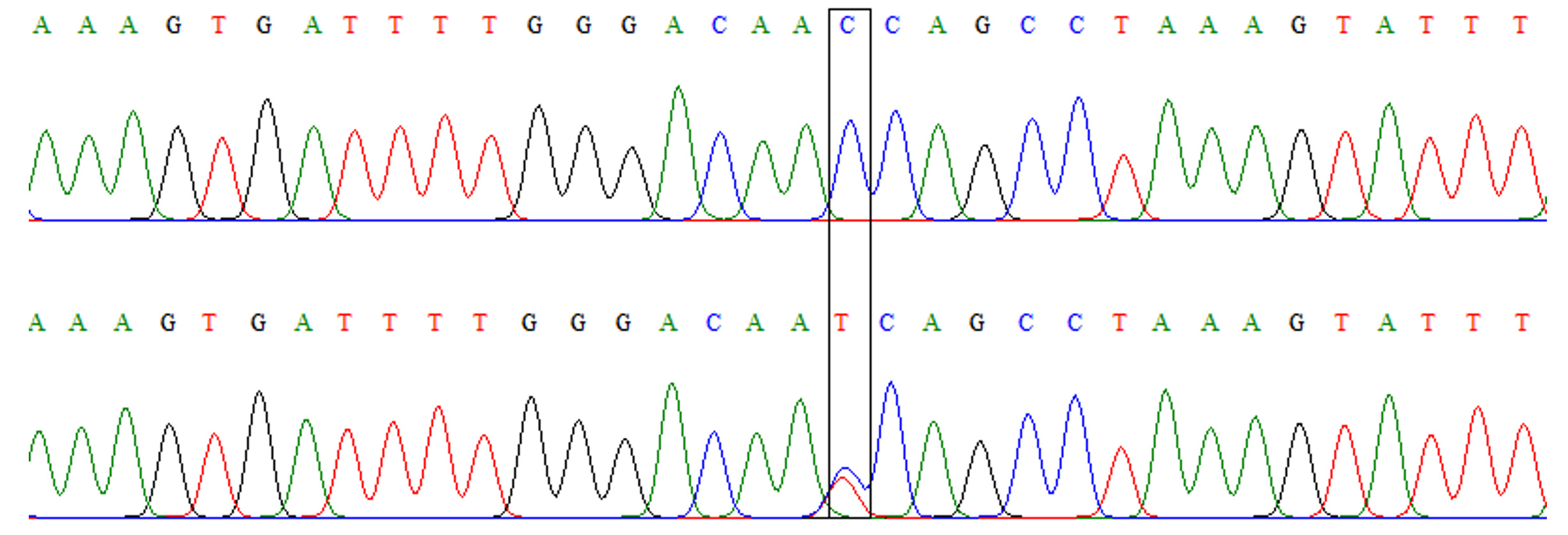 Fig. 3. Chromatograph of DNA sequencing for TLR-4 rs4986791 C/T polymorphism. Upper: wild-type homozygous genotype (CC), lower: heterozygous genotype (CT)
Fig. 3. Chromatograph of DNA sequencing for TLR-4 rs4986791 C/T polymorphism. Upper: wild-type homozygous genotype (CC), lower: heterozygous genotype (CT)Table (3):
Genotypes and alleles of TLR-4 rs4986790 in patients with IgM seropositive anti- toxoplasma Abs and controls.
rs4986790 |
Patients (n=50) |
Controls (n=50) |
P-values |
OR(95%CI) |
|---|---|---|---|---|
Genotypes AA AG GG HWE |
40(80%) 10(20%) 0(0%) 0.432 |
47(94%) 3(6%) 0(0%) 0.827 |
0.037 |
1.0 3.92(1.01-15.22) |
Alleles A G |
90(90%) 10(10%) |
97(97%) 3(3%) |
0.045 |
1.0 3.59(0.96-13.47) |
TLR-4 rs4986791
Similar to TLR-4 rs4986790, this polymorphism also had only two genotypes: CC and TT (Fig. 3). However, the frequencies of these genotypes were comparable between patients and controls without a significant difference (OR=2.09, 95% CI=0.37-11.95, p=0.40). Although the mutant allele (allele T) was slightly more frequent among patients than controls (Table 4), the difference was not a significant (OR=2.04, 95%CI= 0.37-11.41, p=0.407).
Table (4):
Genotypes and alleles of TLR-4 rs4986791 in patients with seropositive IgM anti- toxoplasma Abs and controls.
rs4986791 |
Patients (n=50) |
Controls (n=50) |
P-values |
OR(95%CI) |
|---|---|---|---|---|
Genotypes CC CT TT HWE |
46(92%) 4(8%) 0(0%) 0.768 |
48(96%) 2(4%) 0(0%) 0.885 |
0.40 |
1.0 2.09(0.37-11.95) |
Alleles A G |
96(96%) 4(4%) |
98(98%) 2(2%) |
0.407 |
1.0 2.04(0.37-11.41) |
TLR-4 rs3050716
Also, this polymorphism had only two genotypes (CC and CT).The frequencies of these genotypes were very comparable between patients and controls with no significant difference (OR= 4.26, 95%CI=0.46-39.54, p=0.169). Furthermore, there was no significant difference in the allele frequency between patients and controls (OR=4.13, 95%CI=0.45-37.57, p=0.174) as illustrated in table 5.
Table (5):
Genotypes and alleles of TLR-4 rs3050716 in patients with seropositive IgM anti- toxoplasma Abs and controls.
rs5030716 |
Patients (n=50) |
Controls (n=50) |
P-values |
OR(95%CI) |
|---|---|---|---|---|
Genotypes CC CT TT HWE |
46(92%) 4(8%) 0(0%) 0.768 |
49(98%) 1(2%) 0(0%) 0.885 |
0.169 |
1.0 4.26(0.46-39.54) |
Alleles A G |
96(96%) 4(4%) |
99(98%) 1(1%) |
0.174 |
1.0 4.13(0.45-37.57) |
Haplotype Blocks
Table 6 shows the most frequent six haplotype blocks for the three polymorphisms in the TLR-4 gene. Among these blocks, only ACC was significantly less frequent among patients than controls (85% versus 94%, p=0.038), while frequencies of other haplotypes were very close between patients and controls.
Table (6):
Haplotype blocks of TLR-4 polymorphism in patients and controls.
Haplotype blocks |
Frequency in Patients |
Frequency in controls |
p-value |
|---|---|---|---|
ACC |
85 (85%) |
94 (94%) |
0.038 |
ACT |
2 (2%) |
1 (1%) |
0.561 |
ATC |
2 (2%) |
2(2%) |
1.0 |
GCC |
5 (5%) |
3 (3%) |
0.470 |
CCT |
2 (2%) |
0 (0%) |
0.155 |
GTC |
2 (2%) |
0 (0%) |
0.155 |
The current study showed a significant association between the AG genotype of TLR-4 rs4986790 and susceptibility for toxoplasmosis (OR=3.92, 95% CI=1.01-15.22, p=0.037). This implies that carrier of AG genotype of this polymorphism are at about 4-time higher risk of having the disease compared with AA genotype carriers. On the other hand, there was no significant association between the SNPS rs4986791 and rs3050716 with the susceptibility to toxoplasmosis.
Globally, there are very few studies which addressed this issue. Wujcicka et al. (2017)10 recruited 116 Polish pregnant women including 51 patients with toxoplasmosis and 65 age-matched controls, to investigate the association of 4 polymorphisms in TLR-2, TLR-4 and TLR-9 with toxoplasmosis. The author did not find any significant association between TLR4 rs4986790 or rs3050791 polymorphisms and toxoplasmosis. A previous study in Brazilian children with toxoplasmosis showed a relationship between the presence of C minor allele in TLR9 2848 G>A and the occurrence of ocular toxoplasmosis11,12.
In a very close intracellular protozoan, Rasouli et al .used 112 patients with visceral leishmaniasis (VL) and 155 ethically matched control to explore the association of rs4986790 or rs3050791 SNPs with VL among Iranian patients. There was no significant association of either SNP with the disease. Moreover, the haplotypes constructed from these SNPs were not significantly differ between patients and controls13.
How can rs4986790 SNP alter the structure and/or function of TLR4 is a question the exact answer of which is still a controversial issue. However, mutant allele can exploit one or more of three possible ways to influence TLR4 function; expression, signaling, or ligand binding. The majority of researches in this regard pointed out that expression of TLR4 is not affected by these SNPs14,15.
Accordingly, some authors hypothesized a disruption in the interaction between mutant TLR4 and serum components such as CD14, LBP, or MD-2 which are part of the functional response of TLR14. This disruption results from conformational changes in the receptor. Henckaerts and co-workers proposed saddle-like surface of extracellular domain of mutant TLR4 with the Asp299Gly and Thr399Ile amino acids positioned at opposite ends of the saddle and the concavity between the two amino acids suggests a possible docking site for either ligand or co-receptor that may disrupt the normal function of the receptor. Threonine amino acid at 399 position conserved the branched side chain, but increases the overall steric bulk in this region and possibly precluding ligand/cofactor docking16,17.
Undoubtedly, such conformational changes and disrupting in the ligand docking will alter the signaling pathways of the mutant TLR4. The study of Davoodi and co-workers (2012) revealed that the activity of NF-kB in the mutant TLR4 cells was higher than that of wild type in response to lipopolysaccharide. Besides, there were high levels of interleukin-1 receptor associated kinase (IRAK) accompanied with rapid degradation of this factor upon LPS treatment in wild type compared with mutant TLR4. This implies reduced signaling and less cytokine genes transcription because degradation of IRAK serves as negative feedback mechanism18.
According to these data, it is reasonable to assume that the presence of G allele in The TLR-4 rs4986790 polymorphism is associated with a reduced interaction between TLR-4 and the of pathogen-associated molecular patterns of T. gondii, with eventual reduction in the signaling cascade which limit the immune response. This explains the higher susceptibility of AG genotype carriers compared with AA carriers15.
Although the individual analysis of the each SNP did not show a significant association of rs4986791 or rs3050716 SNPs with toxo-plasmosis, the haplotype analysis did show such association, because the presence of wild type allele from each SNPs had a protective role against toxoplasmosis. Such a results have been reported by Tox 2 who found that the haplotype block GACG of the variants TLR4 2258 G > A, rs4986791 or rs3050716 polymorphisms to be correlated with a decreased risk of T. gondii infection in Polish women19,20.
The current results showing direct association of allele G of the SNP Asp299Gly with susceptibility of toxoplasmosis that may play a role in the induction of abortion.
Acknowledgements
None.
Conflict Of Interest
The authors declares that there is no conflict of interest.
Authors’ Contribution
EA collected samples. AA and HH designed the experiments. ST performed the experiments. AA and HH analyzed the data. ST wrote the manuscript and compiled information from the literature. AA and HH supervised and reviewed the manuscript
Funding
None.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
The consent was obtained from all patients and controls. This study was approved by the institutional review board (IRB) of the College of Medicine -Al-Nahrain University (IRB/74).
- Caroline Paquet R.M., Trois-Riviטres H., Yudin M.D., Toronto O.N. Toxoplasmosis in Pregnancy: Prevention, Screening, and Treatment. J. Obstet Gynaecol. Can., 2013; 35(1eSuppl A): S1-7.
- Skariah S., McIntyre M.K., Mordue D.G. Toxoplasma gondii: determinants of tachyzoite to bradyzoite conversion. Parasitol Res., 2010; 107(2): 253–60.
- Iwasaki A. and Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science, 2010; 327: 291–295.
- Iwasaki A. and Medzhitov R. Toll-like receptor control of the adaptive immune response. Nat. Immunol., 2004; 5: 987-995.
- Schroder N.W.J. and Schumann R. Single nucleotide polymorphisms of toll-like receptors and susceptibility to infectious diseases. Lancet Infect. Dis., 2005; 5: 156164.
- Theodoropoulos G.E., Saridakis V., Karantanos T., Michalopoulos N.V., Zagouri, F. et al. Toll-like receptors gene polymorphisms may confer increased susceptibility to breast cancer development. Breast, 2012; 21: 534–538.
- Wujcicka W., Wilczyסski J., Nowakowska D. Do the placental barrier, parasite genotype and Toll-like receptor polymorphisms contribute to the course of primary infection with various Toxoplasma gondii genotypes in pregnant women? Eur. J. Clin. Microbial. Infect. Dis., 2014; 33(5): 703–709.
- Zare-Bidaki M., Hakimi H., Abdollahi S.H., Zainodini N., Arababadi M.K., Kennedy D. TLR4 in Toxoplasmosis; friends or foe? Microb Pathog., 2014; 69–70: 28–32.
- Han S.J., Melichar H.J., Coombes J.L., Chan S.W., Koshy A.A., Boothroyd J.C., Barton G.M., Robey E.A. Internalization and TLR-dependent type I interferon production by monocytes in response to Toxoplasma gondii. Immunol. Cell Biol., 2014; 92(10): 872–881.
- Saif N., Al Ameeri G., Alhweesh M., Alkadasi M., Zaid A.A.. Seroprevalence of toxoplasmosis in pregnant women in Taiz-Yemen. Int. J. Curr. Microbiol. App. Sci., 2014; 3: 680–690.
- Peixoto-Rangel A.L., Miller EN, Castellucci L., Jamieson S.E., Peixe R.G., Elias L.S., et al. Candidate gene analysis of ocular toxoplasmosis in Brazil: evidence for a role for toll-like receptor 9 (TLR9). Mem. Inst. Oswaldo Cruz, 2009; 104(8): 1187– 90.
- Mahdy M.A., Alareqi L.M., Abdul-Ghani R., et al. A community-base survey of Toxoplasma gondii infection among pregnant women in rural areas of Taiz governorate, Yemen: the risk of waterborne transmission. Inefct. Dis. Poverty, 2017; 6: 26.
- Rasouli M., Keshavarz M., Kalani M., Moravej A., Kiany S., Badiee P. Toll-like receptor 4 (TLR4) polymorphisms in Iranian patients with visceral leishmaniasis. Mol. Biol. Rep., 2012; 39: 10795-10802.
- Wu Y. The neuroimmuno pharmacology of alcohol. Ph.D. thesis. Unversity of Adelaide, South Australia. 2011; pp 157-158.
- Ferwarda B., McCall B., Verheijen K., Kullberg B., van der Ven A. van der Meer J. and Netea M.G. Functional consequences of toll-like receptor 4 polymorphisms. Mol. Med., 2008; 14: 346-352.
- Henckaerts L., Pierick M., Joossens M., Ferrante M., Rutgeerts P. and Vermeire S. Mutations in pattern recognition receptor genes modulate seropositivity to microbial antigens in patients with inflammatory bowel disease. Gut., 2007; 56: 1536-1542.
- Manolakis A.C., Kapsoritakis A.N., Tiaka E.K., Sidiropoulos A. and Gerovassili A. TLR4 gene polymorphism: evidence for protection against type 2 diabetes but not for diabetes-associated ischaemic heart dieases. Eurp. J. Endocrinol., 2011; 165: 261-267.
- Davoodi et al. Molecular detection of methicillin resistant Staphylococcus aureus (MRSA) and methicillin resistant coagulase-negative Staphylococcus (CoNS) in Iran. African Journal of Microbiology Research, 2012; 6(16): pp. 3716-3721.
- Wujcicka W., Gaj Z., Wilczynski J., Nowakowska D. Possible role of TLR4 and TLR9 SNPs in protection against congenital toxoplasmosis. Eur. J. Clin. Microbial. Infect Dis., 2015; 34(10): 2121–9.
- Rallabhandi P., et al. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J. Immunol., 2006; 177: 322–32.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


