Honeybees are significant to agriculture and global ecosystems due to their role as key pollinators. The honeybee’s gut microbiota is necessary for maintaining their health, providing nutrition and protection against pathogens. The objective is to develop effective strategies to promote the well-being of honeybee species. This paper comprehensively reviews current literature utilizing advanced genomic techniques to characterize bee gut microbial diversity. It examines culture-independent and culture-dependent methods to classify microorganisms inhabiting the bee gut. Their symbiotic relationships and contributions to critical bee physiological processes are also explored. The gut microbiome holds an indispensable role in bee health by regulating immune function, nutrient absorption and defense against pathogens. Specific bacterial taxa like Lactobacillus, Bifidobacterium, Snodgrassella, Apibacter, Frischella and Gilliamella exhibit probiotic, antimicrobial and symbiotic properties that safeguard bee gut homeostasis. The unique microbial composition of honey, influenced by the bee gut microbiota and environment, holds potential prebiotic and probiotic benefits for human health. Maintaining a balanced bee gut microbiome through microbiome engineering could strengthen bee resistance to diseases, thereby addressing worldwide bee population declines. Further unravelling the health impacts of honey microbes could uncover novel therapeutic applications and advance sustainable apiculture and human nutrition initiatives.
Honeybee, Microbiome, Probiotics, Symbiosis
Honeybees are social insects from the Hymenoptera order and are classified under the genus Apis, are renowned for their remarkable ability to produce and store honey and other beneficial substances. The microbiome is crucial for maintaining health and well-being of both bees and humans, comprising all microorganisms inhabiting a specific area, including bacteria, archaea, viruses, fungi and protozoans.1,2 It has recently been demonstrated that honeybees have a unique and species-specific microbiome, which may offer protection from parasites and pathogens.3,4 Extensive studies have shown the pivotal role of the gut microbiome in bees, influencing critical functions such as digestion, immune system development, and antimicrobial protection.5 Similarly, in humans, gut microbes perform crucial functions in managing immune responses and vital metabolic processes.6,7 Genetic analysis of bee gut microbiomes provides valuable insights into their composition, diversity, and potential functions, utilizing sophisticated metagenomic techniques.8,9 This approach enables scientists to identify specific bacterial taxa crucial for maintaining bee health.
The gut microbiome is indispensable for bee health, contributing significantly to carbohydrate digestion, infection defense, and overall immune system enhancement.10 Moreover, factors such as nutrient content and secondary chemicals present in pollen influence the bee gut microbiome, potentially impacting bee survival and well-being.11 In human health, the gut microbiome serves a pivotal role in metabolizing essential nutrients and maintaining intestinal barrier function.12 Dysbiosis, a disruption in the balance of the gut microbiome, poses risks to both human and bee health.13 In bees, dysbiosis is induced by American and European Foulbrood which lead to impaired immune function, reduced nutrient absorption, and heightened susceptibility to illnesses.14 Similarly, dysbiosis in humans is associated with health issues such as Inflammatory Bowel Disease, Irritable Bowel Syndrome, Metabolic Syndrome and Obesity, Type 2 Diabetes and clostridium difficile Infection.15-19
Understanding the intricate interplay between the honeybee gut and honey microbiome and their impact on sustainable bee and human health is necessary for devising effective strategies to promote the well-being of both species.
Strategies for the study of honeybee gut microbiome
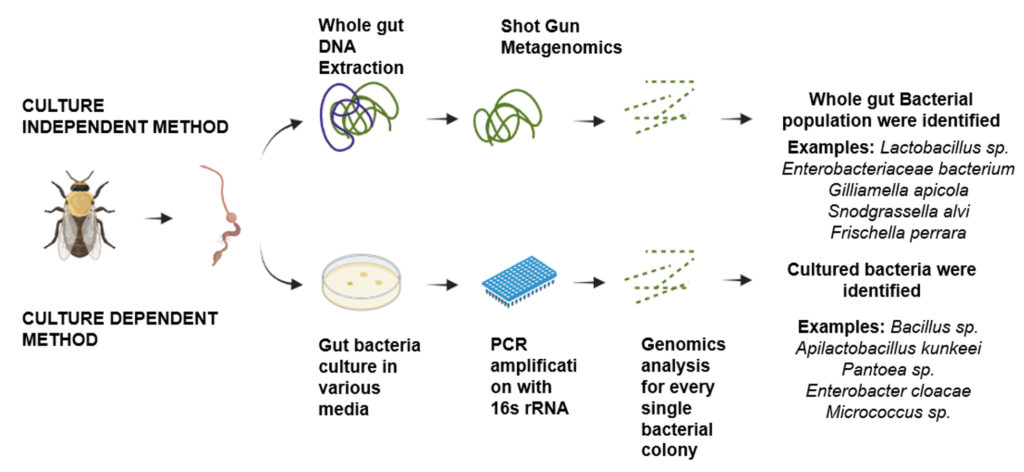
The majority of current methods for classifying microorganisms in honeybee gut are genomic in nature. This means that they depend on identifying a certain DNA sequence to a particular microbial group at different levels of resolution, such as species, phylum, or even strain.20 The amplicon-based technique is characterized by standard protocols that begin with complete genomic DNA isolation sourced from a microbial ecological sample and continue with the sequencing of the whole genome, specific genes, or genomic regions. The two types of genomic methodologies are culture-dependent and culture-independent, depending on where the microbial sample came from Figure 1.21
Culture dependent method
The term “culture-dependent methods” describes microbial sampling techniques wherein gut homogenates undergo particular culture conditions prior to genomic analysis. The primary approaches for establishing the taxonomy of gut bacteria in honeybees before the widespread adoption of Sanger sequencing and next-generation sequencing were culture-based techniques. This led to limited identification because certain species were preferred under certain circumstances and those hard to culture in the laboratory were not represented.21,22 The honeybee core microbiota species may now be characterized genetically, morphologically, and biochemically, including distinct strains of the species, through culture-based methods.23 Through culture dependent method, the gut bacterial flora can also be identified by culturing in the Luria Bertani (LB) agar for Gram-negative bacteria, Nutrient agar (NA), Brain Heart Infusion (BHI) agar, a general-purpose non-selective media suitable for a broad range of organisms. Gluconobacter (GB) agar media is more selective for acetic acid bacteria like Gluconobacter, and De Man, Rogosa and Sharpe (MRS) agar is highly selective for lactic acid bacteria, particularly Lactobacillus species.24
Culture-independent method
Culture-independent methods analyze DNA and RNA of microorganisms directly obtained from samples, bypassing the need for intermediary laboratory culture processes required by culture-dependent methods. This mitigates species-related biases that can be cultured. The utilization of amplicon sequencing for a universally conserved marker gene, such as the 16S ribosomal RNA (rRNA), represents the predominant methodology for delineating the microbial taxonomy within a specified ecological niche. Total nucleic acid sequencing, or metagenomics, can yield more detailed information, including information about the metabolic and functional capacities of the microbiota. Through the application of culture-independent methodologies, it has become achievable to determine both the structural composition and the relative prevalence of the microbiome.25,26
For the purpose of taxonomic identification of honeybee microbiota species using sequencing, there are two primary methods available: marker gene sequencing based on amplicon analysis and shotgun metagenomics.3,27 The initial phase involves the application of high-throughput sequencing techniques on metagenomic samples, which is traditionally followed by the alignment of sequencing reads to a recognized reference database abiotic stressor. The utilization of the amplicon-based marker gene methodology is prevalently observed in both culture-dependent and culture-independent techniques, attributable to its comparative simplicity. This method primarily involves amplification of gene through PCR and sequencing of specific fungal and bacterial markers. In bacterial studies focusing on taxonomy and phylogeny, amplifying the 16S rRNA gene is the prevailing choice. This gene is favored due to its widespread presence across bacterial species, and the entirety of its sequence contains a sufficient degree of variation to differentiate among the majority of bacterial species.28-30 The 16S ribosomal RNA gene encompasses nine distinct “hypervariable regions” (V1-V9), which are bordered by conserved sequences. This configuration facilitates the enhancement of amplification through the utilization of universal primers.21,31
The study of identifying microbial eukaryotes in the bee gut has not been extensively explored. Amplicon sequencing, utilizing the 18S rRNA gene and Internal Transcribed Spacer (ITS) regions, is a powerful approach to identify and classify eukaryotic communities in environmental samples. The 18S rRNA gene is highly conserved across eukaryotes, making it an ideal target for universal primers, while the ITS regions provide higher taxonomic resolution for fungi and other microeukaryotes.
In this method, specific primers are designed to amplify regions of the 18S gene (often the V4-V6 regions) or ITS, allowing the sequencing of eukaryotic DNA. High-throughput sequencing platforms, such as Illumina, are frequently used due to their ability to process large amounts of data efficiently. This approach offers insights into the diversity and relative abundance of eukaryotic taxa within a given community. Recent studies have optimized primer sets and protocols to minimize bias and improve taxonomic resolution when using the 18S rRNA and ITS regions. For instance, a study highlighted the development of degenerate primers that capture a broad range of eukaryotic taxa, which were then applied using Illumina sequencing for diverse environmental samples. This method is widely used in microbial ecology and environmental monitoring to assess eukaryotic diversity in ecosystems ranging from marine environments to terrestrial soils. This approach, while efficient for taxonomic profiling, is often complemented by metagenomics to gain insights into the functional potential of the microbial community.3,32 ITS (Internal Transcribed Spacer) approach have revealed a diverse fungal microbiome associated with honeycomb and the gut of different honeybee species, which play distinct ecological roles within the hive. In honeybee guts, Ascomycota and Basidiomycota dominate, with species such as Kodamaea, Zygosaccharomyces, and Wallemia showing higher abundance. In contrast, the honeycomb exhibits a different fungal profile, featuring Bipolaris, Metschnikowia, Trichoderma, and Starmerella, suggesting environmental adaptation of fungi within the hive ecosystem. The fungal diversity plays roles in nutrient cycling, pathogen defense, and symbiosis with the bees themselves. Interestingly, some fungi in the honeybee gut and honeycomb, such as Aspergillus flavus and Fusarium solani, are linked to both honeybee health and environmental interactions, underscoring the intricate relationship between the fungal microbiome and the bees’ physiology. Further insights have also identified bacterial-fungal symbioses, such as Bacillus velezensis, present in fungi from honeybee-associated samples. This highlights the complexity of microbial interactions within the hive ecosystem, contributing to overall hive health and productivity.33,34 In conclusion, the authors noted that ITS regions provided adequate resolution for identifying eukaryotic elements at the species level with high precision.35,36
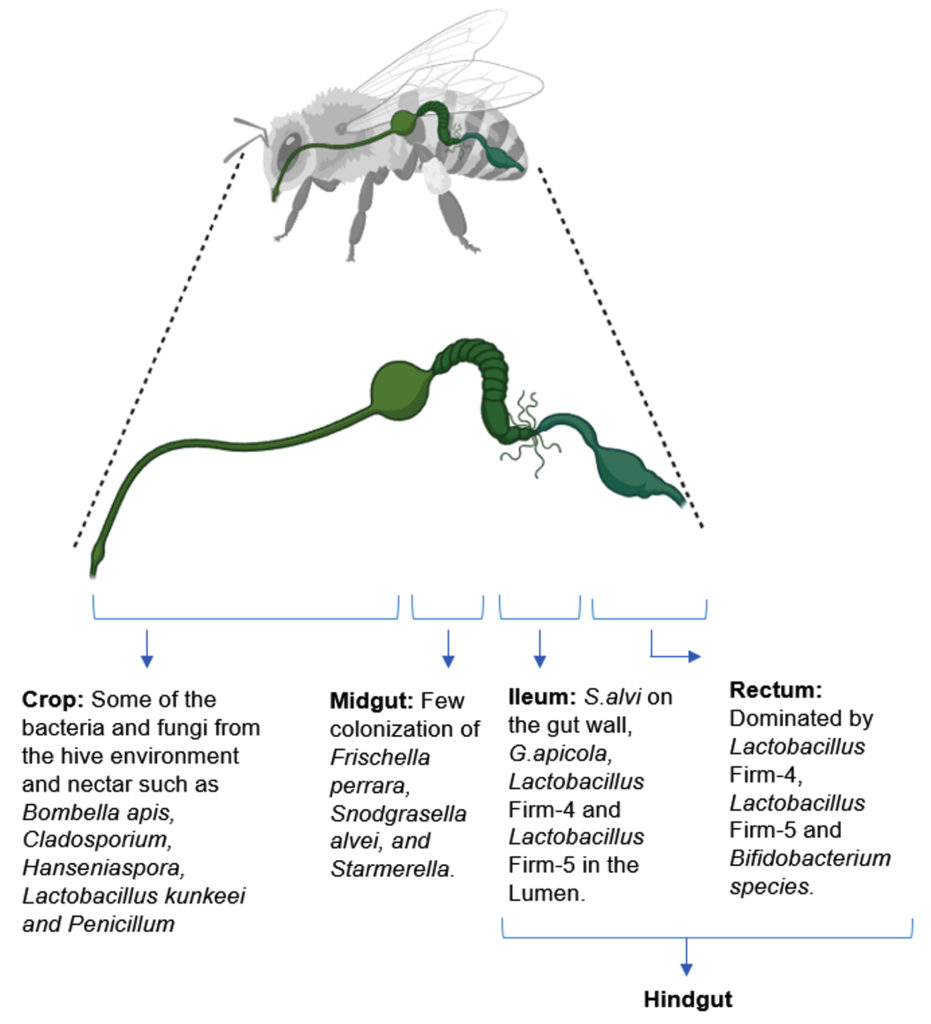
Exploring bacterial diversity within the honeybee gut is a captivating field of research.8,37 Recent investigations indicate that gut microbial community of honeybees comprises a diverse array of bacteria, yeasts, and fungi.38 These microorganisms engage in intricate interactions with the bee host and among themselves, contributing to the bee’s developmental processes.39 Exposure to pesticides or other environmental stressors has the potential to disrupt the bee gut microbiome, detrimentally affecting bee health and ultimately causing declines in honeybee populations.37 The gut bacteria within honeybees play a crucial role in breaking down complex carbohydrates, such as pollen and nectar into simpler forms, immune system modulation, defense against pathogens, behavioral regulation and protection against environmental stress.40-43 Figure 2 illustrates the different regions of the honeybee gut and their associated bacterial communities.44
The well-being and metabolic functions of honeybees rely heavily on their gut microbiome. A substantial part, around 95%, in the gut microbial community is comprised of a select group of phylotypes, including Actinobacteria (such as Bombiscardovia and Bifidobacterium), Bacteroidetes (like Apibacter), Gammaproteobacteria (including Gilliamella and Frischella), Firmicutes (such as Bombilactobacillus, Apilactobacillus, and Lactobacillus), Betaproteobacteria (like Snodgrassella), and Alphaproteobacteria (including Commensalibacter, Bartonella, and Bombella). Research indicates that certain bacterial taxa, such as Lactobacillus, Bombilactobacillus, Snodgrassella, Bifidobacterium, and Gilliamella, considered core microbiomes of honeybees, play vital roles such as defense against pathogens and carbohydrate digestion, highlighting a symbiotic relationship (Table 1).10
Table (1):
Microorganisms found in honey bee gut
No. |
Microbe |
Function |
Symbiosis |
Examples |
References |
|---|---|---|---|---|---|
1. |
Gilliamella apicola |
Digestion of pollen and honey, production of lactic acid |
Mutualistic – benefits both the bee and the bacteria |
Aids in breaking down complex sugars in pollen and honey, providing the bee with essential nutrients |
49 |
2. |
Bifidobacterium asteroideum |
Synthesis of vitamins and short-chain fatty acids, immune system support |
Mutualistic |
Contributes to the bee’s vitamin B production and gut health, potentially boosting its immune response |
50 |
3. |
Lactobacillus Firmontanus |
Production of lactic acid, inhibition of harmful bacteria |
Mutualistic |
Creates an acidic environment in the gut that protecting the bee from pathogens |
22 |
4. |
Snodgrassella alvi |
Nitrogen fixation, production of essential amino acids |
Mutualistic |
Fixes atmospheric nitrogen into a form usable by the bee, and synthesizes essential amino acids that the bee cannot produce itself |
49 |
5. |
Bombella intestini |
Production of B vitamins, degradation of complex carbohydrates |
Mutualistic |
Provides the bee with B vitamins, and helps break down complex carbohydrates into simpler sugars that the bee can absorb |
51 |
6. |
Candidatus Bartonella apis |
Transmission of bee viruses, potential role in queen development |
Symbiotic – complex relationship with benefits influences carbohydrate metabolism, specifically aiding in the digestion of plant-derived polysaccharides. |
May play a role in the transmission of certain bee viruses, but also potentially contributes to queen development and reproduction |
52 |
7. |
Melissococcus pluton |
Production of lactic acid, inhibition of harmful bacteria |
Mutualistic |
Creates an acidic environment in the gut that protecting the bee from pathogens |
53 |
8. |
Frischella perrara |
Synthesis of vitamins and short-chain fatty acids, immune system support |
Mutualistic |
Contributes to the bee’s vitamin B production and gut health, potentially boosting its immune response |
54 |
9. |
Apibacter invictus |
Production of antimicrobial compounds, protection against pathogens |
Mutualistic |
Produces compounds that prevent the growth of harmful bacteria, safeguarding the bee’s gut health |
55 |
In Homeostatic signalling, Short Chain Fatty Acids (SCFAs) are a key energy source for honeybees, especially for cells lining the gut. These acids can regulate metabolic pathways and energy balance, crucial for maintaining gut health. SCFAs have been shown to enhance immune responses by modulating signalling pathways involved in immune cell activity. In honeybees, they help regulate inflammation and immune homeostasis. SCFAs promote the integrity of the gut epithelium by enhancing tight junction protein expression, helping protect against harmful pathogens and toxins.45,46
In defense Mechanisms, SCFAs lower gut pH, creating an inhospitable environment for pathogenic bacteria, thereby preventing infections. This acidic environment favors beneficial microbes and inhibits harmful ones. SCFAs can activate pathways like the NF-kB signaling, leading to enhanced production of antimicrobial peptides and immune effectors, which play a defensive role against gut pathogens.43,47
Through 16S ribosomal RNA gene sequencing, it has been proven that the bee’s gut microbiome incorporates only 8-10 phylotypes, with the majority belonging to the phyla Proteobacteria, Firmicutes, and Actinobacteria which together, they represent more than 95% of all 16S rRNA sequences.48 Various samples have exhibited the presence of well-known pathogens such as Nosema sp., Serratia marcescens, Ascosphaera apis and Paenibacillus larvae affecting honeybees.28
Impact of honeybee gut microbiome on bee health
The gut microbiome in honeybees serves a role similar to that in mammals, contributing to host physiology such as metabolism and immunological functions.41,56 In honeybees, the gut microbiome is comprised of a few bacterial phylotypes spread throughout the hindgut, midgut, and foregut.48 Despite insects having fewer commensal bacteria in their guts compared to mammals, these bacteria can still exert a significant influence on insect health. Understanding the gut microbiome’s role in bee health is essential, as it can greatly reduce the prevalence of bee diseases.
A metagenomic investigation uncovered the capability of Gilliamella apicola to degrade pectin, an important constituent of the inner pollen wall known as intine.57 Current studies employing untargeted metabolomics have characterized the metabolic processes within the honeybee gut microbiome. Specifically, the gut region primarily inhabited by G. apicola is where galacturonate, the primary product of pectin breakdown, accumulates.57 The honeybee gut microbiome utilizes a diverse range of substrates derived from pollen. These results indicate that the bee gut microbiome has evolved to utilize food components that the host does not metabolize and absorb, resulting in their accumulation in the hindgut.47 The microbiome-induced metabolic changes alter the physical and chemical characteristics of the gut environment. In the presence of the microbiome, both pH and redox potential decrease along the bee gut, with a specific reduction in oxygen levels observed in the ileum.58 Understanding how the microbiome influences specific host phenotypes is crucial, although currently, the transmission of metabolites to the host remains unknown. In mammals, metabolites like butyrate move from the intestines to various host tissues, impacting colon cell function. Similar functions have been proposed in bees, though they require validation.
In recent research, the honeybee gut microbiome has been shown to play a crucial role in bee health through metabolic processes that enhance nutrient absorption and protection from pathogens. For example, Snodgrassella alvi, a core honeybee gut symbiont, helps colonize the bee gut using host-derived organic acids, such as citrate and glycerate, while modulating tryptophan metabolism to produce protective compounds like anthranilate. This symbiotic relationship supports the immune system, gut health, and overall resilience of bees to environmental stresses.59
Additionally, honeybee gut bacteria, including Gilliamella apicola and Snodgrassella alvi, form biofilms that offer a defense against pathogens, and these bacteria have also demonstrated resistance to antibiotics commonly used in apiculture, such as tetracycline, highlighting their role in antimicrobial defense. These findings underscore the importance of the honeybee gut microbiome in maintaining colony health, resilience to pathogens, and possible environmental impacts through horizontal gene transfer of antibiotic-resistant genes.60
Lactobacillus species in the honeybee gut influence behaviors related to learning and memory by modulating tryptophan metabolism. Specifically, Lactobacillus Firm-5 converts tryptophan into indole derivatives, activating the host aryl hydrocarbon receptor (AhR), which promotes learning and memory abilities. Additionally, antibiotic treatments disturb gut microbiota, impairing olfactory memory and behavioral performance under field conditions. This shows that a healthy, conventional gut microbiota is crucial for both physiological and cognitive processes in bees.61
Recent research highlights the crucial role of honeybee gut microbes in supporting bee health by regulating metabolic processes, behavioral functions, and brain chemistry. Specific bacterial species like Gilliamella apicola aid in carbohydrate and glycerophospholipid metabolism, while Lactobacillus Firm-4 and Firm-5 enhance amino acid pathways. These gut bacteria also influence neurotransmitter levels such as dopamine and serotonin, which impact sensory sensitivity and taste-related behaviors. Additionally, Lactobacillus species play a role in modulating gene expression related to neural functions like olfactory perception, essential for foraging and social behaviors in bees.61
Since the bee gut microbiome can significantly lower the prevalence of bee diseases, understanding the function of the gut microbiome in maintaining bee health is essential. In order to keep bees safe from diseases and pathogens, maintaining their gut microbiome is essential.37 In bumblebees, it was demonstrated that the gut microbiome leads to a notable decrease in the presence of the gut parasite Crithidia bombi.62 Both microbiome transplants and monocolonization experiments revealed that the gut bacteria in bees trigger the host immune system. F. perrara has an impact on the pylorus’s homeostasis and gut immunity. In addition, the incidence of F. perrara varies throughout colonies and that higher concentrations of this bacteria have been linked to altered diets and compromised host development, this could have an impact on bee health.48
Research has shown that the gut microbiome of bumblebees and honeybees contributes in a certain manner as a resistance mechanism. Microbiome-free B. terrestis inoculated with wild-type workers faecal matter showed greater resistance to the Crithidia bombi gut parasite than with uninoculated bees in two different experiments.63,64 Instead of the colony from which the bees originated, the colony source had a greater influence on the microbiome transplant’s ability to provide protection, indicating that varying gut microbiome compositions can offer different levels of protection. Bombus terrestris maintains a social core gut microbiota, similar to honeybees. Further research is necessary to determine whether individual members of the community provide pathogen protection to bumble bees, as these investigations were unable to identify the strains that underlie the protection.64 Fungi may help bees by competing with harmful pathogens for resources, thereby reducing the growth of spoilage microbes in bee provisions. Some studies have shown that certain fungi can inhibit the growth of bee pathogens. For example, fungi like Aspergillus, Cladosporium, and others isolated from honeybee provisions have demonstrated the ability to inhibit the growth of Ascosphaera apis, a pathogen affecting bee larvae. However, the effectiveness of different fungal strains can vary, and not all fungi exhibit this inhibitory ability.65
Antibiotic properties rendered by the gut microbes
Bacillus species provide benefits to honeybees by producing a range of enzymes and antibiotic-like substances. These substances play a dual role, aiding in carbohydrate digestion and inhibiting the proliferation of harmful organisms within the bee gut.66 Additionally, pathogens have been observed in the honeybee gut, with the predominant segment of these grouped contigs associated with the trypanosomatid parasite Lotmaria passim.67 Gilliamella apicola and Snodgrassella alvi have been studied extensively for their roles in antibiotic resistance. These bacteria form biofilms in the honeybee gut, aiding in the defense against pathogenic bacteria and contributing to antimicrobial resistance (AMR). Recent studies have demonstrated that Gilliamella species exhibit resistance to tetracycline, commonly used in apiculture, leading to the accumulation of antibiotic-resistant genes (ARGs) within bee gut microbiomes.68 Lactobacillus spp. and Bifidobacterium spp. possess antimicrobial properties that inhibit harmful pathogens, not only within the bees but also in the hive and external environments. These microbes produce bacteriocins and other antimicrobial compounds that protect the colony from diseases like American foulbrood, caused by Paenibacillus larvae.69
Probiotic properties rendered by the gut microbes
The bacteria residing in the honeybee gut are predominantly categorized as probiotics, playing a crucial role in maintaining bee health.69 American foulbrood (AFB) and European foulbrood (EFB) are significant ailments affecting honeybee broods, leading to substantial economic losses in the apiculture sector worldwide due to declines in bee populations and honey yields. These diseases can be prevented through the effectiveness of probiotic bacteria naturally present in the bee gut. EFB and AFB are caused by gram-positive bacteria, specifically Paenibacillus larvae and Melissococcus plutonius. Principal groups of probiotic bacteria, including Bifidobacteria, Bacillus species and Lactic Acid Bacteria (LAB) possess the ability to alleviate microbiome dysbiosis associated with antibiotics and immune deficiencies in adult worker bees. Certain Lactobacillus species found in the
honeybee gut have demonstrated their probiotic properties and positive impact on host health.70 Probiotics, as live bacteria ingested in appropriate amounts, significantly improve honeybee immunity in apiculture, resulting in enhanced disease resistance, increased honey production, wax gland development, colony loss, reduced mortality, and decreased nutritional stress.71 Additionally, Probiotic bacteria in honeybees also provide significant advantages such as stimulating fat body development and food gland function.72
The gastrointestinal tract of Apis mellifera is the origin for the probiotic characteristics of LAB.73 They discovered that LAB can thrive in the intestines of honeybees and can withstand pH fluctuations throughout the digestive system. Another study demonstrated that two Lactiplantibacillus plantarum strains, isolated from the Indian honeybee Apis cerena indica, possess probiotic properties. These included the ability to survive in simulated gastrointestinal conditions, the ability to withstand acid and bile tolerance and the ability to aggregate and be hydrophobic.74 LAB species are among the many commensal bacteria found in honeybees’ guts that have been identified as potentially effective probiotics. These bacteria may be used as a dietary supplement for humans and animals as well as to help honeybees recover from illness and become more resilient to it.75,76 Other than LAB species, the Bacillus species also been identified to have the probiotic properties.66 The evidence indicates that the gut of honeybees serves as a diverse repository for numerous LAB species, originating from a wide array of environmental origins. The predominant species in LAB are Lactobacillus species; however, sufficient numbers of Bifidobacterium and Enterococcus species are also present. The probiotic qualities of these bacteria have been thoroughly investigated, and it has been shown that they are essential for both the survival and healthy operation of the bee colony.
Antimicrobial (fungal/bacterial) properties rendered by the gut microbes
The gut microbiome of honeybees perform a crucial role in enhancing their overall well-being by bolstering their resistance to diseases through immune regulation and the production of diverse antimicrobial substances.69 Bacillus species exhibit beneficial characteristics such as the formation of antimicrobial peptides (bacteriocins), immune stimulation, and adhesion.77 Several studies have investigated the antibacterial properties of specific gut-associated Bacillus species against European foulbrood (EFB) and American foulbrood (AFB).75 Recently, honey’s antibacterial qualities are connected to the honey microbiome’s effects in suppressing different human and foodborne pathogens.25 The composition of microbial communities in honey samples exhibited significant antimicrobial activity, revealing traces of antimicrobial bacteria and a diverse origin of pollen in honeybee samples collected from various geographical locations in Greece. Various bacteria were identified in both honeybee guts and honey, with Bradyrhizobium exhibiting antagonistic effects against pathogens.78
Diversity of microbiome in honey
Microbes capable of thriving in honey must possess resistance to its high sugar concentration, acidity, and other antibacterial properties. Honey produced by bees may contain a diverse range of microbial entities, including bacteria like Bacillus and Clostridium species, as well as yeasts (Table 2). Certain lactic acid bacteria in honey have shown antibacterial activity and contribute to maintaining a healthy gut microbiome.79 The presence of various microorganisms in honey not only contributes to its unique taste but also holds potential health benefits for humans.37 Saccharomyces, Bacillus, and Micrococcus are easily extracted from honeycomb. While the mold Aspergillus may be dormant in honey, sugar-tolerant yeasts originate from flowers and soil. Microbes present in comb honey primarily result from pollen contamination and the feces of larvae fed by worker bees. However, these microbes struggle to thrive in honey for extended periods due to its high sucrose concentration and antimicrobial properties.
Table (2):
Yeast, moulds and bacteria found in honey
| Yeast | Functions | References |
|---|---|---|
| Ascophaera | Ascosphaera apis is a significant pathogen for the Apis mellifera, the Western honey bee leading to chalkbrood disease, which holds economic importance. | 80,81 |
| Nematospora | Phytopathogen against honey bee larvae which is found in honey. | 82 |
| Debaryomyces hansenii | Probiotic property in gut microbiome modulation | 83 |
| Zygosaccharomyces rouxii | Fermentation processes, overall flavor and preservation of the honey. | 84 |
| Zygosaccharomyces mellis | High sugar tolerant yeast that leads to deterioration of the honey | 18,81 |
| Aureobasidium pullulans | A very high pullulan producing yeast-like fungus | 85 |
| Cryptococcus neoformans | Decomposer or breaking down organic matter | 86 |
| Moulds | ||
| Aspergillus | Induces Stonebrood disease in honey bees | 87 |
| Bettsia alvei | Xerophilic fungi that thrive in low moisture content | 88 |
| Bacteria | ||
| Bacillus sp. | Enterotoxin producer | 81,89 |
| Clostridium sp. | Enterotoxin producer | 89,90 |
Study on microbial community structure among honey samples of different pollen origin such as fir, cotton, fir-oak, and Arbutus unedo honeys were done.25 In fir honey, the most common taxon identified was Lactobacillus kunkeei. Cotton honey primarily harboured Lysobacter, Meiothermus, Pseudomonas and Streptococcus as the most abundant microorganisms. Conversely, fir-oak honey exhibited Lonsdalea, the microorganism associated with acute oak decline, and Zymobacter, known for fermenting under low oxygen and high osmotic pressure conditions, as predominant types. Methylotrophic bacteria were found abundantly across different geographic and pollen origins. Additionally, Bacilli/anoxybacilli, sphingomonads, pseudomonads, paracocci, and lysobacters were also identified in the honey samples. The analysis suggests that the microbial components detected in the honey samples mainly originate from the indigenous gut microbiome of honeybees and the microbiome present in the flowering plants they visit. This diverse range of bacteria includes potentially beneficial strains with probiotic properties, as well as pathogens capable of affecting both animals and plants.
Aside from the microbial taxa mentioned previously, additional Bacillus species such as B. amyloliquefaciens, B. licheniformis, B. subtilis, B. cereus, B. pumilus, B. thuringiensis, and B. megaterium, were also detected in honey samples. It’s worth noting that bacterial strains of the B. cereus species are known for producing enterotoxins, whereas those from other Bacillus species are generally considered safe.89
Honey microbiome on human health
During the honey production process, honeybees ingest nectar and utilize enzymes to transform it. Additionally, they incorporate certain symbiotic microorganisms from their gastrointestinal tract, which may offer potential health benefits to humans.91 Unlike the human microbiome, which typically remains stable over time, regular intake of new symbiotic microorganisms is essential to ensure their ability to colonize the human body and sustain their beneficial effects. Honey contains microorganisms which include probiotics, and consuming such microorganisms can provide nourishment and health benefits to humans.92
In honey harvested directly from bee hives, a variety of bacteria were isolated, including Gluconobacter oxydans, Pseudomonas spp., and Bacillus spp. Notably, G. oxydans has demonstrated resistance to 2% bile salts and 100% survival at pH 5.0 and 2.0 after 3 hours of interaction, highlighting its resilience in challenging conditions.93 Additionally, these bacteria possess the ability to assimilate cholesterol, potentially reducing its absorption by the body, making them promising probiotic candidates for use in food products. Moreover, given honey’s high fructose content, certain bacteria residing in honey may rapidly break down fructose. These microorganisms, referred to as fructophilic lactic acid bacteria, exhibit a marked preference for the metabolic utilization of fructose in comparison to glucose. Notably, bacteria like Lactobacillus kunkeei, among fructophilic lactic acid bacteria, generate bacteriocins, functioning as a defensive barrier against competing microorganisms. Lactobacillus spp. was obtained from both the stomachs of honeybees and honey samples. They were then evaluated for their inhibitory effects against Escherichia coli and Salmonella enterica, showing significant inhibition.94 A prebiotic is a dietary supplement that cannot be digested and alters the equilibrium of the gut microbiota by promoting the proliferation and function of helpful organisms while inhibiting harmful bacteria.95 Honey has been identified as a suitable sweetening agent in fermented dairy products, facilitating the proliferation of essential bacterial strains including Lactobacillus acidophilus, Streptococcus thermophilus, Bifidobacterium bifidum and Lactobacillus delbrueckii, all of which are vital for maintaining gastrointestinal health, without obstruction.96
In conclusion, recent advancements in biotechnology offer promising avenues for improving the overall health of honeybees through microbiome engineering, strengthening their resistance to viral and bacterial infections. Extensive evidence underscores the critical role of the gut microbiome in honeybee health, with correlational analyses providing valuable insights and laboratory investigations driving experimental work. However, there remains a need for field research conducted in real apiary environments to fully understand the complexities of bee microbiomes. Furthermore, the role of honey microbiomes in both bee and human health cannot be understated. The unique microbial composition of honey, influenced by factors such as bee gut microbiota and environmental conditions, may contribute to the antimicrobial properties and potential health benefits associated with honey consumption for humans. As honey serves as a source of prebiotics and probiotics, its consumption may promote gut health and enhance overall well-being in humans. The evolving understanding of bee gut microbial communities, alike to those found in humans and other mammals, suggests significant advancements in research are on the prospect. Honeybee’s pivotal role in pollinating a substantial portion of the world’s crops and wild flowering plants, their declining populations have prompted an urgent examination of factors impacting their well-being, including their microbiomes. Thus, elucidating the details of this microbial community holds promise for enhancing bee health and addressing broader questions regarding the symbiotic relationship between hosts and microorganisms, thereby contributing to sustainable bee and human health initiatives.
ACKNOWLEDGMENTS
The authors acknowledge the Centre for Plant Molecular Biology and Biotechnology, Tamil Nadu Agricultural University, for the infrastructure facility.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SM conceptualized the study and applied methodology. VRS performed samples collection. KH performed data curation. MS visualization and investigated the study. KH wrote the original draft. KH, SM, MS, NS and VRS wrote the manuscript. NS and VRS reviewed the manuscript. NS edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was partly supported by a Research Grant from the National Bee Board under the National Beekeeping and Honey Mission of the Ministry of Agriculture and Farmer’s Welfare, Government of India.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in this manuscript.
ETHICS STATEMENT
Not applicable.
- Chhun A, Moriano-Gutierrez S, Zoppi F, Cabirol A, Engel P, Schaerli Y. An engineered bacterial symbiont allows noninvasive biosensing of the honey bee gut environment. Plos Biology. 2024;22(3):e3002523.
Crossref - Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiological reviews. 2010;90(3):859–904.
Crossref - Bukin YS, Mikhailov IS, Petrova DP, Galachyants YP, Zakharova YR, Likhoshway YV. The effect of metabarcoding 18S rRNA region choice on diversity of microeukaryotes including phytoplankton. World J Microbiol Biotechnol. 2023;39(9):229.
Crossref - Koch H, Abrol DP, Li J, Schmid-Hempel P. Diversity and evolutionary patterns of bacterial gut associates of corbiculate bees. Molecular ecology. 2013;22(7):2028-2044.
Crossref - Raymann K, Moran NA. The role of the gut microbiome in health and disease of adult honey bee workers. Curr opin insect sci. 2018;26:97-104.
Crossref - Zhang G, Zhang W, Cui X, Xu B. Zinc nutrition increases the antioxidant defenses of honey bees. Entomol Exp Appl. 2015;156(3):201-210.
Crossref - Feng W, Huang J, Zhang Z, et al. Understanding of waggle dance in the honey bee (Apis mellifera) from the perspective of long non-coding RNA. Insects. 2022;13(2):111.
Crossref - Anderson KE, Copeland DC. The honey bee “hive” microbiota: meta-analysis reveals a native and aerobic microbiota prevalent throughout the social resource niche. Frontiers in Bee Science. 2024;2:1410331.
Crossref - Tuddenham S, Sears CL. The intestinal microbiome and health. Current opinion in infectious diseases. 2015;28(5):464-470.
Crossref - Cornet L, Cleenwerck I, Praet J, et al. Phylogenomic analyses of Snodgrassella isolates from honeybees and bumblebees reveal taxonomic and functional diversity. Msystems. 2022;7(3):e01500-21.
Crossref - Vanderplanck M, Michez D, Albrecht M, et al. Monitoring bee health in European agro-ecosystems using wing morphology and fat bodies. One Ecosystem. 2021;6:e63653.
Crossref - Duranti S, Vivo V, Zini I, et al. Bifidobacterium bifidum PRL2010 alleviates intestinal ischemia/reperfusion injury. PLoS One. 2018;13(8):e0202670.
Crossref - Khan KA, Al-Ghamdi AA, Ghramh HA, et al. Structural diversity and functional variability of gut microbial communities associated with honey bees. Microbial pathogenesis. 2020;138:103793.
Crossref - Ye M-H, Fan S-H, Li X-Y, et al. Microbiota dysbiosis in honeybee (Apis mellifera L.) larvae infected with brood diseases and foraging bees exposed to agrochemicals. R Soc Open Sci. 2021;8(1):201805.
Crossref - Mohajeri MH, Brummer RJM, Rastall RA, et al. The role of the microbiome for human health: from basic science to clinical applications. Eur j nutr. 2018;57:1-14.
Crossref - Gurung M, Li Z, You H, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51
Crossref - Ho JML, Mattia JR, Bennett MR. Tunable NF-κB Oscillations in Yeast. Cell Systems. 2017;5(5):440-442.
Crossref - Liu G, Tao C, Zhu B, et al. Identification of Zygosaccharomyces mellis strains in stored honey and their stress tolerance. Food sci biotechnol. 2016;25:1645-1650.
Crossref - Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat rev Gastroenterol hepatol. 2020;17(4):223-237.
Crossref - Romero S, Nastasa A, Chapman A, Kwong WK, Foster LJ. The honey bee gut microbiota: strategies for study and characterization. Insect mol biol. 2019;28(4):455-472.
Crossref - Thakur A, Kumar SM, Saranya N, Nakkeeran S, Srinivasan M, Subramanian S. Characterisation of the gut bacteriome of hill and plain race of indian honey bee Apis cerana Fabricius. Indian J Entomol. 2023;85(1):19-27.
Crossref - Martinson VG, Danforth BN, Minckley RL, Rueppell O, Tingek S, Moran NA. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol ecol. 2011;20(3):619-628.
Crossref - Corby-Harris V, Snyder L, Meador CAD, Naldo R, Mott B, Anderson KE. Parasaccharibacter apium, gen. nov., sp. nov., improves honey bee (Hymenoptera: Apidae) resistance to Nosema. J econ entomol. 2016;109(2):537-543.
Crossref - Ganeshprasad D, Lone JK, Jani K, et al. Gut bacterial flora of open nested honeybee, Apis florea. Front Ecol Evol. 2022;10:837381.
Crossref - Stavropoulou E, Remmas N, Voidarou C, et al. Microbial community structure among honey samples of different pollen origin. Antibiotics. 2023;12(1):101.
Crossref - Kwong WK, Moran NA. Gut microbial communities of social bees. Nat rev microbiol. 2016;14(6):374-384.
Crossref - Jovel J, Patterson J, Wang W, et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front microbiol. 2016;7:459.
Crossref - Vega MF, Libonatti C, Ramos OY, Basualdo M. Characterization of a microbial community isolated from honey bee colonies. Rev Argent de Microbio. 2024;56(3):265-269.
Crossref - Nguyen BK, Mignon J, Laget D, et al. Honey bee colony losses in Belgium during the 2008–9 winter. J Apic Res. 2010;49(4):337-339.
Crossref - Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J clin microbiol. 2007;45(9):2761-2764.
Crossref - Chakravorty S, Helb D, Burday M, Connell N, Alland D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J microbiol methods. 2007;69(2):330-339.
Crossref - Blaalid R, Kumar S, Nilsson RH, Abarenkov K, Kirk PM, Kauserud H. ITS 1 versus ITS 2 as DNA metabarcodes for fungi. Molecular ecology resources. 2013;13(2):218-224.
Crossref - Callegari M, Crotti E, Fusi M, et al. Compartmentalization of bacterial and fungal microbiomes in the gut of adult honeybees. npj Biofilms Microbiomes. 2021;7(1):42.
Crossref - Cui P, Kong K, Yao Y, et al. Community composition, bacterial symbionts, antibacterial and antioxidant activities of honeybee-associated fungi. BMC microbiology. 2022;22(1):168.
Crossef - Gancarz M, Hurd PJ, Latoch P, et al. Dataset of the next-generation sequencing of variable 16S rRNA from bacteria and ITS2 regions from fungi and plants derived from honeybees kept under anthropogenic landscapes. Data in Brief. 2021;36:107019.
Crossref - Yun J-H, Jung M-J, Kim PS, Bae J-W. Social status shapes the bacterial and fungal gut communities of the honey bee. Scientific reports. 2018;8(1):2019.
Crossref - Olofsson TC, Vásquez A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr microbiol. 2008;57:356-363.
Crossref - Guo Y, Diao Q-Y, Dai P-L, et al. The effects of exposure to flupyradifurone on survival, development, and foraging activity of honey bees (Apis mellifera L.) under field conditions. Insects. 2021;12(4):357.
Crossref - Pickard JR, Soroker V, Stevanovic J. The 60 th volume of the Journal of Apicultural Research-a look into the past and future. 2021;
Crossref - Li Z, Huang Q, Zheng Y, et al. Identification of the toxic compounds in Camellia oleifera honey and pollen to honey bees (Apis mellifera). J Agric Food Chem. 2022;70(41):13176-13185.
Crossref - Powell JE, Lau P, Rangel J, Arnott R, De Jong T, Moran NA. The microbiome and gene expression of honey bee workers are affected by a diet containing pollen substitutes. PLoS One. 2023;18(5):e0286070.
Crossref - Leipart V, Ludvigsen J, Kent M, et al. Identification of 121 variants of honey bee Vitellogenin protein sequences with structural differences at functional sites. Protein Science. 2022;31(7):e4369.
Crossref - Raymann K, Coon KL, Shaffer Z, Salisbury S, Moran NA. Pathogenicity of Serratia marcescens strains in honey bees. MBio. 2018;9(5):10.1128/mbio.01649-18.
Crossref - Smutin D, Lebedev E, Selitskiy M, Panyushev N, Adonin L. Micro” bee” ota: Honey bee normal microbiota as a part of superorganism. Microorganisms. 2022;10(12):2359.
Crossref - Zheng H, Perreau J, Powell JE, et al. Division of labor in honey bee gut microbiota for plant polysaccharide digestion. Proc Natl Acad Sci. 2019;116(51):25909-25916.
Crossref - Bonilla-Rosso G, Engel P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr opin microbiol. 2018;43:69-76.
Crossref - Kešnerová L, Emery O, Troilo M, Liberti J, Erkosar B, Engel P. Gut microbiota structure differs between honeybees in winter and summer. The ISME journal. 2020;14(3):801-814.
Crossref - Engel R. The influence of land use on the pollen diet of honey bee (Apis mellifera) colonies in Ellis County, Kansas. 2020.
Crossref - Kwong WK, Moran NA. Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int j syst evol microbiol. 2013;63(Pt_6):2008-2018.
Crossref - Scardovi V, Zani G, Trovatelli LD. Deoxyribonucleic acid homology among the species of the genus Bifidobacterium isolated from animals. Archiv für Mikrobiologie. 1970;72(4):318-325.
Crossref - Yun J-H, Lee J-Y, Hyun D-W, Jung M-J, Bae J-W. Bombella apis sp. nov., an acetic acid bacterium isolated from the midgut of a honey bee. Int J Syst Evol Microbiol. 2017;67(7):2184-2188.
Crossref - Liu J, Zhang R, Tang R, et al. The Role of honey bee derived aliphatic esters in the host-finding behavior of Varroa destructor. Insects. 2022;14(1):24.
Crossref - Lang H, Liu Y, Duan H, Zhang W, Hu X, Zheng H. Identification of peptides from honeybee gut symbionts as potential antimicrobial agents against Melissococcus plutonius. Nat Commun. 2023;14(1):7650.
Crossref - Kubota A, Kawai R, Li D, et al. Enzymatic and structural characterization of β-fructofuranosidase from the honeybee gut bacterium Frischella perrara. Appl Microbiol Biotechnol. 2022;106(7):2455-2470.
Crossref - Kwong WK, Steele MI, Moran NA. Genome sequences of Apibacter spp., gut symbionts of Asian honey bees. Genome Biol. Evol. 2018;10(4):1174-1179.
Crossref - Naccache C, Najjari A, Djebbi S, Soui A, Kharrat I, Khemakhem MM. Gut microbiota composition of Apis mellifera L. populations from Tunisia. J Apic Res. 2023:1-10.
Crossef - Zheng H, Nishida A, Kwong WK, et al. Metabolism of toxic sugars by strains of the bee gut symbiont Gilliamella apicola. MBio. 2016;7(6):10.1128/mbio. 01326-16.
Crossref - Zheng H, Powell JE, Steele MI, Dietrich C, Moran NA. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci. 2017;114(18):4775-4780.
Crossref - Quinn A, El Chazli Y, Escrig S, et al. Host-derived organic acids enable gut colonization of the honey bee symbiont Snodgrassella alvi. Nat Microbiol. 2024;9(2):477-489.
Crossref - Tilocca B, Greco V, Piras C, et al. The bee gut microbiota: Bridging infective agents potential in the one health context. Int J Mol Sci. 2024;25(7):3739.
Crossref - Zhang Z, Mu X, Cao Q, Shi Y, Hu X, Zheng H. Honeybee gut Lactobacillus modulates host learning and memory behaviors via regulating tryptophan metabolism. Nat Commun. 2022;13(1):2037.
Crossref - Folly AJ, Koch H, Stevenson PC, Brown MJF. Larvae act as a transient transmission hub for the prevalent bumblebee parasite Crithidia bombi. J Invertebr Pathol. 2017;148:81-85.
Crossref - Weinhold A, Grüner E, Keller A. Bumble bee microbiota shows temporal succession and increase of lactic acid bacteria when exposed to outdoor environments. Front Cell Infect Microbiol. 2024;14:1342781.
Crossref - Koch H, Cisarovsky G, Schmid-Hempel P. Ecological effects on gut bacterial communities in wild bumblebee colonies. Journal of Animal Ecology. 2012;81(6):1202-1210.
Crossref - Rutkowski D, Weston M, Vannette RL. Bees just wanna have fungi: a review of bee associations with nonpathogenic fungi. FEMS Microbiology Ecology. 2023;99(8):fiad077.
Crossref - Tootiaie S, Moharrami M, Mojgani N. Honeybee gut: reservoir of probiotic bacteria. Probiotic bacteria and postbiotic metabolites: role in animal and human health. 2021;2:221-236.
Crossref - Regan T, Barnett MW, Laetsch DR, et al. Characterisation of the British honey bee metagenome. Nat commun. 2018;9(1):4995.
Crossref - Sun H, Li H, Zhang X, et al. The honeybee gut resistome and its role in antibiotic resistance dissemination. Integrative Zoology. 2023;18(6):1014-1026.
- Moharrami M, Mojgani N, Bagheri M, Toutiaee S. Role of honey bee gut microbiota in the control of American foulbrood and European foulbrood diseases. Arch Razi Inst. 2022;77(4):1331.
Crossref - Daisley BA, Pitek AP, Chmiel JA, et al. Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. The ISME journal. 2020;14(2):476-491.
Crossref - Ramos OY, Basualdo M, Libonatti C, Vega MF. Current status and application of lactic acid bacteria in animal production systems with a focus on bacteria from honey bee colonies. J appl microbiol. 2020;128(5):1248-1260.
Crossref - Szymaś B, Łangowska A, Kazimierczak-Baryczko M. Histological structure of the Midgut of honey bees (Apis Mellifera L.) Fed Pollen Substitutes Fortified with Probiotics. Journal of Apicultural Science. 2012;56(1):5-12.
Crossref - Pachla A, Ptaszyńska AA, Wicha M, et al. Insight into probiotic properties of lactic acid bacterial endosymbionts of Apis mellifera L. derived from the Polish apiary. Saudi J Biol Sci. 2021;28(3):1890-1899.
Crossref - Honey CC, Keerthi TR. Probiotic potency of Lactobacillus plantarum KX519413 and KX519414 isolated from honey bee gut. FEMS microbiology letters. 2018;365(4):fnx285.
Crossref - Forsgren E, Locke B, Sircoulomb F, Schäfer MO. Bacterial diseases in honeybees. Current Clinical Microbiology Reports. 2018;5:18-25.
Crossref - Lamei S, Stephan JG, Nilson B, et al. Feeding honeybee colonies with honeybee-specific lactic acid bacteria (Hbs-LAB) does not affect colony-level Hbs-LAB composition or Paenibacillus larvae spore levels, although American foulbrood affected colonies harbor a more diverse Hbs-LAB community. Microbial ecology. 2020;79(3):743-755.
Crossref - Duc LH, Hong HA, Barbosa TM, Henriques AO, Cutting SM. Characterization of Bacillus probiotics available for human use. Appl environ microbio. 2004;70(4):2161-2171.
Crossref - Tsadila C, Amoroso C, Mossialos D. Microbial diversity in bee species and bee products: Pseudomonads contribution to bee well-being and the biological activity exerted by honey bee products: A narrative review. Diversity. 2023;15(10):1088.
Crossref - Audisio M, Benítez-Ahrendts M. Lactobacillus johnsonii CRL1647, isolated from Apis mellifera L. bee-gut, exhibited a beneficial effect on honeybee colonies. Beneficial Microbes. 2011;2(1):29-34.
- Cornman RS, Bennett AK, Murray KD, Evans JD, Elsik CG, Aronstein K. Transcriptome analysis of the honey bee fungal pathogen, Ascosphaera apis: implications for host pathogenesis. BMC genomics. 2012;13:1-13.
Crossref - Xiong ZR, Sogin JH, Worobo RW. Microbiome analysis of raw honey reveals important factors influencing the bacterial and fungal communities. Front Microbiol. 2023;13:1099522.
Crossref - Sood S, Steinmetz H, Beims H, et al. Paenilarvins: Iturin family lipopeptides from the honey bee pathogen Paenibacillus larvae. Chembiochem. 2014;15(13):1947-1955.
Crossref - Angulo M, Reyes-Becerril M, Medina-Córdova N, Tovar-Ramírez D, Angulo C. Probiotic and nutritional effects of Debaryomyces hansenii on animals. Appl microbiol biotechnol. 2020;104:7689-7699.
Crossref - Chen S, Tang Q, Geng J, et al. Detection of Viable Zygosaccharomyces rouxii in Honey and Honey Products via PMAXX-qPCR. Journal of Food Quality. 2022;2022(1):8670182.
Crossref - Jiang H, Xue S-J, Li Y-F, et al. Efficient transformation of sucrose into high pullulan concentrations by Aureobasidium melanogenum TN1-2 isolated from a natural honey. Food chemistry. 2018;257:29-35.
Crossref - Ergin Ç, Ilkit M, Kaftanoǧlu O. Detection of Cryptococcus neoformans var. grubii in honeybee (Apis mellifera) colonies. Mycoses. 2004;47(9-10):431-434.
Crossref - Foley K, Fazio G, Jensen AB, Hughes WOH. The distribution of Aspergillus spp. opportunistic parasites in hives and their pathogenicity to honey bees. Veterinary microbiology. 2014;169(3-4):203-210.
Crossref - Rodríguez-Andrade E, Stchigel AM, Terrab A, Guarro J, Cano-Lira JF. Diversity of xerotolerant and xerophilic fungi in honey. IMA fungus. 2019;10:1-30.
Crossref - Silva MS, Rabadzhiev Y, Eller MR, Iliev I, Ivanova I, Santana WC. Microorganisms in honey. Honey analysis. 2017;500:233-257.
Crossref - Lanh PT, Duong BTT, Thu HT, Hoa NT, Van Quyen D. Comprehensive analysis of the microbiome in Apis cerana honey highlights honey as a potential source for the isolation of beneficial bacterial strains. PeerJ. 2024;12:e17157.
Crossref - Endo A, Salminen S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst Appl Microbiol. 2013;36(6):444-448.
Crossref - Anadón A, Martínez-Larrañaga MR, Arés I, Martínez MA. Prebiotics and probiotics: An assessment of their safety and health benefits. 2016:3-23.
Crossref - Begum SB, Roobia RR, Karthikeyan M, Murugappan RM. Validation of nutraceutical properties of honey and probiotic potential of its innate microflora. LWT-Food Science and Technology. 2015;60(2):743-750.
- Vanderplanck M, Gilles H, Nonclercq D, Duez P, Gerbaux P. Asteraceae paradox: Chemical and mechanical protection of Taraxacum pollen. Insects. 2020;11(5):304.
Crossref - Sanz ML, Polemis N, Morales V, et al. In vitro investigation into the potential prebiotic activity of honey oligosaccharides. J agric food chem. 2005;53(8):2914-2921.
Crossref - Bansal V, Medhi B, Pandhi P. Honey–a remedy rediscovered and its therapeutic utility. Kathmandu Univ med j (KUMJ). 2005;3(3):305-309.
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.