ISSN: 0973-7510
E-ISSN: 2581-690X
Of all viruses causing liver pathology, Epstein-Barr virus (EBV) has been one of the most ubiquitous. Presumably, a co-infection of both EBV and hepatitis C virus (HCV) negatively influences the prognosis of HCV-infected patients. Hence, we endeavoredto estimate the impact of EBV infection on treatment with interferon plus ribavirin in chronic HCV patients. Eighty-four chronic HCV patients and sixteen control subjects (with no symptoms of HCV infection) were enrolled in this study. Viral loads were assessed using reverse transcriptase polymerase chain reaction, while anti-EBV IgM levels were determined by ELISA. Chronic HCV patients with negative EBV IgM exhibited higher response rates (85.7%) to interferon than those with positive EBV IgM (62%). Furthermore, HCV RNA load was remarkably higher in EBV-seropositive patients (with a P-value of 0.0001). Chronic HCV infection cannot be considered in isolation; as other pathogens such as EBV may have far-ranging effects against the disease prognosis. We concluded that EBV co-infection can deteriorate the response to interferon in patients with chronic HCV infection, and that EBV could enhance the replication of HCV. Accordingly, EBV treatment should be considered when designing specific protocols for treatment of such patients.
Epstein-Barr Virus, Hepatitis C, Interferon-Ribavirin Therapy.
Hepatitis C virus (HCV) is considered as one of the most global epidemics. In 2015, around 175 million people were HCV positive and this number escalates annually where 23.7 new HCV infections occur in 100,000 of the human population1. Every year, approximately 700,000 persons die as a result of HCV related comorbidities, which include but not limited to; hepatocellular carcinoma, cirrhosisand liver failure2. Egypt has the highest prevalence rate of HCV worldwide, with around 15% of the population HCV infected. The high infection rate of HCV within the Egyptian community makes it a pressing health problem with the highest priority to the Egyptian Government3,4. Meanwhile, the World Health Organization (WHO) has set a global target to reduce both the new infection and death rate by 90% and 65% respectively by 20301.
Notably, the efficacy of antiviral therapy can be influenced by various determinants including both viral and host factors. Among the viral determinants are the viral quasi species, genotype and the pretreatment viral load. On the other hand, host factors include age, sex, immune system competence and co-infections e.g. with other viruses5,6.
Viral co-infections not only affect the response to treatment but also can indeed alter the evolvement of human viral infections7. In addition to hepatitis A, B, C, D and E, other viral agents like EBV, herpes simplex virus, cytome-galovirus (CMV), and adeno viruses are highly considered as hepatotropic8. Of these viruses, EBV plays an essential and complicated role in liver damage. Being a member in the herpes family, EBV can establish a latent infection in over 90% of human adults9. Like other members of the herpes family, EBV exhibits alternating episodes of latency and reactivation10. It remains latent inside B-lymphocytes and may cause immortalization and malignant transformation of these cells11. In immunocompetent persons, EBV infection is characterized by a transient viremia followed by a robust T-cell response, which keeps the infection in a latent state12. Failure or suppression of the immune system leads to reactivation which is observed during stress or co-infection with otherviruses13,14.
Revolutionary changes in HCV treatment have occurred over the past five years upon approval of several direct-acting antivirals(DAA). Current treatment consists of drug combinations of a short duration and high tolerability and cure rates in the 95% range. Nonetheless, it has been demonstrated that 1-15% of patients fail to achieveSVR15. Variable factors were found to be associated with this reduced response such as high pretreatment viral load, previous treatment failure and infection with HCV genotype 3. In addition, host factors such as liver cirrhosis, gender, interferon lambda 4 (IFNL4) and interferon gamma-inducible protein 10 (IP10) levels may affect the response to antiviral agents16,17.
Although the classical HCV treatment in adults with pegylated interferon (PegIFN) and ribavirin is now replaced by the new DAAs, it is still the only recommended HCV treatment for children older than 2 years of age and adolescents, in addition to being an alternative regimen for certain genotypes18.
The association of EBV infection with viral hepatitis has been studied in previous research; nevertheless, the effect of EBV/HCV co-infection in response to treatment with interferon is not clear yet. The purpose of our study was to detect EBV infection frequency among chronic HCV patients and to explore the effect of EBV infection on the response of those patients to interferon-plus ribavirin therapy.
Participants
This study was performed at the Egyptian National Hepatology and Tropical Medicine Research Institute during the period from June 2013 to June 2014. Laboratory tests were done in the Microbiology Department of the Institute.
Participants in this study were divided into two groups; group A and group B. Group A consisted of 84 chronic HCV patients (positive for both HCV Ab and HCV RNA) who received combined treatment of PegIFN plus ribavirin (Pegasys, Hoffman, La Roche, Switzerland) for 12 weeks. These patients were subjected to reverse transcriptase polymerase chain reaction (RT-PCR) assay that is specific for HCV detection and quantification. The patients were under surveillance at the Institute’s gastroenterology outpatient clinic, and as part of their routine evaluation, they under went a percutaneous liver biopsy. On the other hand, Group B consisted of 16 control subjects who were negative for anti-HCV.
According to serological results, group A was divided into 2 subgroups; subgroup A1 included 21 chronic HCV patients having EBV co-infection (+ve EBV IgM) and subgroup A2 included 63 chronic HCV patients without EBV co-infection.
According to the serological results of group B, there were 9 subjects with +ve EBV IgM (subgroup B1), and 7 subjects with -ve EBV IgM (subgroup B2).
None of the patients or control groups had received immuno-suppressive or antiviral therapy before. They were all Egyptians and unrelated to each other. Hepatitis C viral loads were assessed using RT-PCR before and after 12 weeks of the IFN therapy.
Ethical consideration
Informal written and oral approval was considered before the study from each patient.Study approval was issued and maintained by the local ethical committee at the institute.
Fully automated CobasAmpliprep/ CobasTaqman device (Roche Diagnostics, Switzerland) was used for detecting HCV RNA19,20.
RNA extraction
HCV viral particles were lysed via treatment with high temperature combined with a protease treatment and a lysis buffer. These treatments and temperature conditions induce the release of DNA and RNA and inhibits the activity of RNases in blood samples. HCV RNA was mixed with proteases and the glass beads, the lyses buffer was added and incubated. Thereafter, the mixture was incubated, and the HCV RNA and HCV QS RNA were compelled to the exterior of the magnetic glass beads. Unbound materials, e.g. proteins, salts, and other contaminations, were detached by washing the magnetic glass beads.
Following beads separation and the washing steps, the adsorbed RNAs were separated at a high temperature with an aqueous layer. The processed specimen with the magnetic glass beads and the released HCV RNA and HCV QS RNA, were all mixed to the amplification buffer and moved to COBAS TaqMan Analyzer.
Reverse Transcription and PCR Amplification
Fully automated CobasAmpliprep/ CobasTaqman device (Roche Diagnostics company, Switzerland) with a recognition limit of 15 IU/ml was used for detecting HCV RNA. The RT-PCR (Reverse transcription process and the PCR amplification reaction) was performed with the recombinant established stable enzyme from thermophilic bacterial species DNA Polymerase enzyme (Z05) in the presence of metal ions and buffer that is required for activity. And sinceZ05 has both reverse transcriptase activity and DNA polymerase activity, both reverse transcription and amplification occur together allowing amplicon detection in real time. Subsequently, the samples were added to the amplification buffer mix in the specified K-tubes where reverse transcription and amplification take place. Primers were annealed to the specific loci in high temperature to allow the amplification process.
Calculation of results
HCV RNA intensity in the samples were calculated by COBAS TaqMan Analyzer through comparing the HCV light intensity to the HCV QS intensity for each sample and each control. In the annealing PCR phase on the COBAS TaqMan Analyzer, the specimens were subjected to illumination and excitation via filtered light and filtered emission. Fluorescence readings were gathered for every specimen.
The analyses data were converted by the machine to the built-in software and stored in its database. The light intensity data were analyzed to produce new numbers that reflect the HCV RNA concentration. Additionally, the machine standardization in the COBAS AmpliPrep/COBAS TaqMan HCV Test were utilized to estimate the titer assessment of both the sample and the control depending on the HCV RNA and HCV QS RNA reads.The titer data were testified in International Units/mL (IU/mL).
Serological analysis of EBV infection
Anti-EBV IgM against viral capsid antigen (VCA) in the samples was estimated by the enzyme linked immunosorbent assay (ELISA) technique using Diametra – Italy micro-assay kit. Samples were processed and treated as described in the manufacturer’s instructions.
Histological assessment of the biopsy specimens
Liver biopsies were obtained percutaneously through ultrasound-guided Menghini needle 14G with 1.6 mm internal diameter. The biopsies then were fixed in a formalin solution; microtome was used to generate thin sections (5 µm each) of the paraffin coated biopsies. For handling of the specimens, slides were stained with 5 levels hematoxylin and eosin, and 5 levels Masson’s trichrome stain, in a total of 10 levels for each specimen.
All specimens were inspected by two medical pathology specialists and categorized by consensus for all abnormal histological results. The histological activity index was determined according to METAVIR scoring system as follows21.
Grading of disease activity
This includes four categories of necro-inflammatory activity
A0: no activity
A1: mild activity
A2: moderate activity
A3: severe activity
Fibrosis grades
F0: No fibrosis
F1: Portal fibrosis without septa
F2: Portal fibrosis with rare septa
F3: Numerous septa but no cirrhosis
F4: Cirrhosis
Statistical methods
Statistical evaluation of our data was performed by the statistical program for social science (SPSS) version 15. Both Chi-square test and the One-way ANOVA (analysis of variance) were used to compare qualitative and quantitative variables respectively. In addition, the Student’s t-test was used to detect the significance of difference between two means of variables containing continuous data. A P-value <0.05 was interpreted as a significant difference. Mean and standard deviation were represented the quantitative data, while the percentage was used to represent the qualitative data.
According to our serological data, group A was divided into 2 small groups (subgroups); the A1 subgroup and A2 subgroup. Subgroup A1 included 21 chronic HCV patients (8 females and 13 males) having EBV co-infection. Subgroup A2 included 63 chronic HCV patients (16 females and 47 males) without EBV co-infection.
According to the serological results of group B (control group), there were 9 subjects with +ve EBV IgM (subgroup B1) including 3 females and 6 males, and 7 subjects with -ve EBV IgM (subgroup B2) including 3 females and 4 males.
Pathological and demographic data
In order to investigate if any of the demographic or pathological factors was co-related with co-infection, a comparison was carried out between subgroup A1(with HCV/EBV co-infection) and A2 (without co-infection), and is outlined in Table 1. The data describe that no significant differences were observed between the two subgroups regarding age, sex or the histological characteristics of the liver biopsy. These data reflect that there is no correlation between co-infection and the demographic or pathological state.
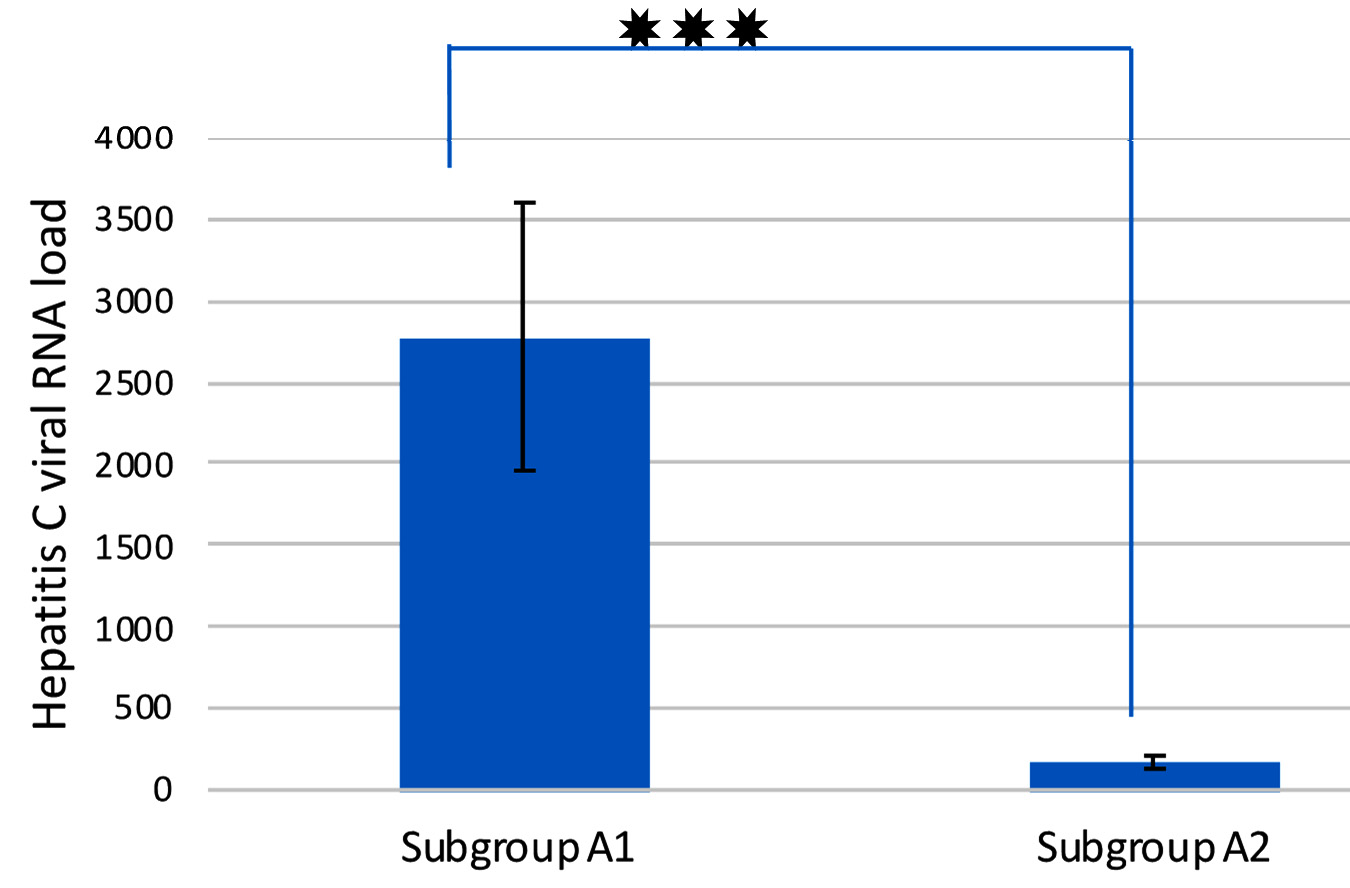
Comparison of Hepatitis C viral RNA loads inEBV co-infected versus mono-infected patients
In order to examine if a significant variance in HCV load exists between co-infected and mono-infected patients, hepatitis C viral loads were assessed using RT-PCR. The study showed that there was a statistically significant difference between the two subgroups as regards HCV RNA load (P value = 0.0001) as shown in figure1.Subgroup A1 (EBV +ve) showed more than 10-fold increase in HCV RNA load than the subgroup A2 (EBV -ve).
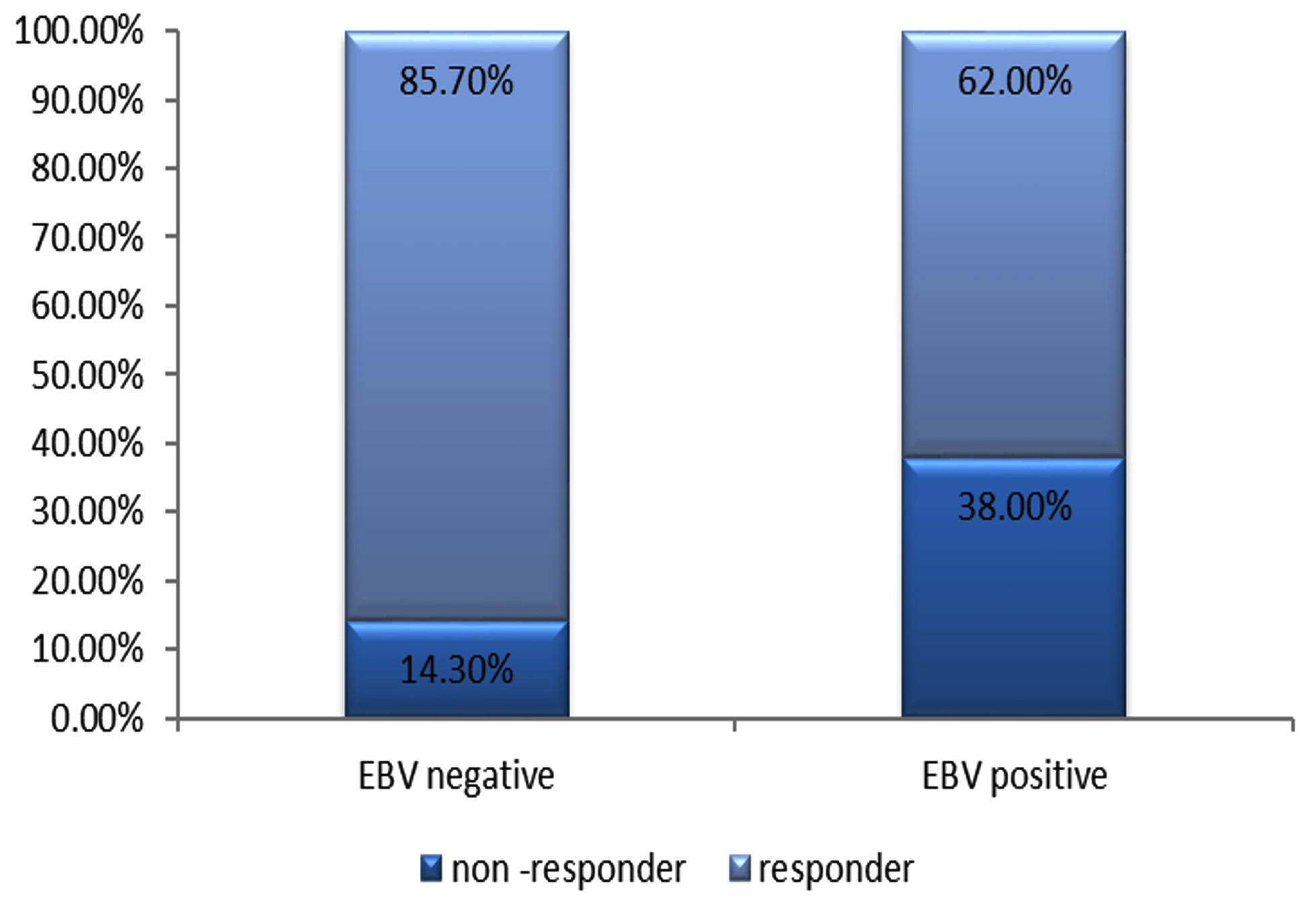 Fig. 2. Response to treatment with PegIFN + ribavirin among chronic HCV patients (EBV +ve and EBV –ve patients).
Fig. 2. Response to treatment with PegIFN + ribavirin among chronic HCV patients (EBV +ve and EBV –ve patients).Response to IFN therapy
The response to PegIFN + ribavirin therapy was detected, where hepatitis C viral loads were assessed using RT-PCR before and after 12 weeks of therapy. Responders were described as patients who exhibited a negative result for HCV RNA after 12 weeks of therapy. The current study revealed that there was a statistically significant difference between subgroup A1 and subgroup A2 regarding the response to therapy.
According to HCV RNA assay, 85.7% of group A were responders, compared to only 62% in group B(Figure 2). On the other hand, around 75% of non-responders were from subgroup A1 (EBV/HCV co-infected patients).
Table (1):
pathological and Demographic data ofgroup A (chronic HCV patients).
Subgroup A1 (n=21) |
Subgroup A2 (n=63) |
P-value |
|
|---|---|---|---|
Sex |
|||
Male |
13 |
47 |
0.26 |
Female |
8 |
16 |
|
Age (years) |
|||
Mean ± SD |
45.9 ± 9.7 |
41.9 ± 8.8 |
0.08 |
Fibrosis grade |
|||
F1 |
10 |
37 |
0.6 |
F2 |
8 |
20 |
|
F3 |
3 |
6 |
|
Necrosis grade |
|||
A1 |
13 |
49 |
0.1 |
A2 |
7 |
14 |
|
A3 |
1 |
0 |
Table (2):
pathological and Demographic data ofgroup A (chronic HCV patients).
Responders |
Non-responders |
P-value |
|
|---|---|---|---|
Age (years) |
|||
Mean ± SD |
41.5± 2.3 |
48 ± 2.8 |
0.005 |
EBV IgM |
|||
Mean ± SD |
7± 5.8 |
9.5 ± 4.8 |
0.02 |
Fibrosis grade |
|||
F1 |
39 |
8 |
0.7 |
F2 |
21 |
7 |
|
F3 |
7 |
2 |
|
Necrosis grade |
|||
A1 |
51 |
11 |
0.1 |
A2 |
16 |
5 |
|
A3 |
0 |
1 |
Table (3):
Comparison between subgroup A1 (patients) and subgroup B1(controls) regarding serum concentrations of EBV IgM.
EBV IgM concentration
(AU) |
Subgroup A1
(EBV +ve and HCV +ve) (n=21) |
Subgroup B1
(EBV +ve and HCV-Ab -ve) (n=9) |
P-value |
|---|---|---|---|
Mean ± SD |
15.6 ± 5.3 |
16.4 ± 2.96 |
0.16 |
Median (Range) |
14 (12 –31) |
17 (12-20) |
Correlation between response to treatment and different parameters
Comparison between the responders and the non-responders to treatment showed that there were no significant differences between the two groups as regards the grade of fibrosis and the necro-inflammatory score. In contrast, a statistically significant difference was identified between the two groups as regards their mean age and serum EBV IgM concentration (table 2). Higher serum EBV IgM concentration and older age were linked to greater risk of no response(P-value = 0.02).Comparison between those in group A (patients) and those in group B(controls) regarding EBV IgM concentration in serum showed a statistically insignificant difference between the two groups (Tables 3 & 4).
HCV coinfections with HBV, HIV, CMV, and EBV have been reported. In this study, we investigated the frequency of EBV infection among recently diagnosed chronic HCV patients who have not received antiviral or immuno-suppressive therapy before enrollment. We also assessed the response of HCV patients co-infected with EBV to the combined therapy of IFN + ribavirin.
Our data showed that EBV IgM was detected in 25% of the enrolled HCV-infected patients. An inverse relationship was found between EBV/HCV co-infection and the response toIFN therapy. Co-infection has greatly reduced the response to the treatment. HCV patients with positive EBV IgM showed markedly lower response rate (62%) to the antiviral treatment compared to those with negative EBV IgM(85.7%). These results showed an agreement with those of Gerakari et al.22 who evaluated the relation between latent CMV and EBV co-infection and its impact on liver pathology in HCV patients as well as their response to PegIFN plus ribavirin therapy. They reported that latent CMV and EBV infection may play a role in the clinical outcome of liver disease in chronic HCV patients and may worsen their response to combination therapy. These results are coinciding with those of Bader El Din et al. 23 who investigated the impact of co-infection with CMV on the response to antiviral therapy among chronic HCV patients. They found that CMV co-infection, which is a herpes family member as EBV, was associated with a lower response rate to combined therapy. In another study performed by Shoman et al.24 who studied the impact of co-infection with EBV on biochemical parameters and immune response in chronic HCV patients, it was suggested that EBV and chronic HCV co-infection may lead to down regulation of the immune response in patients. They recommended introducing treatment of EBV infection among chronic HCV patients to decrease the risk of rapid progression to liver cirrhosis and hepatocellular carcinoma.
However, the current study results are conflicting with those of Strnad-Trojan et al.25 who studied a patient with X-linked lympho-proliferative disorder infected with EBV and HCV. Clearance of both EBV DNA and HCV-RNA was achieved after a course of IFN a-2b and ribavirin. This discrepancy could be attributed to HCV genotype as the reported patient had HCV genotype 1b while in Egypt the predominant genotype is 4a26.
In this study, hepatitis C viral RNA loads were higher in EBV co-infected patients. These results support the previous results of Sugawara et al.27 and Morrison et al.28 who examined the impact of EBV co-infection on HCV replication, and found that EBV can enhance the replication of HCV in vitro. They also concluded that HCV replication was greatly potentiated by EBNA1 protein. These results are also in line with that of Petrova et al.11. In their study, Petrova et al. examined the impact of EBV infection among chronic HBV and chronic HCV patients. They showed that patients with reactivated EBV infection had higher levels of HCV RNA and lower levels of HBV DNA, when compared to patients without reactivation. Worth mentioning, earlier studies on dual viral infections demonstrated an inverse relationship between both HCV and HBV, any of them can suppress the cell infection or nucleic acid replication of the other one29.
However, the current study results are contradicting with those of Challine et al.30 who investigated the co-infection of HCV and EBV in AIDS patients. They prospectively studied 135 latent EBV infected patients. Among these patients, around 20% were positive for HCV RNA. There was no relationship between the viral loads of EBV, HCV and HIV. No variance on EBV markers between HCV positive and negative cases. There was no relation between EBV and HCV viral load.This discrepancy of results could be explained by differences between study populations; as their patients were all AIDS patients while our patients were HIV-free.
In the meantime, the age of the patient is an important factor related to the positive responsiveness to IFN treatment in chronic HCV. Generally, younger patients are more likely to respond positively to IFN therapy than older patients31,32,33.
Such hypothesis was sustained by the outcome of the present study where the mean age of responders was 41, while the mean age of non-responders was 48. These findings reiterate the results of Hayashi et al.31 who reported that women with age around 39 and younger than that are more responsive to IFN therapy than those more than 40 years old. Also, in another study by Iqbal et al.33 they found that IFN therapy was more effective in patients below 40 years than in patients aged 40 or more.
There is a number of possible explanations including undermined cellular, humoral, and innate immune responses which may be responsible for the deteriorated responses to therapy in older patients. Also, older HCV patients are likely to have more progressive liver problems, such as fibrosis and cirrhosis34.
Hence, the current study suggests that EBV co-infection decreases the response of chronic HCV patients to IFN therapy.
Recommendations
Chronic infection with HCV cannot be considered in isolation; as other pathogens such as EBV may have far-ranging effects against the prognosis of the disease. Patients with chronic HCV should be examined for co-infection with EBV.
Co-infection with EBV can deteriorate the response of chronic HCV patients to the antiviral treatment. EBV treatment should be considered when designing specific protocols for treatment of such patients.
Acknowledgments
We would like to express our heartfelt thanks to Dr. Omar Zayed for English editing of the manuscript and submission process .
Conflicts Of Interest
The authors declare that there are no conflicts of interest.
Authors’ Contribution
AZ, SM NM, LM and AA designed the experiments. AZ and SM performed the experiments. NM and LM analyzed the data and wrote the manuscript. AA supervised and reviewed the manuscript. AZ, SM NM, LM and AA read and approved the manuscript.
Funding
None.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
Informal written and oral approval was considered before the study from each patient. Study approval was issued and maintained by the local ethical committee at the National Hepatology and Tropical Medicine Research Institute.
- WHO (World Health Organization): Hepatitis C fact sheet No. 164 [Internet]. Geneva: World Health Organization; 2017. http://www.who.int/mediacentre/factsheets/fs164/ en/.
- WHO (World Health Organization): Guidelines for the screening, care and treatment of persons with Hepatitis C infection [Internet]. Geneva: World Health Organization; 2016. Available from: http://apps.who.int/iris/bitstream/10665/205035/1/9789241549615_eng.pdf.
- Esmat G. Hepatitis C in the Eastern Mediterranean Region. East Mediterr. Health J., 2013; 19: 587-588.
- El-Zanaty F., Way A. Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health, El-Zanaty and Associates, and Macro International, Cairo, 2009.http://dhsprogram.com/pubs/pdf/FR220/FR220.pdf.
- Ferenci P. Current treatment for chronic hepatitis C. Curr. Treat. Options. Gastroenterol, 2004; 7(6): 491–9.
- Martinot-Peignoux M., Marcellin P., Pouteau M., Castelnau C., Boyer N., et al. Pretreatment serum hepatitis C virus RNA levels and hepatitis C virus genotype are the main and independent prognostic factors of sustained response to interferon alfa therapy in chronic hepatitis C. Hepatology, 1995; 22: 1050-6.
- Palma T., Doonan P.B., Trager N.N., Kasman L.M. A systematic approach to virus-virus interactions. Virus Res., 2010; 149: 1-9.
- Gallegos-Orozco J.F., Rakela-Broder J. Hepatitis viruses: not always what it seems to be. Rev. Med. Chile, 2010; 138: 1302-1311.
- Mihaela P., Victor K. Epstein-Barr virus: Silent companion or causative agent of chronic liver disease. World J. Gastroenterol, 2010; 16: 4130-4134.
- Henry H., Balfour J.R., Priya V. Primary Epstein–Barr virus infection: impact of age at acquisition, co-infection and viral load. J. Infect. Dis., 2013; 207: 1787-1789.
- Petrova M., Kamburov V., Nikolovska D., Kosseva O., Nikolova M., Krastev Z. Epstein-Barr virus: is there any contribution to chronic hepatitis B and C. Liver Int., 2010; 30: 488-489.
- Cohen J.I., Kimura H., Nakamura S., Ko Y.H., Jaffe E.S. Epstein-Barr virus-associated lympho-proliferative disease in non-immuno-compromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann. Oncol., 2009; 20: 1472-1482.
- Gandhi M.K., Tellam J.T., Khanna R. Epstein-Barr virus-associated Hodgkin’s lymphoma. Br. J. Haematol., 2004; 125: 267-281.
- Gredmark S, Jonasson L, Van Gosliga D, Ernerudh J, Soderberg-Naucler C. Active cytomegalovirus replication in patients with coronary disease. Scand. Cardiovasc J. 2007; 41: 230-234.
- Pawlotsky J.M. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology, 2014; 146: 1176-1192.
- Sarrazin C., Dvory-Sobol H., Svarovskaia E.S., Doehle B.P., Pang P.S., et al. Prevalence of Resistance-Associated Substitutions in HCV NS5A, NS5B, or NS3 and Outcomes of Treatment with Ledipasvir and Sofosbuvir. Gastroenterology, 2016; 151: 501-512.
- Benitez-Gutierrez L., Barreiro P., Labarga P., de Mendoza C., Fernandez Montero J.V., et al. Prevention and management of treatment failure to new oral hepatitis C drugs. Expert Opin-Pharmacother, 2016; 17: 1215-1223.
- Edwards D., Coppens D., Prasad T., Rooka L. and Iyera J.: Access to hepatitis C medicines. Bull. World Health Organ., 2015; 93: 799–805.
- Cobas Ampliprep Cobas TaqMan: COBAS AmpliPrep/COBAS TaqMan HCV Test, 2008. Retrieved from https://www.accessdata.fda.gov/cdrh_docs/pdf6/P060030c.pdf
- Zitzer H., Heilek G., Truchon K., Susser S., Vermehren J., Sizmann D., et al.: Second-Generation CobasAmpliPrep/CobasTaqMan HCV Quantitative Test for Viral Load Monitoring: a Novel Dual-Probe Assay Design. J. ClinMicrobiol., 2013; 51(2): 571–577.
- Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology; 1996; 24:289-293.
- Gerakari S., Zouboulis-Vafiadis I., Chrysos G., Daikos G., Themeli-Digalaki K., et al. The effect of co-infection by hepatotropic and hepatomimetic viruses in physical evolution of HCV hepatitis. The Internet Journal of Infectious Diseases, 2011; 9: 1-9.
- Bader El-Din N.G., El-Meguid M.A., Tabll A.A., Anany M.A., Esmat G., et al. Human cytomegalovirus infection inhibits response of chronic hepatitis C virus infected patients to interferon-based therapy. J. Gastroentero.Hepat., 2011; 26: 55-62.
- Sahar S., Mohamed N., Ashraf T., Hussam G., Sherif E. Assessment of immunological changes in Epstein-Barr virus co-infection in Egyptian chronic HCV patients. Mem. Inst. Oswaldo Cruz, Rio de Janeiro, 2014; 109: 722-727.
- Strnad-Trojan N., Linde R., Reichenbach J. Trojan J., Zeuzem S., Zielen S.: Treatment of HCV infection with interferon alpha-2b and ribavirin in a patient with X-linked lymphoproliferative syndrome. Eur. J. Pediatr., 2006; 165: 348–350.
- Sievert W., Altraif I., Razavi H., et al. Asystematic review of hepatitis C Virus epidemiology in Asia, Australia and Egypt. Liver International, 2011; 3223: 61-80.
- Sugawara Y., Makuuchi M., Kato N., Shimotohno K., Takada K. Enhancement of hepatitis C virus replication by Epstein-Barr virus-encoded nuclear antigen 1. EMBO, 1999; 18: 5755-5760
- Morrison T.S., Mauser A., Wong A. Inhibition of IFN-g signaling by EBV immediate-early protein. Immunity, 2001; 15: 787-797.
- Jardi R., Rodriguez F., Buti M., Costa X., Cotrina M., Galimany R., et al. Role of hepatitis B, C, and D viruses in dual and triple infection: In uence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral. Hepatology, 2001; 34: 404-410.
- Challine D.., Buisson, Cadilhac M., et al. Hepatitis C Virus Epstein-Barr Virus interaction in patients with AIDS. Journal of Medical Virology, 2002; 67(4): 510-515.
- Hayashi J, Kishihara Y., Ueno K., Yamaji K., Kawakami Y., Furusyo N., et al.: Age-Related Response to Interferon Alfa Treatment in Women Vs Men with Chronic Hepatitis C Virus Infection. Arch. Intern. Med., 1998; 158(2): 177-181. doi: 10.1001/archinte.158.2.177.
- Gao B., Hong F., and Radaeva S. Host Factors and Failure of Interferon Treatment in Hepatitis C Virus. Hepatology, 2004; 39: 880–890.
- Iqbal S., Khalil-Ur-Rahman, Sheikh M.A., Arshad M. Response of different HCV genotypes to interferon therapy in different age groups of chronic Hepatitis-C patients.J. Ayub Med. Coll. Abbottabad, 2014; 26: 310-315.
- Ginaldi L., Loreto M.F., Corsi M.P., Modesti M., De Martinis M. Immunosenescence and infectious diseases. Microbes. Infect., 2001 3: 851–857.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.