ISSN: 0973-7510
E-ISSN: 2581-690X
The invasion and spread of cancer cells are two of the most notable characteristics of malignant tumors. Recent studies suggest that the epithelial-mesenchymal transition (EMT) has been linked to this significant occurrence. It is linked to the absence of the epithelial brow and the presence of mesenchymal facial hair. The aims of the present study were to explore the immunohistochemical staining of vimentin and E-cadherin ex vivo as EMT markers and assess their potential as predictive biomarkers for transitional cell cancer (TCC). In this study, 55 paraffin-embedded biopsies from TCC patients and 10 autopsies that appeared to be normal were included. Immunohistochemistry was used to produce patterns of vimentin and E-cadherin expression. When compared to female patients, the expression of E-cadherin and vimentin significantly increased with increasing age in male patients (> 50 years). In contrast to the considerable rise in vimentin expression in higher grades and stages of the tumor, E-cadherin expression was significantly reduced with tumor stage and grade. The findings of this study reveal that elevated vimentin and reduced E-cadherin are important indicators associated with a poor prognosis for TCC.
HCV, Vimentin, E-cadherin, Bladder Cancer, IHC
Human urinary bladder carcinoma (UBC) is one of the top 10 most common cancer types globally. The majority of bladder cancers are transitional cell carcinomas (TCC).1 The urothelial cells that generally line the inside of the bladder are where bladder cancer first develops.2 Other but less typical kinds of bladder inner lining cancer include squamous cell carcinoma and adenocarcinoma.3 Bladder cancer is one of the most prevalent health issues in the world, with industrialized nations having the greatest incidence rates.4-6 In the US, TCC ranks fourth among men’s cancers and tenth among women’s cancers, accounting for almost 95% of bladder malignancies.7 The grading of cellular differentiation was used to classify the cellular morphology of TCC; well-differentiated cells were graded as 1, moderately-differentiated cells as grade 2, and poorly-differentiated cells as grade 3. The bladder cancer stage is determined by the degree of tumor infiltration into the bladder wall; stages Tis, Ta, and T1 represent noninvasive types that are only present in the inner lining of the bladder epithelium, tumor invasion of the muscle, or perivesical invasion.2 Bladder cancer risk factors include smoking, which is a major factor, occupational exposure to carcinogens, dietary risk factors, pollution, sex, race, and medical conditions. However, for a more accurate assessment of the effects, each risk factor must be taken into account in the context of genetic-environmental interactions.8,9
Hepatitis C virus (HCV), a member of the genus Hepacivirus in the family Flaviviridae, is present in around 160 million people, and a significant portion of this population develops hepatocellular carcinoma (HCC).10-12 Moreover, HCV infection has been linked to several malignancies, including head and neck cancer and oral squamous cell carcinoma.13 According to Gordon et al., those with chronic hepatitis C have nearly double the risk of acquiring renal cell carcinoma.14 Although it was discovered that HCV may be responsible for the impairment of the DNA damage repair system through its propensity to interfere with cellular processes involved in identifying and responding to DNA damage, the chronic inflammatory microenvironment of HCV may be the cause of such tumors.12,15 The membrane-associated glycoproteins known as epithelial cadherins (E-cad), usually referred to as “classical” cadherins of type I, are produced by epithelial cells and play a crucial role in tissue morphogenesis and remodeling. They are a part of the broad cadherin family that regulates cell-to-cell adhesion.16 It has been demonstrated that the majority of epithelial malignancies lose E-cad partially or completely by mutation, epigenetic silencing, or increased expression of non-epithelial cadherins, as in cases of gastric cancer (GC), breast cancer (BC), and colorectal cancer (CRC).17
Vimentin, a member of type III intermediate filament protein group, is one of the most commonly expressed proteins. It participates in intracellular conformational changes and mechanoprotection in cells of mesenchymal origin, such as leukocytes, endothelial cells, and smooth muscle cells.18,19 The excessive exposure of the apoptotic cell to vimentin could form vimentin autoantibodies (AVA) and consequently induce platelet activation as well as white blood cells.20,21 In many epithelial cancers, including colorectal and prostate cancers, vimentin was found to be overexpressed. This overexpression has been linked to tumor invasion, proliferation, and a poor prognosis. The upregulation of vimentin levels also encourages cellular motility, which signals the initiation of EMT.4,22 The primary characteristics of EMT are the loss of cell-cell contact and the partitioning of intracellular tight junctions, which result in the loss of epithelial characteristics and the acquisition of mesenchymal phenotypes,23 and the release of subpopulations of cells capable of migrating and forming metastatic colonies.24 Vimentin displayed a different pattern of expression in normal transitional epithelium compared to bladder cancer. It was detected in 43% of bladder malignancies but was not observed in all normal urothelial cells.25 There is a dearth of local research addressing the issue concerning patients with TCC carcinoma and a history of HCV.
The present study was aimed at evaluating the expression of vimentin and E-cadherin as biomarkers with a link to tumor stage and grade in transitional cell carcinoma of the bladder in Iraqi patients.
Biopsy Samples
The study samples comprised 55 paraffin-embedded tissues from transitional cell carcinoma patients whose ages ranged from 36 to 70 years, with a mean age of 57 years. Thirteen of the patients were under 50 years old. Men made up about 75% of the samples from the 55 cases. The tissue samples were obtained from the histopathology laboratory archives in Baghdad, Iraq. Afterward, tissue blocks were classified primarily using the histological records of the bladder biopsy specimens. Grades and stages of bladder cancer were assigned based on the tumor, node, and metastasis (TNM) grading system into (GI, GII, and GIII) and (Ta, T1, and T2, respectively).26 A total of 25 patients have tumor grades GI, 18 with GII, and 12 with GIII, while 12, 24, and 19 patients were with Ta, T1, and T2, respectively. A qualified pathologist histopathologically re-examined each tissue sample that was collected to confirm the record information and select the best tissue sections. A total of 10 seemingly normal bladder autopsies from the Institute of Forensic Medicine were obtained after receiving the consent of the deceased’s next of kin. The patient group was age-matched with eight males and two females. Using a microtome, sections of formalin-fixed, paraffin-embedded blocks were cut at a thickness of 4 mm. One section from each block was stained with hematoxylin and eosin (H&E), and four more sections from the same tissue block were placed on glass slides. Using immunohistochemical staining, 4 additional sections of the same tissue block were put on positively charged glass slides to identify vimentin and E-cadherin.
Immunohistochemical Analysis
The paraffin-embedded sections were deparaffinized with xylene and then rehydrated in successively lower alcohol concentrations. To obtain the antigen (epitope), a heating technique was used. As a result, the slides were rinsed in citrate buffer (0.01 mol/L, pH 6.0) and heated at 100°C in the oven. The slides were pre-treated with 3% of H2O2 for 15 min at room temperature to stop the endogenous peroxidase activity and then rinsed with phosphate-buffered saline (PBS) for 3-5 min. E-cadherin monoclonal antibody (NCH-38) and anti-vimentin monoclonal antibody, clone V9 (DAKO, USA), were used in the process. Using a 1:100 dilution of the primary antibody, tissue samples were incubated for two hours at 4°C in a humid environment. Sections were washed with PBS before being incubated with the secondary antibody for 45 minutes. Sections were then twice-washed with PBS for 5 minutes, stained with hematoxylin and diaminobenzidine (DAB) chromogen, dried, and covered with a cover slip. Samples of negative control were prepared simultaneously in duplicate, except for the primary antibody, which was replaced with PBS. The positive controls were provided with the kits mentioned above.
Statistical Analysis
The statistical analysis system (SAS; 2012, version 9.1) was utilized in this study to examine how various factors affected the study variables. The Chi-square test was performed to compare the number of positive samples.27
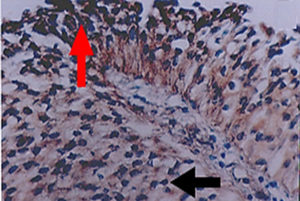
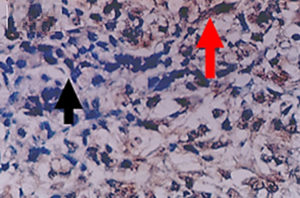
The present study discovered a significant increase in the number of TCC carcinoma patients with HCV, as shown in Table 1. According to the grade of cancer, which increased from 4 patients only with G1 to 11 patients with G3, as well as the stage of cancer, where only 1 patient had Ta and 13 had T2. According to the tumor grade, the cytoplasmic expression of E-cadherin was detected in 40% of TCC patients (Figure 1). Concerning E-cadherin, the results obtained showed that there is an inversely significant relationship between the number of TCC carcinoma patients whose samples were positive for E-cadherin and the grade of the disease. This was evident by a decrease in the number as the grade progressed. There was a reduction from 15 patients in G1 to only 2 patients in G3 patients. As shown in Table 2 the number of E-cadherin-positive samples decreased significantly and inversely with progression in disease stages. On the other hand, 40% of TCC patients showed vimentin expression (Figure 2). The number of patients with positive samples for this marker increased with the increase in both the grade and stage of the disease, from 6 to 9 patients in G1 and G3, respectively, and from 3 patients with Ta TCC to 7 patients with T2 (Table 2). Additionally, vimentin expression recorded a significant positive relationship with TCC development.
Table (1):
HCV incidence in TCC samples according to grades and stages.
Grade |
TCC Cancer Patients |
HCV Positive |
Stage |
TCC Cancer Patients |
HCV Positive |
|---|---|---|---|---|---|
G1 |
25 |
4 |
Ta |
12 |
1 |
G2 |
18 |
9 |
T1 |
24 |
10 |
G3 |
12 |
11 |
T2 |
19 |
13 |
Total |
55 |
24 |
Total |
55 |
24 |
p<0.001 |
p<0.001 |
HCV: Hepatitis C virus; TCC: Transitional cell carcinoma
Figure 1. Positive (red pointer) and negative (black pointer) cytoplasmic expression of E-cadherin in transitional cell carcinoma (40x)
Figure 2. Positive (red pointer) and negative (black pointer) cytoplasmic expression of vimentin in transitional cell carcinoma (40x)
The relationship between markers under study with the age and gender of TCC patients from whom samples were obtained was analyzed. In this situation, patients with positive results were divided into two age groups based on their age: patients under 50 included 13 patient samples, while patients over 50 contained 42 patient samples. In terms of HCV incidence, E-cadherin expression, and vimentin staining, the results showed a significant difference between the above groups. The number of patients rose in the group of people over 50 in terms of HCV incidence, E-cadherin, and vimentin expression. However, the gender-based analysis revealed that females were more likely than males to be infected with HCV and that vimentin expression was higher in positive samples from females than from males. In contrast, E-cadherin expression showed an inverse relationship with the number of positive samples, with males recording a higher number of positive samples than females (Table 3).
Table (2):
Expression of E-cadherin and vimentin in TCC samples according to grades and stages.
Grade |
TCC Cancer Patients |
E-cadherin Positive |
Vimentin Positive |
Stage |
TCC Cancer Patients |
E-cadherin Positive |
Vimentin Positive |
|---|---|---|---|---|---|---|---|
G1 |
25 |
15 |
6 |
Ta |
12 |
8 |
3 |
G2 |
18 |
5 |
7 |
T1 |
24 |
9 |
7 |
G3 |
12 |
2 |
9 |
T2 |
19 |
3 |
7 |
Total |
55 |
24 |
22 |
Total |
55 |
20 |
17 |
p<0.001 |
p<0.001 |
p<0.001 |
p<0.001 |
TCC: Transitional cell carcinoma
Table (3):
Expression of the study markers in term of age and gender.
| Parameter | Age | Gender | ||||
|---|---|---|---|---|---|---|
| <50 years (N=13) | ≥ 50 years (N=42) | P-value | Male (N=41) |
Female (N=14) |
P-value | |
| HCV | 6 | 18 | p<0.01 | 16 | 8 | p<0.01 |
| E-cadherin | 5 | 15 | p<0.01 | 18 | 4 | p<0.001 |
| Vimentin | 7 | 10 | p<0.001 | 15 | 6 | p<0.001 |
HCV: Hepatitis C virus
Several studies have shown that the prevalence of TCC increases with age and is more common in men than women.7,28-30 This observation is consistent with the results of the present study, which found that 76% (42 patients out of 55) of the study samples belonged to patients over the age of 50, and 75% of TCC patients were men. This may be caused by a variety of factors, including aging, which increases the likelihood that cellular processes that could lead to neoplastic transformation will intensify and manifest, cumulative exposure to carcinogens in the environment, especially if they smoke, and decreased ability to empty the bladder.31 In addition, men’s higher exposure to environmental carcinogens including cigarettes and industrial pollution has been linked to the gender discrepancy in bladder cancer incidence.32 Also, other factors such as those of a genetic and hormonal nature may make women more susceptible to the disease.33
The Hepatitis C virus is considered one of the oncoviruses that are connected to the development of cancer. These extrahepatic cancers include lymphoma,12 renal cell carcinoma (RCC),14 TCC, and head and neck squamous cell carcinoma.34 Consequently, the current study revealed a high proportion of HCV-positive cases in the TCC samples (24/54). The current study’s results support the findings of Hemmaid et al. regarding the association between HCV infection and more advanced bladder TCC, including higher grades and stages as well as being more invasive.34 Although, the exact mechanism by which HCV causes bladder cancer is still controversial, some hypotheses have emerged, that suggest that hyper-telomerase expression as well as activity in bladder cancer tissue occur in correlation with HCV.34 Also, it was hypothesized that HCV disrupts the mechanism of DNA damage repair by binding to the host protein RAD51AP1 and disrupting its function in mammalian cells.35 Moreover, HCV may interfere with cytotoxic T-cell mediated apoptosis, which is vital for the maintenance of host immunity and normal tissue, and can lead to renal oncogenesis.36
Although bladder cancer treatment approaches have advanced considerably, unfavorable biological processes, particularly those that are prone to invasion and metastasis, continue to complicate clinical treatment, resulting in ineffective therapy and a bad prognosis.37 Several molecular markers are employed to detect bladder cancer using the immunohistochemical technique since they show great potential for predicting prognosis in TCC.38,39 E-cadherin and vimentin, two markers associated with EMT, were examined in the current study to examine their expression and potential as prognostic indicators for TCC. The cytoplasmic expression of E-cadherin decreased with the progression of the stages and grades of the disease, whereas the expression of vimentin was demonstrated to increase, according to the stage and grade of TCC. These differences were statistically significant. The results are consistent with several studies.4,38,40-42
According to Zhao et al., the expression of E-cadherin was inversely correlated with the progression of tumor grade. As tumor stage increased, E-cadherin expression significantly decreased, whereas vimentin showed an opposite expression distribution to that of E-cadherin and significantly increased with the tumor stage.40 Another study demonstrated a statistically significant correlation between lower expression of E-cadherin and unfavorable clinicopathological parameters, indicating that expression of E-cadherin may be one of the beneficial prognostic markers in individuals with upper tract urothelial cancer.43 With a positive correlation with the increase in disease stage, vimentin expression was observed to be overexpressed in around 69% of TCC patients, rising from 43% in patients with G1 to nearly 94% in patients with G3 TCC.4 Several studies have demonstrated that decreased E-cadherin expression and increased vimentin are linked to a poor prognosis for individuals with bladder,38,40 colorectal cancer,44 and breast carcinoma.45 It is commonly accepted that EMT is necessary for tumor invasion and metastasis because it gives cells improved properties for motility and invasion.5,42 During such EMT-related events, the expression of cadherin switches from epithelial to mesenchymal.46 Finally, molecular techniques have been recommended to identify infectious diseases47-56 and cancers.57-65
Since the expression of vimentin and E-cadherin in TCC tissue showed a substantial relationship with tumor grade and stage, the findings of this study reveal that employing these markers in combination as prognostic markers for TCC may be of interest.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
AVAILABILITY OF DATA
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, University of Baghdad, Baghdad, Iraq, with reference number CSEC/0322/0032 dated March 20, 2022.
- Van der Weyden L, O’Donnell M, Plog S. Histological Characterization of Feline Bladder Urothelial Carcinoma. J Comp Pathol. 2021;182:9-14.
Crossref - Redondo-Gonzalez E, de Castro LN, Moreno-Sierra J, et al. Bladder carcinoma data with clinical risk factors and molecular markers: A cluster analysis. Biomed Res Int. 2015;168682.
Crossref - Martin JW, Carballido EM, Ahmed A, et al. Squamous cell carcinoma of the urinary bladder: Systematic review of clinical characteristics and therapeutic approaches. Arab J Urol. 2016;14(3):183-191.
Crossref - Rahmani AH, Babiker AY, AlWanian WM, Elsiddig SA, Faragalla HE, Aly SM. Association of Cytokeratin and Vimentin Protein in the Genesis of Transitional Cell Carcinoma of Urinary Bladder Patients. Dis Markers. 2015;204759.
Crossref - Luo Y, Zhu YT, Ma LL, et al. Characteristics of bladder transitional cell carcinoma with E-cadherin and N-cadherin double-negative expression. Oncol Lett. 2016;12(1):530-536.
Crossref - Malek-Hosseini Z, Khezri A, Amirghofran Z. Circulating Levels of M30 and M65 Molecules in Transitional Cell Carcinoma of the Bladder and Their Relation to Tumor Progression. Iran J Cancer Prev. 2016;9(2):e4086.
Crossref - Al-Husseini MJ, Kunbaz A, Saad AM, et al. Trends in the incidence and mortality of transitional cell carcinoma of the bladder for the last four decades in the USA: a SEER-based analysis. BMC Cancer. 2019;19(1):46.
Crossref - Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234-241.
Crossref - Bashaweeh RK, Alfaraj GA, Alwatban JJ, Allabboudy LM, Alghabban SA, Alkhateeb S. Urothelial carcinoma of the bladder with abnormal inguinal metastasis: A case report. Urol Case Rep. 2021;35:101535.
Crossref - Goossens N, Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 2015;21(2):105-114.
Crossref - Goto K, Suarez AAR, Wrensch F, Baumert TF, Lupberger J. Hepatitis C Virus and Hepatocellular Carcinoma: When the Host Loses Its Grip. Int J Mol Sci. 2020;21(9):3057.
Crossref - Yi Z, Yuan Z. Hepatitis C Virus-Associated Cancers. Adv Exp Med Biol. 2017;1018:129-146.
Crossref - Mahale P, Sturgis EM, Tweardy DJ, Ariza-Heredia EJ, Torres HA. Association Between Hepatitis C Virus and Head and Neck Cancers. J Natl Cancer Inst. 2016;108(8):DJW035.
Crossref - Gordon SC, Moonka D, Brown KA, et al. Risk for renal cell carcinoma in chronic hepatitis C infection. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1066-1073.
Crossref - Mitchell JK, Lemon SM, McGivern DR. How do persistent infections with hepatitis C virus cause liver cancer? Curr Opin Virol. 2015;14:101-108.
Crossref - Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem. 2020;295(8):2495-2505.
Crossref - Repetto O, De Paoli P, De Re V, Canzonieri V, Cannizzaro R. Levels of soluble E-cadherin in breast, gastric, and colorectal cancers. Biomed Res Int. 2014;408047.
Crossref - Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033-3046.
Crossref - Usman S, Waseem NH, Nguyen TKN, et al. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers. 2021;13(19):4985.
Crossref - Arreola-Diaz R, Majluf-Cruz A, Sanchez-Torres LE, Hernandez-Juarez J. The Pathophysiology of The Antiphospholipid Syndrome: A Perspective From The Blood Coagulation System. Clin Appl Thromb Hemost. 2022;28:10760296221088576.
Crossref - Leong HS, Mahesh BM, Day JR, et al. Vimentin autoantibodies induce platelet activation and formation of platelet-leukocyte conjugates via platelet-activating factor. J Leukoc Biol. 2008;83(2):263-271.
Crossref - Liu Y, Wang Y, Chen C, et al. LSD1 binds to HPV16 E7 and promotes the epithelial-mesenchymal transition in cervical cancer by demethylating histones at the Vimentin promoter. Oncotarget. 2017;8(7):11329-11342.
Crossref - Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Molecular Cell Biol. 2014;15(3):178-196.
Crossref - Wu Y, Sarkissyan M, Vadgama JV. Epithelial-Mesenchymal Transition and Breast Cancer. J Clin Med. 2016;5(2).
Crossref - Ding X, Wang Y, Ma X, et al. Expression of HMGA2 in bladder cancer and its association with epithelial-to-mesenchymal transition. Cell Prolif. 2014;47(2):146-151.
Crossref - Rosai J. Ackerman’s surgical pathology . 8th (ed). 1996;1(11):85-1211 .
- SAS. Statistical Analysis System, User’s Guide. Statistical. Version 9.1th ed. USA: Inst. Inc. Cary. N.C. 2012.
- Al-Thuwaini MM, Enayah SH, Ali Alwzy MAA, Husieen Hafeh AAA. Assessment the Incidence of Transitional Cell Carcinoma (TCC) of the Bladder Cancer. Int J Pharm Qual Assur. 2019;10(02):355-360.
Crossref - Gunlusoy B, Ceylan Y, Degirmenci T, et al. The potential effect of age on the natural behavior of bladder cancer: Does urothelial cell carcinoma progress differently in various age groups? Kaohsi J Med Sci. 2016;32(5):261-266.
Crossref - Gupta P, Jain M, Kapoor R, Muruganandham K, Srivastava A, Mandhani A. Impact of age and gender on the clinicopathological characteristics of bladder cancer. Indian J Urol. 2009;25(2):207-210.
Crossref - Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU International. 2010;105(3):300-308.
Crossref - Brandt MP, Gust KM, Mani J, et al. Nationwide analysis on the impact of socioeconomic land use factors and incidence of urothelial carcinoma. Cancer Epidemiol. 2018;52:63-69.
Crossref - Lutz CT, Livas L, Presnell SR, Sexton M, Wang P. Gender Differences in Urothelial Bladder Cancer: Effects of Natural Killer Lymphocyte Immunity. J Clin Med. 2021;10(21):5163.
Crossref - Hemmaid KZ, Awadalla A, Elsawy E, et al. Impact of Hepatitis C Virus (HCV) infection on biomolecular markers influencing the pathogenesis of bladder cancer. Infect Agent Cancer. 2013;8(1):24.
Crossref - Nguyen TTT, Park EM, Lim YS, Hwang SB. Nonstructural Protein 5A Impairs DNA Damage Repair: Implications for Hepatitis C Virus-Mediated Hepatocarcinogenesis. J Virol. 2018;92(11).
Crossref - Ma Y, Huang Z, Jian Z, Wei X. The association between hepatitis C virus infection and renal cell cancer, prostate cancer, and bladder cancer: a systematic review and meta-analysis. Sci Rep. 2021;11(1):10833.
Crossref - Wang Z, Tang ZY, Yin Z, et al. Metadherin regulates epithelial-mesenchymal transition in carcinoma. OncoTargets Ther. 2016;9:2429-2436.
Crossref - Baumgart E, Cohen MS, Silva Neto B, et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res. 2007;13(6):1685-1694.
Crossref - Rasteiro AM, E SEL, Oliveira PA, Gil da Costa RM. Molecular Markers in Urinary Bladder Cancer: Applications for Diagnosis, Prognosis and Therapy. Vet Sci. 2022;9(3):107.
Crossref - Zhao J, Dong D, Sun L, Zhang G, Sun L. Prognostic significance of the epithelial-to-mesenchymal transition markers E-cadherin, vimentin and twist in bladder cancer. Int Braz J Urol. 2014;40(2):179-189.
Crossref - Moussa RA, Khalil EZI, Ali AI. Prognostic Role of Epithelial-Mesenchymal Transition Markers “E-cadherin, β-Catenin, ZEB1, ZEB2 and p63” in Bladder Carcinoma. World J Oncol. 2019;10(6):199-217.
Crossref - Singh R, Ansari JA, Maurya N, Mandhani A, Agrawal V, Garg M. Epithelial-To-Mesenchymal Transition and Its Correlation With Clinicopathologic Features in Patients With Urothelial Carcinoma of the Bladder. Clin Genitourin Cancer. 2017;15(2):e187-e97.
Crossref - Favaretto RL, Bahadori A, Mathieu R, et al. Prognostic role of decreased E-cadherin expression in patients with upper tract urothelial carcinoma: a multi-institutional study. World J Urol. 2017;35(1):113-120.
Crossref - Niknami Z, Muhammadnejad A, Ebrahimi A, Harsani Z, Shirkoohi R. Significance of E-cadherin and Vimentin as epithelial-mesenchymal transition markers in colorectal carcinoma prognosis. EXCLI Journal. 2020;19:917-926.
Crossref - Attia AS, Mohamed AH, Hegazy AA, et al. Prognostic value of aquaporin-3, vimentin and E-cadherin expression in invasive breast carcinoma: an immunohistochemical study. Middle East Journal of Cancer. 2020.1;11(4):423-437.
- Bryan RT. Cell adhesion and urothelial bladder cancer: the role of cadherin switching and related phenomena. Philos Trans R Soc Lond B Biol Sci. 2015;370(1661):20140042.
Crossref - Hashim ST, Fakhry SS, Rasoul LM, Saleh TH, Alrubaii BA. Genotyping toxins of Clostridium perfringens strains of rabbit and other animal origins. Trop J Nat Prod Res. 2021;5(4):613-616.
Crossref - Eyad HN, Adbulateed YA, Lafi SA. Abnormal presentation of TB patients: anthropological study, Ann Trop Med and Public Health. 2019; 22(6): S186.
Crossref - Abdulateef Y. Role of PSA in Diagnosis of Chronic Prostatitis. Indian Journal of Forensic Medicine & Toxicology. 2020;14(1):774-779.
- Allami RH, Hassoon AH, Abdulateef YM, Ghani AA. Genetic Association of Angiotensin-converting enzyme 2 ACE-2 (rs2285666) Polymorphism with the Susceptibility of COVID-19 Disease in Iraqi Patients. Trop J Nat Prod Res. 2023;7(2):2346-2351
- Saleh T, Hashim S, Malik SN, Al-Rubaii BAL. The impact some of nutrients on swarming phenomenon and detection the responsible gene RsbA in clinical isolates of Proteus mirabilis. Int J Pharm Sci Res. 2020;1(6):437-444.
Crossref - Shehab ZH, Laftah BA. Correlation of nan1 (Neuraminidase) and production of some type III secretion system in clinical isolates of Pseudomonas aeruginosa. Biomed Res. 2018;15(3):1729-1738.
- Al-Rubii, BAL. Cloning LasB gene of pseudomonas aeruginosa eiastase 10104-2aI in E. coli BL21 and E.coli DH5? and investigated their effect on the stripping of vero cells. Pak J Biotechnol. 2017;14(4):697-705.
- Rasin KH, Algabar FA, Al-Obaidi M, Krayfa AH. Evaluation of Medical Waste Management During The Covid-19 In Diyala Governorate. Environmental Engineering & Management Journal. 2022 ;21(12):1931–1944
- Jiad AL, Ismael MK, Muhsin SS, Al-Rubaii BA. ND2 Gene Sequencing of Sub fertile Patients Recovered from COVID-19 in Association with Toxoplasmosis. Bionatura. 2022;7(3):45.
Crossref - Rasoul LM, Nsaif MM, Al-Tameemi MT, Al-Rubaii BA. Estimation of primer efficiency in multiplex PCR for detecting SARS-Cov-2 variants. Bionatura. 2022;7(3):48.
Crossref - Abbas MS, Ahmed AG, Ali SQ, AL-Rubaii BA. Immunological inflammatory factors in patients diagnosed with COVID-19. Biomedicine. 2023; 43(1):230-235.
Crossref - Ahmed AA, Khaleel KJ, Fadhel AA, Al-Rubaii BAL. Chronic Myeloid Leukemia: A retrospective study of clinical and pathological features. Bionatura.
Crossref - Ali SM, Lafta BA, Al-Shammary AM, Salih HS. In vivo oncolytic activity of non-virulent newcastle disease virus Iraqi strain against mouse mammary adenocarcinoma. AIP Conference Proceedings. 2021;2372:030010.
Crossref - Ali SM, Laftah BA, Al-Shammary AM, Salih HS. Study the role of bacterial neuraminidase against adenocarcinoma cells in vivo. AIP Conference Proceedings. 2021;2372:030009.
Crossref - Hamoode RH, Alkubaisy SA, Sattar DA, Hamzah SS, Saleh TH, Al-Rubaii BAL. Detection of anti-testicular antibodies among infertile males using indirect immunofluorescent technique. Biomedicine. 2022;42(5):978-982.
Crossref - Rasoul LM, Marhoon AA, Albaayit SFA, Ali RW, Saleh,TH, Al-Rubaii BAL. Cytotoxic effect of cloned EGFP gene on NCI-H727 cell line via genetically engineered gene transfer system. Biomedicine. 2022;42(5):938- 942.
Crossref - Rasoul LM, Allami RH, Alshibib AL, laftaah Al-Rubaii BA, Sale TH. Expression and cytotoxic effect of recombinant Newcastle Disease Virus (rNDV) vector expressing enhanced green fluorescent gene in JHH5 cell line. Biomedicine. 2023; 43(1):205-209.
Crossref - Jawad NK, Numan AT, Ahmed AG, Saleh TH, Al-Rubaii BA. IL-38 gene expression: A new player in Graves’ ophthalmopathy patients in Iraq. Biomedicine. 2023;43(1):210-215.
Crossref - Bresam S, Al-Jumaily RM, Karim GF, Al-Rubaii BA. Polymorphism in SNP rs972283 of the KLF14 gene and genetic disposition to peptic ulcer. Biomedicine. 2023; 43(1):216-220.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.