ISSN: 0973-7510
E-ISSN: 2581-690X
This study aimed to assess the effect of titanium nanoparticles (TiNPS) addition into soft denture lining material on Candida albicans (C. albicans) adherence ability, hardness, shear bond strength and spectrophotometer color absorption of the lining material. TiNPS with 2% concentration by weight( according to the pilot study) were added into acrylic-based heat cured soft denture liner. One hundred and twenty different specimens were prepared and divided into four groups according to the test to be performed. The ability of C. albicans to adhere on the soft liner/ TiNPS composite was evaluated in three different periods, also hardness, shear bond strength and light absorption by liner material were measured, the results showed there were significant decrease in adhered Candida cells number on the surface of the experimental specimens in comparison with the control specimens in each incubation period, and significant decrease in the hardness of the experimental specimens. The strength of the shear bond between the soft liner and the acrylic denture base showed non-significant difference but a significant increase in light absorption percentage was observed in all experimental specimens. Thus it can be concluded that the addition of TiNPS can provide soft liner material with antifungal properties, reduced hardness and unaffected shear bond strength with increased opacity of the liner material.
Soft Denture Liner, Candida albicans, Titanium Nanoparticles.
When the underling residual ridge resorb, the denture will lose its accurate adaptation on this resorbed ridge. In order to improve denture’s fitting to the underling supporting tissues, retention, stability, occlusion and patient’ appearance, denture should be relined1.
Soft denture liners act as a layer of absorbing cushion, they can absorb and distribute functional forces which transmitted to the underling irritated, damaged and thin bearing mucosa thus, maintain and restore the integrity of these inflamed tissues. Mostly soft denture liners are used for patients who cannot tolerate the hard base of their complete or partial dentures, so the liner material will make denture wearing more comfortable and accepted by them2.
Also by application of soft denture liners, this can enhance patient’s masticatory ability, biting forces and chewing efficiency by improving denture retention3.
However, these liners have many of drawbacks which appear during their clinical use and affect adversely on their serviceability by altering their structure and properties4, one of the most important drawbacks of these liners, the colonization of such materials by pathological microorganisms especially C. albicans which can adhere and go to penetrate inside the material causing denture induced stomatitis or denture sore mouth5.
Denture stomatitis etiology is multifactorial and despite these etiological factors, C. albicans has been considered the primary pathological microorganism that most widely isolated from the oral cavity of patients suffering from denture stomatitis6.
Furthermore, they have been found that these materials were more prone to colonization by microorganisms than acrylic denture base materials and this attributed to their rough surface and the physical/chemical affinity with the microorganisms7.
Many methods had been used in attempts to clean and disinfect the denture surfaces from the colonized microorganisms which include chemical and mechanical methods, but all these attempts showed an adverse effects on the acrylic denture and the soft lining materials themselves8.
Many attempts had been made to overcome all these problems and prevent the colonization of the soft lining material by microorganisms and one of them, was the incorporation of antifungal agents within the soft liner itself and addition of nanoparticles was one of these antifungal agents9.
Titanium nanoparticles (TiNPs ) are considered an antifungal agents and they were used in many applications such as treatment of skin infections 10.
In the present study TiNPs were incorporated into acrylic-based heat cured soft denture lining material in an attempt to minimize the microbial growth of C. albicans and evaluating whether this addition would significantly affecting some of the mechanical and physical properties of the soft liner itself.
A pilot study of C. adherence and hardness tests was conducted and three concentrations of the nano material were used (1.5%, 2% and 3% ) to select the most appropriate and effective concentration of TiNPs for each test and according to the results of this pilot study for both tests, 2% of TiNPs was the most appropriate concentration because 2% concentration showed favorable reduction in C. albicans with minimum effect on the mechanical properties of the soft liner material. Therefore, the main study was conducted by addition of titanium nano powder (Sky Spring, USA) with concentration of 2% by weight into polyethyl methacrylate soft denture liner (PEMA) (Vertex™ Soft, Vertex-Dental, Netherlands). One hundred and twenty different specimens were prepared and divided into four groups according to the tests to be performed.
Scanning electron microscope (SEM S50. FEI, Netherland) was taken for both control (PEMA) and experimental (containing 2% TiNPS) specimens to show the degree of TiNPS dispersion within the PEMA matrix.
To investigate the chemical interaction between TiNPS and PEMA, Fourier Transform Infrared Spectroscopy (FTIR) analysis (IR Prestige-21Shimadzu, Japan) was conducted.
Fabrication of soft liner specimens loaded with TiNPs to be used in the evaluation of adherence ability of C. albicans:
Disk shaped plastic patterns measuring 10mm in diameter and 2mm in thickness11, were used to prepare silicone-stone mold in order to fabricate soft liner specimens. The soft lining material was mixed, packed and cured according to manufacturer’s instructions. For experimental specimens TiNPs were added into the liner monomer and dispersed by using probe sonication apparatus (BRANSON, USA) for 3 minutes to break them into individual nanoparticles12.
The mixture was cooled down by placing the container in a cooling bath (cold-water bath), to prevent bulk heating of the liquid during sonication which can cause substantial liquid evaporation, or the degradation of the material13. After complete curing the specimens were finished, polished and autoclaved to be sterile.
Isolation of C. albicans
C. albicans can be found in different regions of the human being, but to be more accurate in this study, C. albicans was isolated from the oral cavity of 20 patients asking treatment for their complaint which was denture stomatitis with oral thrush14.
By using a sterile cotton swab, the oral tissue lesions were scrubbed gently and then inoculating a primary isolation medium such as sabouraud dextrose agar (SDA)15,16. These taken swabs were cultured and incubated aerobically at 37°C for 24 – 48 hrs.17, and can be preserved in 4°C for other investigations and tests.
Identification of C. albicans
It was identified by colony morphology as it develops as distinct, creamy, smooth and convex colonies on SDA18,19, and by microscopical examination using Gram stain method20, furthermore, germ tube formation procedure was used21, and the final verification was made by biochemical method by using analytical profile index (API) Candida system (bioMérieux).
Evaluating adherence ability of C. albicans
To test the antifungal efficacy (AFE) of soft liner/TiNPs composites on the adherence ability of C. albicans, C. albicans was diluted in 0.9 NaCl and a yeast suspension of about 107 colony forming unit (CFU/ml) (0.5 McFarland standards) was prepared by using a McFarland densitometer22. The sterile soft lining specimens were deposited in sterile bottles containing 20ml of the prepared suspension and incubated for 1hr. at room temperature. After incubation, the specimens removed from the suspension and rinsed twice by phosphate buffered saline solution (PBS) for one minute with gentle rocking to eliminate all the non-adhered cells, dried by filter paper and fixed with methanol, then stained by crystal violet from 30-60 seconds, and rinsed again with (PBS) solution for 30 seconds, then dried with filter paper to be examined under inverted light microscope23. In four fields of view, adhered cells were enumerated for each specimen. The result expressed as yeast cells/mm2. This procedure was repeated after 14 days and 30 days of specimens’ storage in artificial saliva at 37ºC.
Shore A hardness test
Discs shaped soft liner specimens with diameter of about 30 mm and 3mm thickness24, were prepared in order to measure soft liner’s hardness by using Shore A durometer (Time group-TH200, China). The distance between the durometer indenter and the sample was 20mm and the contact time after penetration was 5 seconds, five indentations were made for each sample and their mean were obtained25.
Shear bond strength test
To evaluate shear bond strength of soft lining material to acrylic denture base, acrylic blocks with specified dimensions (75mm × 25mm × 5 mm length, width, depth respectively) with stopper of depth about 3mm needed to be made26. Heat cured acrylic resin (Spofa dental, Czech) blocks were made, mixing, packing and curing was done according to manufacturer’s instructions, Each specimen consisting of two blocks of acrylic base which set over each other forming a space between them measured (25mm × 25mm × 3mm length, width, depth respectively) to be filled with wax. Then the whole specimen was invested into silicon material to fabricate a mold for final specimen curing. Wax elimination procedure was done to be replaced with soft lining material and specimen’s curing was carried out.
By using instron testing machine (WDW- 20, Laryee Technology Co., Ltd., BEIJING), the specimens were tested and the maximum load required for failure was recorded to calculate the value of bond strength for each specimen according to (ASTM specification D-638m, 1986) formula27.
![]()
Spectrophotometer color absorption
Disk shaped soft liner specimens with 50 ± 1mm in diameter and 0.5 ± 0.05mm in thickness according to ADA specifications No.1228, were used in the measurement of light absorption percentage by using UV-visible spectrophotometer (UV-1800, Biotech engineering management).
Statistical analysis
The results of the study were analyzed by using SPSS (statistical package for social science) computer software (version 21).
The following statistics were employed:
1- Descriptive statistic which include:
- Mean
- Standard deviation (S.D)
- Standard error (S.E)
2- Inferential statistics:
- Independent t-test.
- One-way ANOVA (analysis of variance) test.
A “P” value of > 0.05 was considered statically non-significant, d” 0.05 was considered significant and d” 0.01 was considered as highly significant.
FTIR analysis showed that there was no chemical interaction between PEMA and TiNPs (fig.1). SEM images showed that there were fair dispersion and areas of aggregation of some TiNPs in the PEMA (fig.2 A and B). The experimental specimens showed significant decrease in the number of adhered Candida cells on their surface in comparison with the control specimens for each incubation periods of the study (table 1). A non-significant difference between the experimental specimens as the incubation time in artificial saliva increase (fig.3A, B, C and D), (table 2).
Table (1):
Descriptive statistics and independent t-test showing the difference in the mean values for the studied groups of C. adherence test
Groups |
Mean |
N |
SD |
SE |
t-test |
MD |
Sign. |
|---|---|---|---|---|---|---|---|
Day- (0) control Day – (0) 2% |
15.3 5 |
10 10 |
5.12185 1.24722 |
1.61967 0.39441 |
6.179 |
10.30000 |
0.000 |
Day-(14) control Day-(14) 2% |
16.7 4 |
10 10 |
3.65300 2.44949 |
1.15518 0.77460 |
9.131 |
12.70000 |
0.000 |
Day-(30) control Day-(30) 2% |
15.7 3.7 |
10 10 |
5.27152 2.00278 |
1.66700 0.63333 |
6.729 |
12.0000 |
0.000 |
Table (2):
One-way ANOVA for Comparison of the means of C. albicans adherence test results for the experimental groups and for different incubation periods of the study
Groups |
Sum of Squares |
df |
Mean Square |
F |
Sig. |
|---|---|---|---|---|---|
Between Groups |
9.267 |
2 |
4.633 |
1.202 |
0.316 |
Within Groups |
104.100 |
27 |
3.856 |
||
Total |
113.367 |
29 |
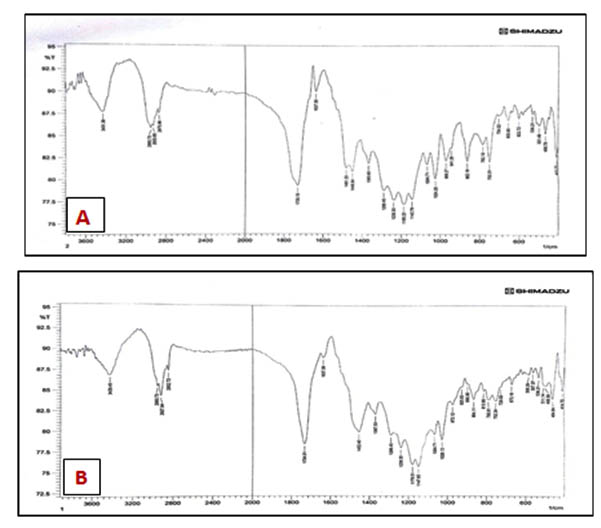
Fig. 1. FTIR spectrum of (A) PEMA, (B) PEMA/TiNPS
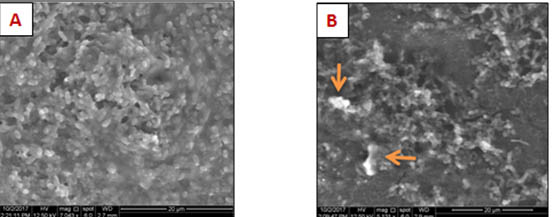
Fig. 2. SEM images for (A) control and (B) experimental specimens
There was significant decrease in the hardness of the experimental specimens (table 3), and a non-significant decrease in the shear bond strength after TiNPS incorporation into the soft liner (table 4), while significant increase in light absorption percentage by the experimental specimens (table 5).
Table (3):
Descriptive statistics and independent t-test showing the difference in the mean values for the studied groups of shore A hardness test
| Groups | Mean | N | SD | SE | t-test | MD | Sign. |
|---|---|---|---|---|---|---|---|
| Control shore A hardness | 56.9000 | 10 | 2.77008 | 0.87598 |
2.738 |
2.86000 |
0.013 |
| 2% shore A hardness | 54.0400 | 10 | 1.79827 | 0.56866 |
Table (4):
Descriptive statistics and independent t-test showing the difference in the mean values for the studied groups of shear bond strength test
| Groups | Mean | N | SD | SE | t-test | MD | Sign. |
|---|---|---|---|---|---|---|---|
| Control shear bond strength | 0.4050 | 10 | 0.10363 | 0.03277 |
0.111 |
0.00580 |
0.913 |
| 2% shear bond strength | 0.3992 | 10 | 0.12844 | 0.04062 |
Table (5):
Descriptive statistics and independent t-test showing the difference in the mean values for the studied groups of light absorption test
| Groups | Mean | N | SD | SE | t-test | MD | Sign. |
|---|---|---|---|---|---|---|---|
| Control light absorption | 2.5940 | 10 | 0.33755 | 0.10674 |
-11.733 |
-6.25300 |
0.000 |
| 2% light absorption | 8.8470 | 10 | 1.65123 | 0.52216 |
SD: Standard deviation, SE: Standard error, MD: Mean difference, df: degree of freedom.

Fig. 3. Fields of experimental and control specimens showing adherent C. albicans cells (A) control specimen with a large number of C. yeast cells (B), (C) and (D): Experimental specimens with a few number of C. yeast cells (10*/0.22)
In this study an attempt was made to get soft lining material resistant to microbial colonization especially against C. albicans. Addition of TiNPS to the soft liner, should not affect adversely on the mechanical and physical properties of the liner material at the same time prevent or reduce for a large extent C. albicans growth.
The statistical results of this study showed there were highly significant decrease in the numbers of adhered C. albicans cells on the surface of the experimental specimens in comparison with the control specimens and this antifungal efficacy of TiNPS increased with increasing nano concentration. Authors documented that there is an electromagnetic attraction between the microorganism which has a negative charge on its surface and the metal oxide nanoparticle which has a positive charge and by this attraction and surface contact, the microbe will die due to its oxidation29.
It was reported that TiNPS cause C. yeast cells death by production of intracellular reactive oxygen species (ROS) causing oxidation of the Coenzyme A and peroxidation of lipids which decrease respiratory activity and thus cell death30. Another explanation, might be due to the cell membrane tearing of fungi caused by TiNPS which disturb its integrity, loss of intracellular substances and suppression of the natural budding process through defecting cell cycle at Pre-mitotic phase (G2/M)31.
Soft liner with decreased hardness is preferable because it will has greater softness and thus increased ability to act as absorbing cushion for occlusal forces32.
The results showed a significant decrease in the hardness of the experimental specimens in comparison with the control specimens.
According to the results of SEM of this study, there were areas of agglomeration of some TiNPS within the acrylic resin matrix because TiNPS dispersion is difficult due to the presence of strong forces between the particles of the nano material called Van der Waal’s forces33, so the reduction in the hardness may be attributed to this fair dispersion of TiNPS in PEMA which adversely affects degree of conversion leading to increased level of residual unreacted monomer that acts as a plasticizer34,35, also these agglomerated TiNPS within the resin matrix can act as stress concentrating centers affecting adversely on the mechanical properties of the polymerized resin material36.
Also the statistical analysis showed that there was a non-significant decrease in the shear bond strength, According to the results of FTIR analyses of this study, no chemical interaction was detected between the TiNPS and PEMA and this was in agreement with many studies which showed that TiO2 is inert chemical material and it rarely interacts with other chemical materials so no chemical reaction which may change the chemical composition of TiO2 itself or the chemical structure of the other mixed material or the surrounding environment37.
As a result there is no chemical interaction between TiNPS and the acrylic denture base material (PMMA) and thus the added TiNPS can act as a barrier that could be interfere with chemical reaction between the PEMA and the PMMA for a little extent especially TiNPS were mixed with the monomer of the PEMA so may interfere with the monomer ability to diffuse into PMMA. The lining material that used in this study has high ability to flow on the denture base thus enhanced the bond strength between the two bonded materials, also according to the used samples, the surface area between the two materials was large and may help to enhance the bonding strength and this could explain the non-significant decrease in the bond strength38, in addition to the small amount of TiNPS that added into the lining material.
The statistical results of this study showed a significant increase for UV-light absorption by the experimental specimens, This is attributed to the presence of TiNPS in the polymer matrix which has good optical and photo catalytic properties and its high ability to absorb UV-light, this type of nanoparticle were able to absorb 95% of UV-light39.
Many studies showed by addition of this nano material will increase the material’s life span, maintain its color and esthetic by the ability of TiNPS to prevent the passage of UV-light, thus reducing the color degradation of the pigments inside the polymer and preventing the color change40,41.
The addition of TiNPS can provide soft liner material with antifungal properties. This addition of nano material lead to reduced liner’s hardness with non-significant effect on the shear bond strength but it can increase the opacity of the liner material.
- Kranjèiæ J, Kosteliæ-Stuniæ M, Èelebiæ A, Komar D, Mehuliæ K, Vojvodiæ D. Denture relining as an indicator of residual ridge resorption. Medicinski Glasnik. 2013; 10(1):126-32.
- Rao CS, Reddy SS, Prasad GM, Reddy KS, Rao NV, Naik MS. A Study to Evaluate and Compare the Shear Bond Strength of Resilient Liners with Heat Cure Denture Base Resins, with and without the Effect of Saliva: An in vitro Study. The journal of contemporary dental practice. 2012; 13(3):394-400.
- Ergun G, Nagas IC. Color stability of silicone or acrylic denture liners: an in vitro investigation. European journal of dentistry. 2007; 1(3):144..
- Pavan S, Arioli Filho JN, Dos Santos PH, Nogueira SS, Batista AU. Effect of disinfection treatments on the hardness of soft denture liner materials. Journal of prosthodontics. 2007; 16(2):101-6.
- Chladek G, Kasperski J, Barszczewska-Rybarek I, ¯mudzki J. Sorption, solubility, bond strength and hardness of denture soft lining incorporated with silver nanoparticles. International journal of molecular sciences. 2012; 14(1):563-74.
- Sharma S, Hegde V. Comparative evaluation of antifungal activity of melaleuca oil and fluconazole when incorporated in tissue conditioner: an in vitro study. Journal of Prosthodontics. 2014; 23(5):367-73.
- Tari BF, Nalbant D. Dogruman Al, F.; Kustimur, S. Surface roughness and adherence of Candida albicans on soft lining materials as influenced by accelerated aging. J. Contemp. Dent. Pract. 2007; 8:18-25.
- Nazirkar G, Bhanushali S, Singh S, Pattanaik B, Raj N. Effect of anatase titanium dioxide nanoparticles on the flexural strength of heat cured poly methyl methacrylate resins: An in-Vitro Study. The Journal of Indian Prosthodontic Society. 2014; 14(1):144-9.
- Iqbal Z, Zafar MS. Role of antifungal medicaments added to tissue conditioners: a systematic review. Journal of prosthodontic research. 2016; 60(4):231-9.
- George SA, Raj MS, Solomon D, Roselin P. Comparative study of the anti-fungal activity of zinc oxide and titanium dioxide nano and bulk particles with anti-fungals against fungi isolated from infected skin and dandruff flakes. Res. Rev. J. Microbiol. Biotechnol. 2014; 3(3):23-30.
- Karakis D, Akay C, Oncul B, Rad AY, Dogan A. Effectiveness of disinfectants on the adherence of Candida albicans to denture base resins with different surface textures. Journal of oral science. 2016; 58(3):431-7.
- Yassir A D .The effect of addition of zirconium Nano particles on antifungal activity and some properties of soft denture lining material. M.Sc. Thesis, college of dentistry, University of Baghdad, 2017
- National Institute of Standards and Technology. Material Measurement Laboratory. Preparation of Nanoparticle Dispersions from Powdered Material Using Ultrasonic Disruption. Special Publication 1200-2 (2012).
- Marsh P. D. and Martin M.: ¯Oral Microbiology . 5th edition. Churchill Livingstone, Edinburgh, UK (2009).
- Byadarahally Raju S, Rajappa S. Isolation and identification of Candida from the oral cavity. ISRN dentistry. 2011; 2011.
- AXÉLL T, SIMONSSON T, BIRKHED D, ROSENBORG J, EDWARDSSON S. Evaluation of a simplified diagnostic aid (Oricult N) for detection of oral candidoses. European Journal of Oral Sciences. 1985; 93(1):52-5.
- Baveja C.: ¯Text Book of Microbiology for Dental Students . 3rd edition Arya Publications, Delhi, India (2010).
- Thein ZM, Samaranayake YH, Samaranayake LP. Characteristics of dual species Candida biofilms on denture acrylic surfaces. Archives of oral biology. 2007; 52(12):1200-8.
- Williams DW, Lewis MA. Oral Microbiology: Isolation and identification of candida from the oral cavity. Oral diseases. 2000; 6(1):3-11.
- Marler LM, Siders JA, Allen SD. Direct smear atlas: a monograph of gram-stained preparations of clinical specimens. Lippincott Williams & Wilkins; 2001.
- Forbes BA, Sahm DF, Weissfeld AS, Bailey WR. Bailey & Scott’s diagnostic microbiology.12th ed. Elsevier: Mosby; 2007.
- Monteiro DR, Gorup LF, Takamiya AS, de Camargo ER, Barbosa DB. Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles. Journal of Prosthodontics. 2012; 21(1):7-15.
- Govindswamy D, Rodrigues S, Shenoy VK, Shenoy S, Shenoy R, Yadav T. The influence of surface roughness on the retention of Candida albicans to denture base acrylic resins–An in vitro study. Journal of Nepal Dentists Association-JNDA| 2014; 14(1).
- Mancuso DN, Goiato MC, Zuccolotti BC, Moreno A, dos Santos DM, Pesqueira AA. Effect of thermocycling on hardness, absorption, solubility and colour change of soft liners. Gerodontology. 2012; 29(2).
- Goiato MC, Zuccolotti BC, Moreno A, Vechiato Filho AJ, Paulini MB, Dos Santos DM. Effect of nanoscale particles incorporation on microhardness of polymers for oral prosthesis. Contemporary clinical dentistry. 2016; 7(3):307.
- Mohammed WS, Salih Z, Fatihallah AA. Comparing Shear Bond Strength Of Auto-polymerized Soft Lining Materials to Acrylic Denture Base Using Different Surface Treatment and Denture Base Materials. Iraqi Dental Journal. 2016 ; 38(1):6-11.
- American society for testing and material, ASTM D, 638-m standard test method for tensile properties of plastics. Philadelphia: American National Standards Institute (1986).
- American National Standards Institute/ American Dental Association Specification No.12 for Denture Base Polymer. Chicago: Council on Dental Materials and Devices(1999).
- Durairaj B, Muthu S, Xavier T. Antimicrobial activity of Aspergillus niger synthesized titanium dioxide nanoparticles. Adv Appl Sci Res. 2015; 6(1):45-8.
- Haghighi F, Roudbar Mohammadi S, Mohammadi P, Hosseinkhani S, Shipour R. Antifungal activity of TiO2 nanoparticles and EDTA on Candida albicans biofilms. Infection, Epidemiology and Microbiology. 2013; 1(1):33-8.
- Kamikawa Y, Hirabayashi D, Nagayama T, Fujisaki J, Hamada T, Sakamoto R, Kamikawa Y, Sugihara K. In vitro antifungal activity against oral Candida species using a denture base coated with silver nanoparticles. Journal of Nanomaterials. 2014; 2014:48.
- Urban VM, Lima TF, Bueno MG, Giannini M, Arioli Filho JN, Almeida AL, Neppelenbroek KH. Effect of the addition of antimicrobial agents on Shore A hardness and roughness of soft lining materials. Journal of Prosthodontics. 2015; 24(3):207-14.
- Chatterjee A. Properties improvement of PMMA using nano TiO2. Journal of applied polymer science. 2010; 118(5):2890-7.
- Ahmed MA, El-Shennawy M, Althomali YM, Omar AA. Effect of titanium dioxide nano particles incorporation on mechanical and physical properties on two different types of acrylic resin denture base. World Journal of Nano Science and Engineering. 2016; 6(03):111.
- Shirkavand S, Moslehifard E. Effect of TiO2 nanoparticles on tensile strength of dental acrylic resins. Journal of dental research, dental clinics, dental prospects. 2014; 8(4):197.
- Sodagar A, Bahador A, Khalil S, Shahroudi AS, Kassaee MZ. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. Journal of prosthodontic research. 2013; 57(1):15-9.
- Trivedi M, Murase J. Titanium Dioxide in Sunscreen. InApplication of Titanium Dioxide 2017. InTech.
- Issa M I. Evaluating the effect of silver nanoparticles incorporation on antifungal action and some properties of soft denture lining material. M.S.c. Thesis, college of dentistry, University of Baghdad, 2014.
- Chatterjee A. Effect of nanoTiO2 addition on poly (methyl methacrylate): an exciting nanocomposite. Journal of applied polymer science. 2010; 116(6):3396-407.
- Andreotti AM, Goiato MC, Moreno A, Nobrega AS, Pesqueira AA, dos Santos DM. Influence of nanoparticles on color stability, microhardness, and flexural strength of acrylic resins specific for ocular prosthesis. International journal of nanomedicine. 2014; 9:5779.
- Goiato MC, Zuccolotti BC, dos Santos DM, Sinhoreti MA, Moreno A. Effect of intrinsic nanoparticle pigmentation on the color stability of denture base acrylic resins. Journal of Prosthetic Dentistry. 2013; 110(2):101-6.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


