The use of metallic nanoparticles (NPs) in various industrial and biomedical fields is increasing exponentially. As a result, research examining the potentially toxic impact of these NPs on human health is also increasing. Cytochrome P450 (P450s) enzymes are important for the endogenous and exogenous molecules metabolism. Inhibition or induction of these enzymes affects xenobiotic detoxification and causes clinically significant drug toxicity or therapeutic failures. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are the most frequently used biomarker for liver injury and their induction is an important indicator of hepatotoxicity. This review aims to understand the existing literature relevant to the effect of metallic NPs on P450s, ALT and AST (aminotransferases) enzymes. It was found that the predominant effect of metallic NPs is the inhibition of the CYP 450 gene and protein expression and induction of aminotransferases, which highlights their potential interaction and induction of drug-associated toxicity as well as their hepatotoxicity. However, further studies are recommended to investigate the effect of NPs size, morphology, surface area, charge, and NPs coating on the expression of these enzymes.
Cytochrome P450, Alanine Aminotransferases, Aspartate Aminotransferases, Metallic Nanoparticles, Induction, Inhibition
Nanotechnology is the scientific branch that is concerned with understanding and applications of materials and assemblies of Nano-size (1-100 nm). This is because these materials have structural, optical, electronic, and magnetic features not found in their macromolecule counterparts.1 Its tiny size and large surface area that increase solubility and bioavailability, and the materials biodegradability of the majority of NPs have all contributed to its great efficacy and safety as a drug delivery vehicle.2
Among the many distinct types of nanomaterials that have been employed for drug delivery, metal-based NPs have captured the interest of scientists because of their specific features (For example, high stability, adjustable shape, porosity, easy method of preparation, simple surface modification, etc.).3 Metal-based NPs are frequently used in therapeutic areas such as wound healing, cosmetic applications,4 cancer therapy, tissue engineering,5 biosensing, and bioimaging.6 Additionally, metal-based NPs offer improved bioavailability, controlled release, and targeted delivery.7
As it is for carbon, organic, and composite-based NPs, elevated human exposure to metal-based NPs has demonstrated a potential hazard to human health. After being ingested, inhaled, or coming into contact with the skin, NPs are prone to build up in sensitive organs like the heart, liver, spleen, kidney, and brain. The main mechanism causing toxicity is the enhancement of the production of reactive oxygen species.8
The liver is the most frequent organ exposed to NPs and the main organ for their detoxification. The large surface-to-volume ratio of NPs could make them more toxic. After penetration, they result in many pathological mechanisms in the liver such as oxidative stress, histopathological alteration, genotoxicity, and inflammation. These mechanisms disrupt the activity of a wide range of liver enzymes including antioxidant enzymes such as catalase, superoxide dismutase, glutathione S-transferase, glutathione reductase, and glutathione peroxidase, alkaline phosphatase (ALP), ALT, and AST. Along with the cytochrome enzymatic system which is the most responsible for liver detoxification ability.9
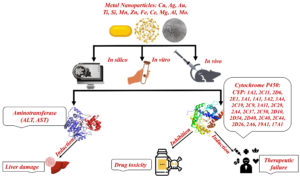
This review focused on the available literature studies on the effect of metal-based NPs on the aminotransferase and P450s enzymes, and thus on their potential effect on drug therapeutic response, drug-associated toxicity, and liver toxicity (Figure).
Figure. Schematic representation of the literature topics studying the effect of metal-based NPs on the aminotransferase and P450s enzymes, and the potential health concern.
Interactions between metallic NPs and cytochrome P450
Human CYPs are a group of functional enzymes primarily expressed in the liver. There are at least 57 different cytochrome enzymes, grouped into 18 families and 43 subfamilies. CYP 450 enzymes are crucial in metabolism of drug, detoxification of xenobiotic, in addition to metabolism of endogenous molecules like steroids and fatty acids. About 80% of clinical drugs are metabolized mainly by the isoforms from the CYP1, 2, and 3 families. External factors, including food, drugs, tobacco, and alcohol, can either inhibit or induce the expression of CYPs and hence affect drug metabolism and the individual response to a drug.10,11 As shown in Table 1, several in vivo, in vitro, and in silico studies have been carried out to investigate the effect of different metallic NPs on P450s enzymes activity.
Table (1):
Metallic NPs effects on CYP activity in in vivo, in vitro, and in silico
NPs |
Size (nm) |
Morphology |
Zeta potential (mV) |
CYP |
Source of CYP |
Effects |
Ref |
|---|---|---|---|---|---|---|---|
Cu |
80 |
Spherical |
NA* |
1A2 2C11 2D6 2E1 3A1 |
In vivo: Male Sprague-Dawley rats liver |
High dose: inhibit mRNA and protein expression (all the studied CYPs) Low dose: enhance mRNA expression (CYP 2E1 and 3A1) |
[12] |
80 |
Spherical |
NA |
1A1 2C11 2D6 2E1 3A1 |
In vivo: Male Sprague-Dawley rats kidney |
Inhibit mRNA and protein expression |
[13] |
|
80 |
Spherical |
NA |
2C11 3A1 1A1 2D6 |
In vivo: Male Sprague-Dawley rats brain |
Inhibition of most CYP450 enzyme expression |
[14] |
|
80 |
Spherical |
NA |
1A2 2C11 2D6 2E1 3A2 |
In vivo: Male Sprague-Dawley rats liver |
Inhibition of gene expression and enzymes activity |
[15] |
|
Ag |
12.42±2.48 |
Spherical |
-43.6±0.7 |
3A4 2C19 2C9 1A2 |
Human CYP450 |
Dose dependent inhibition, the greatest inhibition for CYP3A4 |
[16] |
NA |
NA |
NA |
1A2 2C9 2C19 2D6 2E1 3A4 |
In silico: Molecular docking and quantum mechanical (QM) calculations |
CYP: 2C9, 2C19, and 2D6 strongly interact with Ag3 clusters at a distance of 3 Å |
[18] |
|
35 |
Spherical |
NA |
CYP450 |
In vivo: Aedes aegypti carcasses |
Significant increase |
[19] |
|
15-35 |
Spherical |
NA |
3A11 2C29 |
In vivo: BALB/C mice liver |
Genes of CYP: 3A11 and 2C29: Significant inhibition |
[20] |
|
Commercial colloidal Ag |
25–40 |
NA |
NA |
1A2 2C9 2C19 3A4 2E1 |
Human volunteers serum |
No significant changes |
[17] |
Au |
15±5 |
Spherical |
NA |
1A1 2E1 2D6 |
In vivo: Male rats liver |
Enzymes: CYP: 1A1 and 2E1: significant inhibition CYP2D6: Elevation |
[22] |
10 |
Spherical colloidal monodisperse |
NA |
3A11
2C29 |
In vivo: Mice liver |
Genes: CYP: 3A11 and 2C29: dramatic inhibition |
[20] |
|
Tannic acid-stabilized Au |
5-100 |
Spherical |
−21.3- −36.1 |
2C9 2C19 2D6 3A4 1A2 |
Human liver microsomes (HLMs) |
Enzymes: suppression in concentration, size, and time dependent manner |
[21] |
Polyethyleneimine modified Au |
6.4 ± 0.5 |
Spherical |
13.9 ± 1.4 |
2A4 2C37 2C50 2D10 2D34 2D40 1A2 2C40 2C44 2D26 2E1 3A11 |
In vivo: Mice blood |
Enhancement of hepatic gene expression of CYP: 2A4, 2C37, 2C50, 2D10, 2D34, 2D40, No significant change: CYP: 1A2, 2C40, 2C44, 2D26, 2E1, 3A11 |
[23] |
CA coated Au, PEG coated Au, and CS coated Au |
6.2 ± 0.6, 6.5 ± 0.2, and 6.4 ± 0.5 |
Spherical |
−15.4 ± 2.5, − 10.4 ± 0.6, and 12.5 ± 1.0 |
2A4 2C37 2C50 2D10 2D34 2D40 |
In vivo: Mice liver |
Enzymes: increase level by CA coated and PEG-coated NPs, and no change by CS coated NPs |
[24] |
TiO2 |
NA |
NA |
NA |
2E1 |
In vivo: Rats liver fraction |
Enzyme: CYP2E1: no change in the activity |
[25] |
98.87 |
NA |
NA |
CYP 450 |
In vivo: Vitex agnus-castus leaf tissue |
Enhance CYP 450 gene expression |
[26] |
|
60 ± 10 nm |
NA |
− 27 ± 2.5 |
2E1 |
In vivo: Wistar male albino rats liver |
Significant decrease in CYP 2E1 level |
[27] |
|
PSi: TCPSi, APTES-TCPSi, and Alkyne-THCPSi |
159, 176, and 184 |
Irregular shapes |
-30, +35, and
-30 |
1A2 2A6 2D6 3A4 |
In vitro: Human liver microsomes |
Considerably decreased the enzymes activity |
[28] |
SiO2 |
10-30 |
NA |
NA |
19A1 17A1 |
In vivo: Wistar albino Male Rats testes |
Genes were significantly inhibited |
[29] |
Mn3O4 |
10-25 |
NA |
NA |
1A2 |
In vivo: rats liver |
Upregulation of CYP1A2 gene and enzyme |
[30] |
ZnO |
30 |
Rod to spherical |
NA |
CYP450 |
In vivo: Male Wistar rats spleen |
Dose-dependent manner stimulation of CYP450 |
[31] |
35.65 ± 6.63 |
Near-spherical |
Negative |
CYP450 |
In vivo: Mice liver |
Overexpression of CYP450 enzymes |
[32] |
|
25 |
Spherical |
NA |
CYP450 |
In vivo: Lactating Wistar rats liver |
Significant inhibition of P450s reductase in rats offspring liver tissue |
[33] |
Copper NPs
Huaqiao and colleagues carried out an in vivo study using male Sprague-Dawley rats fed orally with copper NPs (Cu-NPs) for seven days. Cu-NPs (80 nm) in 400 mg/kg daily dose significantly inhibit the expression of the mRNA and the activity of the liver enzymes CYP 2C11, 2E1, 1A2,2D6, and 3A1. However, CYP 2E1 and 3A1 mRNA levels were increased by 100 mg/kg dose of nano-copper.12 The same research group also demonstrated significant inhibition in the level of rats renal activity and mRNA of CP450 enzymes by oral Cu-NPs (80 nm, 200 mg/kg). This effect on CYP450 was associated with the inhibition of nuclear receptors and induction of STAT3/5, Akt, p70S6K, CREB, P38, ERK1/2, and NF-kB signaling pathways. Based on this finding, they believe that by the induction of oxidative stress and inflammatory response in rat kidneys, Cu-NPs can induce TAT, MAPK, and NF-kB Band therefore inhibit CYP450 enzymes.13 The same results were obtained when the research group continued their work to investigate the Cu-NPs effect on CYP 450 in rat brain. Reduction in most of CYP450 enzyme expression in response to high Cu-NPs dosages (80 nm, 200 mg/kg).14 Oral administration of 200 mg/kg/day Cu-NPs to male Sprague–Dawley rats resulted in down regulation of hepatic gene expression, protein, and activity for CYP450 1A2, 3A2, 2C11, 2E1, and 2D6. The signaling pathways of NF-B, MAPK, and STAT5 were found to be involved in the molecular mechanisms causing these effects.15
Silver NPs
The in vitro effect of silver nanoparticle (Ag-NPs) 12.4 nm on human CYP450 was examined by Warisnoicharoen and colleagues. Dose-dependent Inhibition of the CYP450 enzymes activity was observed. CYP3A4 isoform showed the greatest inhibition with 13.52 µM IC50 value followed by CYP2C19, CYP2C9, and CYP1A2 with 14.31 µM, 26.46 µM, and 43.51 µM IC50 values, respectively.16 In a prospective, single-blind controlled study, two weeks of oral administration of a commercial colloidal Ag-NPs product to human volunteers result in a detectable silver in human serum. However, this silver did not produce any significant changes in CYP 450 1A2, 2C9, 2C19, 3A4, and 2E1.17 Nootcharin and colleagues consider the molecular docking and QM calculations to understand the mechanism of deep interaction between Ag-NPs and CYP 450 enzymes specific inhibitor-binding pocket. Among the investigated isoforms (CYP1A2, CYP3A4, CYP2E1, CYP2D6, CYP2C9, and CYP2C19), CYP2D6, CYP2C9, and CYP2C19key amino acidsVal370y, Leu362, and Ile362, respectively, were found to strongly interact with Ag3 clusters at a distance of 3Å.18 After 12 days of exposure to 8 and 10 ppm of Ag-NPs (35 nm) extract from the shell of Cocos nucifera in the second and third instars, respectively, the level of P450s in Aedes aegypti considerably increased.19 Sharp and significant down regulation in the cyp3a11 and cyp2c29 genes expression was observed through real-time polymerase chain reaction for liver biopsies from BALB/C mice subjected to 2 mg/kg/day of intraperitoneal (IP)Ag-NPs (15–35 nm) for 21 days.20
Gold NPs
An in vitro study conducted by Meiling and colleagues research group showed that tannic acid-stabilized gold nanoparticles (Au-NPs) could irreversibly suppress the enzymes CYP3A4, CYP2D6, CYP2C9, CYP2C19, and to a lesser extent, CYP1A2 in a time, size, and concentration-dependent manner. It was also demonstrated that the level of inhibition is determined by the ratio of microsomal protein (the source of CYP450 enzymes)/NP. However, due to the possibility of metabolite and/or probe substrate adsorption to NPs, the observed metabolite production in the in vitro model may not accurately reflect the activity of the enzyme.21 In male rats, the liver enzymes CYP1A1, CYP2E1, and CYP2D6 were found to be significantly inhibited by a low oral dose of Au-NPs 15 nm (4 mg/Kg) for 10 days.22 Intravenous (IV) administration of polyethyleneimine modified Au-NPs(6.4 nm) to mice in 11.5 and 23µg for 1 and 7 days, respectively, led to enhancement of hepatic gene expression of the enzymes Cyp: 2a4, 2c37, 2c50, 2d10, 2d34, 2d40, while no significant alteration in the genes of Cyp: 1a2, 2c40, 2c44, 2d26, 2e1, and 3a11was observed.23 Shuang group studied the effect of Au-NPs coated with either citric acid (CA), polyethyleneglycol (PEG), or chitosan (CS) on the expression of P450s after IV administration of 60-120 µg/mouse for 1 day and 1 week. In this study, mice exposed to a high dose (120 µg/mouse) of CA-coated NPs for 24 hours exhibited elevation of the Cyp: 2a4,2c37, 2c50, 2d10, 2d34, and 2d40 isoforms by 2.5, 13.3, 17.9, 36.0, 14.2, and 33.7 fold, respectively. Then the levels increased dramatically after 7 days of exposure. Similarly, PEG-coated NPs was found to induce these isoforms in mice. However, the levels of these isoforms were neither increased nor decreased in mice treated with chitosan-coated NPs.24 Cyp3a11 and cyp2c29 genes were dramatically inhibited in mice exposed to 2 mg/kg/day of IPAu-NPs (10 nm)for 21 days.20
Titanium dioxide NPs
Pan and colleagues examined Titanium dioxide nanoparticles (TiO2-NPs) effect on CYP2E1 in fraction of rat liver using four concentrations (0.5, 1, 5, and 10 ppm). Measurement of formaldehyde using Nash’s reagent indicated no change in CYP2E1 activity when compared with the control.25 Another study reported the impact of three concentrations of TiO2-NPs (zero, 200, and 800 µg/ml) on CYP 450 gene expression in vitex agnus-castus L. Real-time PCR demonstrated an increase in CYP 450 gene expression by 800 µg/ml concentration.26 In an in vivo study using Wistar male albino rats fed orally with TiO2-NPs (≤ 100 nm) 600 mg/kg for five days demonstrated a significant decline in CYP 2E1 level.27
Silicon NPs
Three different forms of porous silicon nanoparticles (PSi-NPs), including aminopropylsilane-modified Psi, alkyne-terminated thermally hydrocarbonized Psi, and thermally carbonized PSi, were developed and examined against CYP isoforms in human liver microsomes. The study’s findings showed that these PSi-NPs considerably decreased the activity of (CYP1A2, CYP2A6, CYP2D6, and CYP3A4) isoforms.28 The levels of Cyp19a1 and Cyp17a1 genes were significantly inhibited in testes of Wistar albino Male Rats treated orally with 1, 10, and 100 mg/kg body weight/day Silica oxide (SiO2-NPs) 10-30 nm for 22 days. The purpose of this study was to examine the effect of SiO2NPs on the reproductive performance of rats as the testes Cyp19a1 and Cyp17a1 genes are responsible for steroid hormones synthesis and testosterone construction.29
Manganese oxides NPs
Sustained Manganese oxide nanoparticles (Mn3O4-NPs) exposure was found to be responsible for the elevation of CYP1A2. IP administration of 20 mg/Kg/Week/ 2 or 4 months of Mn3O4- NPs 15 nm into the rats indicated upregulation of CYP1A2 gene after 4 months of treatment as indicated by transcription profile analysis and upregulation of CYP1A2 enzyme as indicated by immunohistochemistry assay.30
Zinc NPs
Zinc oxide nanoparticles (ZnO-NPs) 30 nm demonstrated dose-dependent manner stimulation of CYP450 in spleen tissue of male Wistar rat treated with either 50 or 250 mg/kg/week IP for 4 weeks.31 Oral administration of ZnO-NPs to mice at a 25 mg/kg daily dose for 12 weeks resulted in over expression of CYP450 in mice’s liver.32 Lactating Wistar rats treated orally with ZnO-NPs 25 nm (50 mg/kg/day) for 19 days exhibited significant inhibition of P450s reductase in rats in their offspring liver tissue.33
Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST)
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes are two of the liver biochemical tests that are most frequently requested in both inpatient and outpatient settings. ALT is mainly found in hepatocytes (with lesser amount in cardiac, renal, and muscular tissue) and is therefore unique to hepatic injury. It promotes the synthesis of pyruvate and glutamate in the liver which are crucial for production of energy. For males, the normal ALT level is 29-33 IU/L, while for females, it is 19-25 IU/L. Similar to ALT, AST can also be found in the liver but it is more abundant than ALT in heart, brain, renal tissue, and skeletal muscles. AST promotes the metabolism of amino acids and its normal level is < 35 IU/L. In liver diseases, the hepatocytes release the aminotransferase resulting in the elevation of their serum levels.34 Metallic NPs have been evaluated on their ability to induce aminotransferase enzymes by employing various in vivo studies (Table 2).
Table (2):
Metallic NPs effects on aminotransferases activities in vivo
NPs |
Size (nm) |
Morphology |
Zeta potential (Mv) |
Surface area (m2/g) |
Source of aminotransferases activities in vivo |
Effects |
Ref |
|---|---|---|---|---|---|---|---|
CuO |
60-100 |
Irregular morphology |
NA |
NA |
Albino mice serum |
Significant elevation |
[35] |
<20 |
NA |
NA |
NA |
Female rats serum |
Increased the level |
[36] |
|
NA |
NA |
NA |
NA |
Rats serum |
Elevation |
[38] |
|
Cu |
17-41 |
Spherical |
NA |
NA |
BALB/c mice serum |
No significant elevation |
[37] |
Ag |
10-30 |
Spherical |
NA |
NA |
Male Wistar rats blood |
No significant difference in activities |
[39] |
20 |
NA |
NA |
Sprague–Dawley rats serum |
Increase ALT enzyme |
[40] |
||
NA |
NA |
NA |
NA |
Rainbow trout (Oncorhynchus mykiss) hematological parameters |
Significant elevation |
[41] |
|
20-60 |
Spherical |
NA |
NA |
Albino rats serum |
Significant elevation |
[42] |
|
10 |
Spherical |
-33.2 |
NA |
Sprague-Dawley rats serum |
Significant elevation |
[43] |
|
40 |
NA |
NA |
NA |
Male and female BALB/c mice serum |
Significant elevation |
[44] |
|
43.60±6.40 |
NA |
-23.8 |
Swiss albino mice serum |
Significant elevation |
[45] |
||
200-300 |
NA |
NA |
NA |
Male Sprague-Dawley rats serum |
No significant change |
[46] |
|
NA |
NA |
NA |
NA |
Male Wistar rat serum |
Significant elevation |
[48] |
|
<30 |
Spherical |
NA |
NA |
Adult zebrafish liver tissue |
Elevation |
[49] |
|
20-40 |
NA |
NA |
NA |
C. gariepinus blood |
Significant elevation |
[50] |
|
Ag, LMWC-coated Ag, PVP-coated Ag, |
10–30 |
Spherical |
NA |
NA |
Balb/c mice serum |
The most significant inhibition is seen with LMWC-Ag-NPs, followed by PVP-coated Ag NPs. Free Ag-NPs showed the highest values of aminotransferases |
[47] |
Au |
25 |
NA |
NA |
NA |
Male Wistar rats serum |
AST enzyme: increased ALT enzyme: decreased |
[51] |
10 |
NA |
NA |
NA |
Male Wistar rats serum |
Significant elevation |
[54] |
|
-Solid Au
-Porous Au |
35
28 |
Granular |
-51-44.4 |
NA |
Male rabbits serum |
-No effect -Elevation of AST enzyme only |
[52] |
PEG-coated Au |
15 to 35 |
Spherical |
-14.5 |
NA |
Sprague Dawley rats serum |
Greeter induction than uncoated AU-NPs |
[53] |
TiO2 |
100 |
Tetragonal |
NA |
NA |
Mice serum |
Significant elevation |
[55] |
10-15 |
Spherical |
NA |
100-150 |
Male Sprague-Dawley rats serum |
Significant elevation of ALT enzymes No significant change of AST enzyme |
[56] |
|
50-100 |
Spherical |
NA |
NA |
Male Wistar rats serum |
Significant elevation |
[57] |
|
NA |
NA |
NA |
NA |
Male rats serum |
Significant elevation |
[58] |
|
28.8 |
Spherical |
NA |
46.45±2.32 |
Clarias gariepinus serum |
Elevation |
[59] |
|
Si |
70, 300, and 1000 |
Spherical and nonporous |
NA |
NA |
Mice serum |
300 and 1000 nm NPs: no change 70 nm: significant increase |
[60] |
30, 50, and 70 |
Spherical and nonporous |
NA |
NA |
BALB/c male mice serum |
30 nm: the most significant elevation |
[61] |
|
50 |
Amorphous |
<−30 |
NA |
Male Tuck-Ordinary mice |
Significant elevation |
[62] |
|
150 |
Near-spherical |
NA |
NA |
Male Balb/C mice serum |
No significant elevation |
[63] |
|
SiO2 |
NA |
NA |
NA |
NA |
Freshwater fish Oreochromis mossambicus liver tissue |
Decrease |
[64] |
ZnO |
20 |
NA |
-30.9 |
50 |
Sprague Dawley rats serum |
Decrease |
[65] |
<35 |
Polygonal |
35.5 |
NA |
Freshwater snail Biomphalaria alexandrina soft tissue and hemolymph |
Elevation |
[66] |
|
< 100 |
NA |
NA |
NA |
Male Wistar rats serum |
Significant elevation |
[67] |
|
Zn
ZnO |
90.0 ± 2.0
95.0 ± 2.0 |
NA |
NA |
5.34
4.5 – 6.0 |
Male albino Wistar rats serum |
ZnONPs showed greater elevation |
[68] |
ZnO |
≤ 100 |
Spherical and rod |
NA |
NA |
Nile tilapia, Oreochromis niloticus serum |
Significant elevation |
[69] |
20-40 |
NA |
NA |
NA |
Female Swiss albino rats serum |
Elevation |
[70] |
|
NA |
NA |
NA |
NA |
Albino rats serum |
Significant elevation |
[71] |
|
Fe3O4 |
30 |
Spherical |
–18.6 |
NA |
Female Wistar rats serum, liver, and kidney |
Significant elevation in serum and liver
Downregulation in kidney |
[73] |
20-30 |
NA |
NA |
NA |
BALB/c mice serum |
Significant elevation |
[74] |
|
30 |
Spherical |
-10.6 |
NA |
Male Wistar rats blood |
Significant elevation of ALT enzyme |
[77] |
|
NA |
NA |
NA |
NA |
Carp fry serum |
Elevation |
[78] |
|
Dextran-coated Fe3O4 |
9.12±1.46 |
Spherical |
-7.87 |
NA |
Wistar rats serum |
No significant change |
[75] |
Bare coated Fe3O4
And PEG coated Fe3O4 |
15-30 |
NA |
NA |
NA |
Male albino rats serum |
More significant elevation with bare-coated NPs |
[76] |
Oleic acid-Pluronic-coated Fe3O4 |
11±2 |
NA |
-0.22 |
NA |
Male Sprague–Dawley rats serum |
Transient increase |
[72] |
polyacrylic acid polymer coated CeO2 |
10 |
NA |
-33–41 |
NA |
Female Wistar rats serum |
No change |
[79] |
poly acrylic acid polymer coated-CeO2 |
<10 |
NA |
NA |
NA |
Male BALB/c mic serum |
Decrease ALT activity |
[80] |
MgO |
10-15 |
Spherical |
NA |
NA |
Male Wistar rats |
– Significant elevation in AST level – No change in ALT level |
[81] |
NA |
NA |
NA |
NA |
Male Sprague‐Dawley rats serum |
No significant change |
[82] |
|
κ-carrageenan coated selenium |
15− 27 |
Spherical |
NA |
NA |
Wistar rats serum |
Normalize level of both enzymes in liver-intoxicated rats Increase level of AST in healthy rats |
[83] |
Aluminum oxide |
NA |
NA |
NA |
NA |
Albino rats serum |
Elevation |
[84] |
Molybdenum and molybdenum oxide |
NA |
NA |
NA |
NA |
Wistar rats serum |
Elevation |
[85] |
Copper NPs
In an in vivo study, IV administration of half the determined lethal dose (225 mg/kg) of copper oxide nanoparticles (CuO-NPs) (60 to 100 nm) daily for 4 days to albino mice significantly elevated the level of ALT up to 105.67 U/L and AST level up to 306.00 U/L.35 Another study was conducted by Arafaa and colleagues to assess the role of quercetin in reducing the CuO-NPs-induced liver toxicity in female rats. IP administration of CuO-NPs (>20 nm) in doses of 3 mg/kg or 50 mg/kg for one week increased the level of AST and ALT by 16.15, 30.03, or 20.77, 61.42 folds, respectively.36 Interestingly, no significant elevation in aminotransferases activities in vivo enzymes levels was observed in BALB/c mice after administration of the greenly synthesized Cu-NPs (17 and 41 nm) at 1, 2, and 5 mg/kg/day oral doses for two weeks.37 A study similar to Arafaa and colleagues’ study was performed by Yousef and colleagues to evaluate the protective activity of Crocin, the main active principle in saffron, against CuO-NPs-induced hepatotoxicity in rats. In this study, the elevation aminotransferases activities in vivo liver enzymes along with other biomarkers were used as indicators for CuO-NPs-induced rat intoxication.38
Silver NPs
In an in vivo assay employing Male Wistar rats treated topically with Ag-NPs gel (1% m/v, nano-particles size of 10-30 nm) four times a day/28 days, to evaluate their activity to heal a thermally induced burn wound in rat dorsum, no significant change was shown in the level of aminotransferases activities in vivo enzymes in rats blood.39 Feeding of Sprague–Dawley rats with 500 mg/d/kg BW Ag-NPs (20 nm) along with the standard diet for 81 days led to a 12% increase in plasma ALT enzyme level.40 To evaluate Ag-NPs effect of on the aquatic environment, Imani and colleagues employed rainbow trout (Oncorhynchus mykiss) hematological parameters following exposure to 0.1, 0.2, or 0.4 mg/l Ag-NPs solution. After 8 days of treatment, aminotransferases enzymes were significantly elevated with the 0.4 mg/l group experiencing the largest elevations of up to 42.2 and 502.5 for aminotransferases, respectively.41 Acute IP dosing (2,000 mg/kg) of Ag-NPs (20–60 nm) followed by another dose after two days to albino rats resulted in a considerable elevation of aminotransferases to 54.4, and 105 U/L, respectively.42 Acute toxicity of oral Ag-NPs was investigated by Patlolla and colleagues through the administration of high doses(50 or 100 mg/kg/d) of Ag-NPs (10 nm) to Sprague-Dawley rats over a short period of 5 days. According to optical density values, this resulted in significant elevation (< 0.05) of aminotransferases.43 Heydrnejad and colleagues reported that oral administration of Ag-NPs 40 nm (20 or 50 ppm/d/2wks) significantly elevated aminotransferases levels in the serum of both male and female BALB/c mice.44 Swiss albino mice treated to IP 26, 52, or 78 mg/kg of Ag-NPs 43.60 nm for 3 days had significantly elevated aminotransferases levels in their serum, with ALT levels being the highest.45 However, no significant alteration was shown in aminotransferases serum levels in male Sprague-Dawley rats orally exposed to 30, 125, 300, or 700 mg/kg Ag-NPs for 28 days.46 In their study, Peng and colleagues compared the effects of the free uncoated Ag-NPs to those coated with low molecular weight chitosan (LMWC) or polyvinylpyrrolidone (PVP) on the liver toxicity indices aminotransferases. The LMWC-Ag-NPs, followed by the PVP-Ag-NPs demonstrated the most considerable lower levels of aminotransferases, while uncoated NPs displayed the highest values.47 Aminotransferases concentrations in male Wistar rat serum demonstrated a considerable increase following 25 mg/kg of IP Ag-NPs for two weeks.48 A comparative examination of the effects of the feeding of Ag and Au-NPs on adult zebrafish was conducted by Ramachandran and colleagues. They reported that zebrafish exposed to half the determined lethal concentration of Ag-NPs (12.25 µg/L) for 14 days expressed higher liver tissue aminotransferases than those exposed to half the determined lethal concentration of Au-NPs (20.5 mg/L).49 Another study showed that C. gariepinus treated with 100 g/L Ag-NPs for 15 days resulted in a considerable rise in blood enzyme activity of aminotransferases.50
Gold NPs
Conflicting results were obtained for serum aminotransferases levels after the IV administration of Au-NPs 25 nm(0.3619 mg/ml/kg) to male Wistar rats for 3 days. While AST increased by up to 24%, ALT decreased by 43%.51 Another study examined the effects of IV administration of 1 mg/kg/day/3 days of two Au-NPs types—solid Au-NPs (SGNPs) and porous Au-NPs (PGNPs)—on aminotransferases in male rabbits. These two Au-NPs types are 35 nm and 28 nm, respectively. The only result that has been seen is an elevation in AST serum level following PGNPs.52 Patlolla and colleagues compared the effect of poly-ethylene-glycol-coated and free Au-NPs on Sprague Dawley rats serum aminotransferases after oral administration of four different doses for five days. A dose-dependent increase of both enzymes was observed and PEG-coated NPs demonstrated a greater extent of induction than uncoated NPs.53 As for the IP route, administration of 5 µg/2.85 ׳ 1011 Au-NPs 10 nm daily for one week considerably elevated the Wistar male rats serum levels of aminotransferases.54
Titanium dioxide NPs
Serum aminotransferases in mice exposed to different doses (324-2592 mg/kg) of IP TiO2-NPs 100 nm significantly increased after 14 days of administration.55 In another study, male Sprague-Dawley rats injected with different doses (30, 50, 70 mg/kg) and sizes (10-15 nm) TiO2 IP every other day for 3 weeks displayed a significant elevation in serum ALT (P< 0.001), while AST didn’t show considerable change.56 Orazizadeh and colleagues investigated the hepatoprotective effect of glycyrrhizic acid in male Wistar rats, the liver was intoxicated by gavage administration of 300 mg/kg of TiO2 (50-100 nm) for two weeks. Serum aminotransferases significantly elevated as compared to control untreated rats.57 In a similar investigation carried out by Hassaneina and colleagues’ research group to examine thymoquinone ability to protect against TiO2-NPs induced liver toxicity, male rats were intoxicated by a one-time oral dose (300 mg/kg) of TiO2-NPs. This led to a dramatic elevation of serum aminotransferases (P ≤ 0.001).58 TiO2-NPs 28.8 nm have been examined on their ability to induce aminotransferases in Clariasgariepinus. All concentrations used (1-10 mg/L) of TiO2-NPs were able to elevate the levels of aminotransferases in fish serum at all exposure periods (1, 4, and 7 days). The higher the concentration of TiO2-NPs the more pronounced the elevation of the levels of the enzymes.59
Silica NPs
Several diameters of silica nanoparticles (Si-NPs) (70, 300, and 1000 nm) were used by Nishimori and colleagues to assess the impact of NPs size on hepatotoxicity. The 300 and 1000 nm NPs were found to be safe even at higher doses, whereas mice injected IV with 30 mg/kg of 70 nm NPs had severe liver damage and an increase in aminotransferase serum level in a dose-dependent manner.60 Using smaller Si-NPs (30, 50, and 70 nm), the same research group carried out the same study. With the 30 nm size having the most impact, liver damage and an increase in aminotransferases were reported at all sizes in a dose-dependent manner.61 Acute liver toxicity of IP Si-NPs 50 nm was examined by administration of 0.25 mg/kg single dose to Male Tuck-Ordinary mice. Significant elevation of aminotransferases levels was observed with p-values of 0.01 and 0.005, respectively.62 In contrast, after 14 days of treatment with various single doses of Si-NPs of 150 nm(1,2.5,5,10,100,200, and 300 mg/kg), no significant elevation of serum aminotransferases levels was seen in male Balb/C mice.63 Further investigation employed the freshwater fish, Oreochromis mossambicus, the liver tissue aminotransferases expression declined after being exposed to 12 mg/L of SiO2-NPs for one month of.64
Zinc NPs
Pasupuleti and colleagues obtained surprising results after administration of different single oral doses of 5-2000 mg/kg ZnO-NPs20 nm to Sprague Dawley rats. Two weeks after treatment, aminotransferases levels have declined in a dose dependent manner.65 The freshwater snail Biomphalaria alexandrina was employed by Fahmy and colleagues to assess the ZnO-NPs impacts on aquatic ecosystems. The treated snails soft tissue and hemolymph aminotransferases levels increased after three weeks of exposure to sublethal quantities of LC10 (7µg/ml) and LC25 (35µg/ml).66 To test Hesperidin’s ability to protect against ZnO-NPs (< 100 nm) hepatotoxicity, male Wistar rats were administered a single 600 mg/kg of ZnO-NPs through IP. The serum activities of aminotransferases increased noticeably in the group that only received ZnO-NPs.67 When compared to rats that received comparable doses of Zn-NPs, those received ZnO-NPs showed a greater elevation in blood aminotransferases.68 In another study conducted to investigate the potentially hazardous effects of ZnO-NP ≤ 100 nm on aquaculture, it was demonstrated that exposure to ZnO-NP (50 mg/L) for 30 days significantly increased serum aminotransferases of Nile tilapia.69 A significant positive association was found between the doses of ZnO NPs (20 to 40 nm)fed to female Swiss albino rats and aminotransferases serum values after 28 days of daily administration.70 Another experiment was carried out to examine the ability of the natural active compound, Silymarin, to protect against ZnO-NPs-induced hepatotoxicity in albino rats. Rats that were orally intoxicated using ZnO-NPs (50 mg/kg) for 4 weeks demonstrated a remarkable rise in aminotransferases serum levels. ALT increased up to 240 U/L while AST reached the maximum of 360 U/L.71
Iron NPs
The IV injection of 10 mg Oleic acid-Pluronic-coated iron oxide (Fe3O4) MNPs to Male Sprague–Dawley rats caused a transient increase in aminotransferases serum levels after 24 hours of administration.72 The serum and liver aminotransferases levels were significantly increased in female Wistar rats following treatment with 1,000, and 2,000mg/kg oral dose of Fe3O4-NPs 30 nm. However, kidney aminotransferases levels were down regulated.73 BALB/c mice exposed to 150 and 300 g/gr dosages of Fe3O4-NPs (20-30 nm) by oral gavage had considerably higher levels of aminotransferases in their serum, while no significant elevations were observed with the lower doses.74 After 1,7,14, and 28 days of receiving a single dose of 10 mg/kg of dextran-coatedFe3O4-NPs via IV injection, no appreciable modulations were found in Wistar rats serum aminotransferases levels.75 Shakra and colleagues evidenced that coating of Fe3O4-NPs with either bare or PEG didn’t protect against hepatotoxicity and remarkable elevation of aminotransferases resulted after administration of 15 and 30 mg/kg dose via oral gavage to male albino rats daily for one month. The elevation was more pronounced with bare-coated NPs.76 Oral administration of 200 mg/kg single dose Fe3O4-NPs (30 nm) to male Wistar rats resulted in significant upregulation of blood ALT.77 Moreover, Fe3O4-NPs was found to upregulate aminotransferases expression serum levels of carp fry after being exposed to 0.15 mg/l of Fe3O4-NPs.78
Cerium NPs
Cerium oxide nanoparticles (CeO2-NPs) 10 nm have not been found to produce any change in aminotransferases serum levels of female Wistar rats injected IP with 200 mg/kg three times weekly for thirteen weeks.79 CeO2-NPs demonstrated hepatoprotective activity against diethylnitrosamine-induced livertoxicity. Pretreatment of male BALB/c mice with CeO2-NPs (<10 nm) IP at 100 and 200µg/kg daily for eight consecutive days decreased ALT activity by 24% and 23%, respectively.80
Magnesium NPs
In vivo study on male Wistar rats injected with Magnesium Oxide nanoparticles (MgO-NPs) 10-15 nm IP revealed a considerable increase in AST but not ALT at 250 and 500 µg/ml doses every other day for 28 days. But no significant change was observed at lower doses.81 As for orally administered MgO-NPs, male Sprague Dawley rats fed with 40 mg/kg MgO-NPs daily for one month did not demonstrate any remarkable alteration in serum levels of aminotransferases.82
Selenium NPs
Oral administration of a high dose (0.1 mg/g) of k-carrageenan coated selenium NPs to Wistar rats protects against tetrachloride-induced liver damage and normalizes the serum levels of aminotransferases after one week of administration. On the other hand, administration of the same dose to healthy rats increased the level of AST.83
Aluminium NPs
The serum levels of aminotransferases were elevated in albino rats treated with LC90 of aluminum oxide NPs.84
Molybdenum NPs
IP administration of molybdenum NPs at (1 and 25 mg/kg) and molybdenum oxide NPs at (1.2 and 29 mg/kg) increased the Wistar rat’s serum level of aminotransferase after 1, 7, 14, days of administration.85
Several in vivo, in vitro, and in silico studies were carried out to investigate the effects of wide range of metallic NPs on CYP 450 enzymes expressions and activities. The studied CYP 450 were important for drug metabolism, xenobiotic detoxification, and endogenous compounds biotransformation. It was suggested that the majority of metallic NPs have inhibitory effects on gene and protein expression CYP 450s involved in drug metabolism while some have stimulatory effects, which should increase clinical awareness of metallic NPs-drug interactions. It was demonstrated by one study that inhibition of CYP 450 by NPs could be mediated by induction of inflammatory response and oxidative stress. However, the mechanism of interaction between CYP 450 and metallic NPs is not fully understood.
As for the aminotransferase enzymes, most studies regarding their interactions with metallic NPs were based on in vivo studies on rodents. The predominant effect of metallic NPs was a significant elevation of the animals serum aminotransferase. The ALT and to lesser extent AST are applied as biomarker to monitor hepatotoxicity. Their elevation following exposure to metallic NPs highlighted the toxic effect of metallic NPs on liver. Based on the findings of some studies, there was no obvious pattern regarding how coating NPs affected the aminotransferase level.
More research is required to elucidate the effects of NPs characteristics (size, morphology, surface area, zeta potential, and surface coating), route of administration in in vivo studies, in addition to duration of exposure as possible factors able to manipulate the effect of NPs on CYP 450 and aminotransferase.
ACKNOWLEDGMENTS
None.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this review are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Teli MK, Mutalik S, Rajanikant GK. Nanotechnology and Nanomedicine: Going Small Means Aiming Big. Curr Pharm Des. 2010;16(16):1882-1892.
Crossref - Mazayen ZM, Ghoneim AM, Elbatanony RS, Basalious EB, Bendas ER, Pharmaceutical nanotechnology : from the bench to the market. Futur J Pharm Sci. 2022;8(1):12.
Crossref - Garcםa MC, Torres J, Cףrdoba AVD, Longhi M, Uberman PM. Drug delivery using metal oxide nanoparticles. Met Oxides Biomed Biosens Appl. 2022:35-83.
Crossref - Pan S, Goudoulas TB, Jeevanandam J, Tan KX, Chowdhury S, Danquah MK. Therapeutic Applications of Metal and Metal-Oxide Nanoparticles: Dermato-Cosmetic Perspectives. Front Bioeng Biotechnol. 2021;9:724499.
Crossref - Augustine R, Mathew AP, Sosnik A. Metal Oxide Nanoparticles as Versatile Therapeutic Agents Modulating Cell Signaling Pathways: Linking Nanotechnology with Molecular Medicine. Appl Mater Today. 2017;(7):91-103.
Crossref - Mitra J, Mitra J. Exploring the potential of metal oxides for biomedical applications. Metal Oxides for Biomedical and Biosensor Applications.2022:183-203.
Crossref - Kotrange H, Najda A, Bains A, Gruszecki R, Chawla P, Tosif MM. Metal and metal oxide nanoparticle as a novel antibiotic carrier for the direct delivery of antibiotics. Int J Mol Sci. 2021;22(17):95-96.
Crossref - Sengul AB, Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett. 2020;18(5):1659-1683.
Crossref - Waris A, Sharif S, Naz S, et al. Hepatotoxicity induced by metallic nanoparticles at the cellular level: A review. Environ Eng Res. 2023;28(5):220625.
Crossref - Zhao M, Ma J, Li M, et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int J Mol Sci. 2021;22(23):12808.
Crossref - Manikandan P, Nagini S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr Drug Targets. 2018;19(1):38-54, 2017.
Crossref - Tang H, Xu M, Shi F, et al. Effects and mechanism of nano-copper exposure on hepatic cytochrome P450 enzymes in rats. Int J Mol Sci. 2018;19(7):2140.
Crossref - Xu M, Tang H, Zhou X, et al. Effects and mechanisms of sub-chronic exposure to copper nanoparticles on renal cytochrome P450 enzymes in rats. Environ Toxicol Pharmacol. 2018;63:135-146.
Crossref - Wang Y, Tang H, Xu M, et al. Effect of copper nanoparticles on brain cytochrome P450 enzymes in rats. Mol Med Rep. 2019;20(1):771-778.
Crossref - Tang H, Xu M, Luo J, et al. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ Sci Eur. 2019;31(1):30.
Crossref - Warisnoicharoen W, Hongpiticharoen P, Lawanprasert S. Alteration in enzymatic function of human cytochrome P450 by silver nanoparticles. Res J Environ Toxicol. 2011;5(1):58-64.
Crossref - Munger MA, Hadlock G, Stoddard G, et al. Assessing orally bioavailable commercial silver nanoparticle product on human cytochrome P450 enzyme activity. Nanotoxicology. 2015;9(4):474-481.
Crossref - Wasukan N, Kuno M, Maniratanachote R. Molecular Docking as a Promising Predictive Model for Silver Nanoparticle-Mediated Inhibition of Cytochrome P450 Enzymes. J Chem Inf Model. 2019;59(12):5126-5134.
Crossref - Gomathi M, Prakasam A, Chandrasekaran, G. Gurusubramaniam, Revathi K, Rajeshkumar S. Assessment of Silver Nanoparticle from Cocos nucifera (coconut) Shell on Dengue Vector Toxicity, Detoxifying Enzymatic Activity and Predatory Response of Aquatic Organism. J Clust Sci. 2019;30(6):1525-1532.
Crossref - Jarrar Y, Al-Doaiss A, Alfaifi M, Shati A, Al-Kahtani M, Jarrar B. The influence of five metallic nanoparticles on the expression of major drug-metabolizing enzyme genes with correlation of inflammation in mouse livers. Environ Toxicol Pharmacol. 2020;80:103449
Crossref - Ye M, Tang L, Luo M, et al. Size- and time-dependent alteration in metabolic activities of human hepatic cytochrome P450 isozymes by gold nanoparticles via microsomal coincubations. Nanoscale Res Lett. 2014;9(1):642.
Crossref - Al-Hamadani MYI, Alzahrani AM, Yousef MI, Kamel MA, El-Sayed WM. Gold nanoparticles perturb drug-metabolizing enzymes and antioxidants in the livers of male rats: Potential impact on drug interactions. Int J Nanomedicine. 2020;15:5005-5016.
Crossref - Chen H, Zhou S, Zhu M, et al. Gold Nanoparticles Modified With Polyethyleneimine Disturbed the Activity of Drug-Metabolic Enzymes and Induced Inflammation-Mediated Liver Injury in Mice. Front Pharmacol. 2021;12:706791.
Crossref - Zhou S, Li X, Zhu M, et al. Hepatic impacts of gold nanoparticles with different surface coatings as revealed by assessing the hepatic drug-metabolizing enzyme and lipid homeostasis in mice. NanoImpact. 2020;20:100259.
Crossref - Cakmak NK, Zontul C. Determination of the impacts of titanium dioxide nanoparticles on a number of xenobiotic-metabolizing enzymes in rat liver. Sci J Mehmet Akif Ersoy Univ. 2020;3(3):77-83.
- Farahi SMM, Iranbakhsh A, Mahmoodzadeh H, Ebadi M. The effect of titanium dioxide nanoparticles on the relative expression of catalase, P450, SOD, diTDS and WRKY genes of Vitexagnus-castus L. Not Bot Horti Agrobot Cluj-Napoca. 2021;49(4):1-13.
Crossref - Fadda LM, Ali HM, Mohamed AM, Hagar H. Prophylactic administration of carnosine and melatonin abates the incidence of apoptosis, inflammation, and DNA damage induced by titanium dioxide nanoparticles in rat livers. Environ Sci Pollut Res. 2020;27(16):19142-19150.
Crossref - Ollikainen E, Liu D, Kallio A, et al. The impact of porous silicon nanoparticles on human cytochrome P450 metabolism in human liver microsomes in vitro. Eur J Pharm Sci. 2017;104:124-132.
Crossref - Al-khauzay HAL, Al-Husseini AMH. Effect of Silica Nanoparticles on level Cyp19a1and Cyp17a1genes in Male Rats. J Phys Conf Ser. 2019;1294(6).
Crossref - Yue Z, Zhang X, Yu Q, Liu L, Zhou X. Cytochrome P450-dependent reactive oxygen species (ROS) production contributes to Mn3O4 nanoparticle-caused liver injury. RSC Adv. 2018;8(65):37307-37314.
Crossref - Singh N, Das MK, Gautam R, Ramteke A, Rajamani P. Assessment of intermittent exposure of zinc oxide nanoparticle (ZNP)-mediated toxicity and biochemical alterations in the splenocytes of male Wistar rat. Environ Sci Pollut Res. 2019;26(32):33642-33653.
Crossref - Hu H, Guo Q, Fan X, et al. Molecular mechanisms underlying zinc oxide nanoparticle induced insulin resistance in mice. Nanotoxicology. 2020;14(1):59-76.
Crossref - Hussain A, Kumar S, Kaul G. Postnatal distribution of ZnO nanoparticles to the breast milk through oral route and their risk assessment for breastfed rat offsprings. Hum Exp Toxicol. 2020;39(10):1318-1332.
Crossref - Kalas MA, Chavez L, Leon M, Taweesedt T, Surani S. Abnormal liver enzymes: A review for clinicians. World J W J H Hepatol. 2021;13(11):1688-1698.
Crossref - Yaqub A, Anjum KM, Munir A, Mukhtar H, Khan WA. Evaluation of acute toxicity and effects of sub-acute concentrations of copper oxide nanoparticles (CuO-NPs) on hematology, selected enzymes and histopathology of liver and kidney in Musmusculus. Indian J Anim Res. 2018;52(1):92-98, 2018.
Crossref - A. Arafa, Ghanem H, Soliman M, EL-Meligy E. Modulation effects of quercetin against copper oxide nanoparticles-induced liver toxicity in rats. Egypt Pharm J. 2017;16(2):78.
Crossref - Khatami M, Ebrahimi K, Galehdar N, Moradi MN, Moayyedkazemi A. Green synthesis and characterization of copper nanoparticles and their effects on liver function and hematological parameters in mice. Turkish J Pharm Sci. 2020;17(4):412-416.
Crossref - Yousef DM, Hassan HA, Nafea OE, Abd El Fattah ER. Crocin averts functional and structural rat hepatic disturbances induced by copper oxide nanoparticles. Toxicol Res. 2022;11(6):911-919.
Crossref - Melo PS, Marcato PD, Huber SC, et al. Nanoparticles in treatment of thermal injured rats: Is it safe? J Phys Conf Ser. 2011;304(1):012027.
Crossref - Elle RE, Gaillet S, Vide J, et al. Dietary exposure to silver nanoparticles in Sprague-Dawley rats: Effects on oxidative stress and inflammation. Food Chem Toxicol. 2013;60:297-301.
Crossref - Imani M, Halimi M, Khara H. Effects of silver nanoparticles (AgNPs) on hematological parameters of rainbow trout, Oncorhynchusmykiss. Comp Clin Path. 2015;24(3):491-495.
Crossref - Sarhan OMM, Hussein RM. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int J Nanomedicineicine 2014;9(1):1505-1517.
Crossref - Patlolla AK, Hackett D, Tchounwou PB. Silver nanoparticle-induced oxidative stress-dependent toxicity in Sprague-Dawley rats. Mol Cell Biochem. 2015;399(1-2):257-268.
Crossref - Heydrnejad MS, Samani RJ, Aghaeivanda S. Toxic Effects of Silver Nanoparticles on Liver and Some Hematological Parameters in Male and Female Mice (Musmusculus). Biol Trace Elem Res. 2015;165(2):153-158.
Crossref - Al Gurabi MA, Ali D, Alkahtani S, Alarifi S. In vivo DNA damaging and apoptotic potential of silver nanoparticles in swiss albino mice. Onco Targets Ther. 2015;8:295-302.
Crossref - Pourhamzeh M, Mahmoudian ZG, Saidijam M, Asari MJ, Alizadeh MJ. The Effect of Silver Nanoparticles on the Biochemical Parameters of Liver Function in Serum, and the Expression of Caspase-3 in the Liver Tissues of Male Rats. Avicenna J Med Biochem 2016;4(2):e35557.
Crossref - Peng Y, Song C, Yang C, Guo Q, Yao M. Low molecular weight chitosan-coated silver nanoparticles are effective for the treatment of MRSA-infected wounds. Int J Nanomedicine. 2017;12:295-304.
Crossref - Parang Z, Moghadamnia D. Effects of silver nanoparticles on the functional tests of liver and its histological changes in adult male rats. Nanomed Res J. 2018;3(3):146-153.
Crossref - Ramachandran R, Krishnaraj C, Kumar VKA, Harper SL, Kalaichelvan TP, Il Yun S. In vivo toxicity evaluation of biologically synthesized silver nanoparticles and gold nanoparticles on adult zebrafish: a comparative study. 3 Biotech. 2018;8(10):441.
Crossref - Naguib M, Mahmoud UM, Mekkawy IA, Sayed AEDH. Hepatotoxic effects of silver nanoparticles on Clariasgariepinus; Biochemical, histopathological, and histochemical studies. Toxicol Reports. 2020;7:133-141.
Crossref - Bednarski M, Dudek M, Knutelska, et al. The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: In vivo studies. Pharmacol Reports. 2015;67(3):405-409.
Crossref - Aziz AIF, Ihsan A, Nazir A, et al. Novel route synthesis of porous and solid gold nanoparticles for investigating their comparative performance as contrast agent in computed tomography scan and effect on liver and kidney function. Int J Nanomedicine. 2017;12:1555-1563.
Crossref - Patlolla AK, Kumari SA, Tchounwou PB. A comparison of poly-ethylene-glycol-coated and uncoated gold nanoparticle-mediated hepatotoxicity and oxidative stress in spraguedawley rats. Int J Nanomedicine. 2019;14:639-647.
Crossref - Alshammari GM, Abdelhalim MA, Al-Ayed MS, Al-Harbi LN, Yahya MA. The Protective Effect of k-Lipoic Acid against Gold Nanoparticles (AuNPs)-Mediated Liver Damage Is Associated with Upregulating Nrf2 and Suppressing NF-kB. Nutrients. 2022;14(16):3327.
Crossref - Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol. 2009;29(4):330-337.
Crossref - Fatemeh MF, Mohammad F. The Histological and Biochemical effects of Titanium Dioxide Nanoparticle (TiO 2) on the liver in Wistar Rat. Int Res J Biol Sci. 2014;3(6):1-5.
- Orazizadeh M, Fakhredini F, Mansouri E, Khorsandi L. Effect of glycyrrhizic acid on titanium dioxide nanoparticles-induced hepatotoxicity in rats. Chem Biol Interact. 2014;220:214-221.
Crossref - Hassanein KMA, El-Amir YO. Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int J Vet Sci Med. 2018;6(1):16-21.
Crossref - Tuncsoy YO. Impacts of Titanium Dioxide Nanoparticles on Serum Parameters and Enzyme Activities of Clariasgariepinus. Bull Environ Contam Toxicol. 2021;106(4):629-636.
Crossref - Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K. Silica nanoparticles as hepatotoxicants. Eur J Pharm Biopharm. 2009;72(3):496-501.
Crossref - Isoda K, Tetsuka E, Shimizu Y, Saitoh K, Ishida I. Liver Injury Induced by Thirty- and Fifty-Nanometer-Diameter Silica Nanoparticles. Biol Pharm Bull . 2013;36(3):370-375.
Crossref - Nemmar A, Yuvaraju, Beegam S, Yasin J, Kazzam E, Ali B. Oxidative stress , inflammation , and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int J Nanomedicine. 2016;11(1):919-928.
Crossref - Chan WT, Liu CC, Chiang CJS, et al. In vivo toxicologic study of larger silica nanoparticles in mice. Int J Nanomedicine. 2017;12:3421-3432.
Crossref - Sanoopa CP, John N, Chitra KC. Sublethal hepatotoxic effects and biotransformation response in the freshwater fish, Oreochromis mossambicus exposed to silicon dioxide nanoparticles. Biologia (Bratisl). 2022;77(10):2507-2518.
Crossref - Pasupuleti S, Alapati S, Ganapathy S, Anumolu G, Pully NR, Prakhya M. Toxicity of zinc oxide nanoparticles through oral route. Toxicol Ind Health. 2012;28(8):675-686.
Crossref - Fahmy SR, Abdel-Ghaffar F, Bakry FA, Sayed DA. Ecotoxicological Effect of Sublethal Exposure to Zinc Oxide Nanoparticles on Freshwater Snail Biomphalaria alexandrina. Arch Environ Contam Toxicol. 2014;67(2):192-202.
Crossref - Ansar S, Abudawood M, Alaraj ASA, Hamed SS. Hesperidin alleviates zinc oxide nanoparticle induced hepatotoxicity and oxidative stress. BMC Pharmacol Toxicol. 2018;8:65.
Crossref - Sizova EA, Miroshnikov SA, Yausheva EV. Effect of zinc-containing nanoparticles on cytomorphological and biochemical parameters in rats. Trace Elem Electrolytes. 2018;35(4):215-217.
Crossref - Abdeen A, Abdel-latif HMR, Ismail M, Dawood MAO, Hassan AM. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol. 2019;69:44-50.
Crossref - Mohammad GRKS, Seyedi SMR, Karimi E, Homayouni-Tabrizi M. The cytotoxic properties of zinc oxide nanoparticles on the rat liver and spleen , and its anticancer impacts on human liver cancer cell lines. J Biochem Mol Toxicol. 2019;33(7):e22324.
Crossref - Ghareeb OA. Toxicopathological Effects of Zinc Oxide Nanoparticles on the Liver Function Toxicopathological Effects of Zinc Oxide Nanoparticles on the Liver Function and Preventive Role of Silymarin In vivo. Indian J Forensic Med Toxicol. 2021;15(2):3212-3217.
- Jain TK, Reddy MK, Morales MA, Leslie-pelecky DL, Labhasetwar V. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Mol Pharm. 2008;5(2):316-327.
Crossref - Kumari M, Rajak S, Singh SP, et al. Biochemical alterations induced by acute oral doses of iron oxide nanoparticles in Wistar rats. Drug Chem Toxicol. 2013;36(3):296-305.
Crossref - Parivar K, Fard FM, Bayat M, Motabaf M. Evaluation of Iron Oxide Nanoparticles Toxicity on Liver Cells of BALB / c Rats. Iran Red Crescent Med. J. 2016;18(1):e28939.
Crossref - Easo SL, Mohanan PV. Hepatotoxicity evaluation of dextran stabilized iron oxide nanoparticles in Wistar rats. Int J Pharm. 2016;509(1-2):28-34.
Crossref - Shakra MEAE, El-Din RMS, Hamouda SYM, Mohammed AAR, Al-Rahman RAAA. Hepatotoxicity of Bare and Polyethylene Glycol Coated Iron Oxide Nanoparticles and The Protective Role of Virgin Olive Oil in Male Albino Rats. Egypt J Hosp Med. 2019;76(2):3607-3617.
Crossref - Askri D, Ouni S, Galai s, et al. Nanoparticles in foods? A multiscalephysiopathological investigation of iron oxide nanoparticle effects on rats after an acute oral exposure: Trace element biodistribution and cognitive capacities. Food Chem Toxicol. 2019;127:173-181.
Crossref - Hedayati SA, Veisi RS, Shekarabi SPH, Naserabad SS, Bagheri D, Ghafarifarsani H. Effect of Dietary Lactobacillus casei on Physiometabolic Responses and Liver Histopathology in Common Carp (Cyprinus carpio) After Exposure to Iron Oxide Nanoparticles. Biol Trace Elem Res. 2022;200(7):3346-3354.
Crossref - Adebayo OA, Akinloye O, Adaramoye OA. Cerium oxide nanoparticles elicit antitumourigenic effect in experimental breast cancer induced by N methyl N nitrosourea and benzo (a) pyrene in female Wistar rats. J Biochem Mol Toxicol. 2021;35(4):e22687.
Crossref - Adebayo OA, Akinloye O, Adaramoye OA. Cerium Oxide Nanoparticles Attenuate Oxidative Stress and Inflammation in the Liver of Diethylnitrosamine-Treated Mice. Biol Trace Elem Res. 2020;193:214-225.
Crossref - Mazaheri N, Naghsh N, Karimi A, Salavati H. In vivo Toxicity Investigation of Magnesium Oxide Nanoparticles in Rat for Environmental and Biomedical Applications. Iran J Biotechnol. 2019;17(1):1-9.
Crossref - Mekky G, Seeds M, Diab AEAA, et al. The potential toxic effects of magnesium oxide nanoparticles and valproate on liver tissue. J Biochem Mol Toxicol. 2020;35(3):e22676.
Crossref - Lesnichaya M, Karpova E, Sukhov B. Biointerfaces Effect of high dose of selenium nanoparticles on antioxidant system and biochemical profile of rats in correction of carbon tetrachloride-induced toxic damage of liver. Colloids Surfaces B Biointerfaces. 2021;197:111381.
Crossref - Ismail T, Salama MA, Mostafa E-E. Entomotoxic effects of synthesized aluminum oxide nanoparticles against Sitophilusoryzae and their toxicological effects on albino rats. Toxicol Ind Health. 2021;37(10):594-602.
Crossref - Sizova AA, Miroshnikov SA, Kalashnikov VV. Morphological and biochemical parameters in wistar rats influenced by molybdenum and its oxide nanoparticles. Agric Biol. 2016;51(6):929-935.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.