ISSN: 0973-7510
E-ISSN: 2581-690X
Uropathogenic Escherichia coli (UPEC) is prevalent in urinary tract infections (UTIs). UPEC’s biofilm production enables it to invade and persist in the uroepithelium, leading to recurrent UTIs. The biofilm formation is associated with antibiotic resistance. To overcome this resistance, non-conventional compounds must be developed as an alternative to conventional antibiotics. Silver nanoparticles (AgNPs) are significant due to their antibacterial activity against diverse organisms. This study was done to investigate the antibacterial and anti-biofilm effects of AgNPs on UPEC. AgNPs were biosynthesized using Pseudomonas aeruginosa ATCC 27853. AgNPs were characterized using visual inspection and scanning electron microscopy. The Agar well diffusion method was employed to assess the antibacterial activity of AgNPs against UPEC isolates. The study utilized the tissue culture plate method to investigate both the biofilm and anti-biofilm properties of AgNPs. Following incubation, Ps.aeruginosa and silver nitrate (AgNO3) mixture exhibited a colour change from pale yellow to dark brown. The mean size of spherical AgNPs observed under a scanning electron microscope was 24.187 ± 8.019 nm. 130 UPECs were obtained. AgNPs exhibited antibacterial activity at a concentration of 20 µg/ml against all tested UPEC strains. Among UPEC strains that produced biofilms, a significant inhibition of 99.89 ± 0.45% was observed at a higher concentration of 512 µg/ml of AgNPs. Ps.aeruginosa produces nitrate reductase enzyme that can potentially convert AgNO3 to AgNPs. The biosynthesized AgNPs exhibit antibacterial and anti-biofilm activity against all tested UPEC strains.
Pseudomonas aeruginosa, AgNPs, UPEC, Antibacterial Activity, Anti-biofilm Efficacy
Urinary tract infection (UTI) is a major cause of febrile illness in humans, requiring antibiotic treatment.1 Among the various etiological agents, uropathogenic Escherichia coli (UPEC) is mostly responsible for uncomplicated and community acquired urinary tract infection.2 UPEC strains divert from their commensal status as intestinal flora, enter in the urinary tract, exhibit different virulence factors allowing them to cause urinary tract infections. A study was conducted to explore the spread of E. coli isolates from UTI cases, hospital wastewater and municipal wastewater treatment plants.3 According to this study, UTI strains formed a minor proportion of the total bacteria in hospital sewage. Dissemination of hospital-associated strains through municipal wastewater treatment plants causing UTIs was not confirmed.4 Among the various virulence factors of UPEC, biofilm formation is the main virulence factor. Biofilms are the groups of the microorganisms that colonize on a surface and remain enclosed within the extracellular matrix which is produced by them.5 Production of biofilm in UPEC causes the colonization of bacteria following infections in urinary tract.6
Various investigators have noted the close relation between the production of biofilm and multidrug resistance in UPEC.7 Biofilm acts as a barrier for penetration of an antibiotic, and thus promotes multidrug resistance in bacteria.8 It is stated that bacteria enclosed within the biofilms are more resistant to antibiotics, which causes limitations in conventional antibiotic therapies.5 These limitations push forward the use of non-conventional alternative nanosized compounds to treat such infections caused by biofilm forming bacteria. These nanoparticles (NPs) having a size 1–100 nm, exhibit more surface to volume ratio and thus, they have more interaction with the microorganisms which increase their antimicrobial activity.9
Among various metal nanoparticles, silver nanoparticles (AgNPs) play an important role in different branches of medical field because of their antibacterial as well as anti-biofilm activity against various organisms, also they are less toxic to human cells.10 If the bacterial cells are treated with AgNPs, they cannot colonize to form biofilms. Thus, AgNPs show antibacterial activity along with the prevention of biofilm formation.11
This research intended to study the antibacterial as well as anti-biofilm property of biosynthesized AgNPs against uropathogenic E.coli.
An experimental study was carried out from February – December 2021. ATCC strain of Pseudomonas aeruginosa (27853) was used to synthesize AgNPs under the optimized culture parameters (silver nitrate- AgNO3– concentration -0.4 gm/L, incubation temperature- 70°C, at alkaline pH, and incubation time- 96 hours).12-15 For characterization of AgNPs, the colour of culture mixture containing Pseudomonas aeruginosa and AgNO3, was observed by visual inspection, and Scanning Electron Microscopy was used to reveal the size and shape of AgNPs. 130 uropathogenic E.coli (UPEC) were isolated and species level identification was done according to the standard recommended guidelines.16
Study of antibacterial property of AgNPs against UPEC by agar well diffusion method17,18
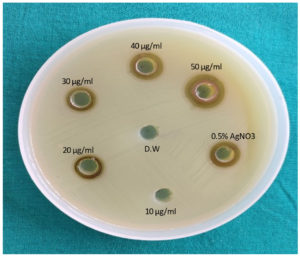
Mueller Hinton agar (MHA) was used to study antibacterial property of AgNPs. AgNPs in the concentrations of 10, 20, 30, 40 and 50 µg/ml were used in this assay. 0.5% AgNO3 was used as positive control and sterile distilled water was used as negative control.
Detection of biofilm formation by TCPM19,20
For this assay, E. faecalis ATCC 29212 and Pseudomonas aeruginosa ATCC 27853 were considered as positive and negative controls, respectively. Detection of biofilm formation by TCPM was firstly described by Christensen et al. 200 µl of the overnight culture (at 37°C) of test organisms in Brain Heart Infusion (BHI) broth was added in sterile microtiter plate having 96 wells. Plate was incubated at 37°C for 24 hours, then phosphate buffer saline was used to wash all the wells of the plate. After drying in an inverted position, 1% crystal violet was used to stain all the wells for 15 min, following the solubilization of crystal violet with 200 µl of ethanol and acetone mixture. To determine final O.D., A600 filter of ELISA microtiter plate reader was used. Final O.D >1 was considered as positive for biofilm formation.
Detection of anti-biofilm activity of AgNPs
This method was originally described by Gurunathann et al.21 and Barapatre et al.22 In ELISA microtiter plate, AgNPs were diluted and 2-fold dilutions were done from 512 µg/ml to 1 µg/ml following the addition of 180 µl of Mueller Hinton Broth (MHB) and 10 µl of test organism to all the wells. Plate was incubated at 37°C for 24 hours, then phosphate buffer saline was used to wash all the wells of the plate. After drying in inverted position, 1% crystal violet was used to stain all the wells for 15 min, following the solubilization of crystal violet with 200 µl of ethanol and acetone mixture. The absorbance was measured at 620 nm. 0.5% AgNO3 and cells without AgNPs were considered as positive and negative controls, respectively. This experiment was repeated for three times and the average of the three O.Ds was considered as final O.D.
To calculate the percentage of biofilm inhibition, the following formula was used:
% of Biofilm Inhibition (B.I.) = [ 1- (OD 620 of cells treated with AgNPs)/(OD 620 of non- treated control)] x 100
As shown in Figure 1, the colour of the broth containing Pseudomonas aeruginosa and AgNO3 solution, turned yellow to brown, after using optimum culture parameters.
Figure 1. Showing the formation of AgNPs (change in colour from pale yellow to dark brown)
A- Culture supernatant of P. aeruginosa + AgNO3 solution
B- Culture supernatant of P. aeruginosa + AgNO3 solution after 96 hours of incubation
Optimum culture parameters such as pH, incubation time, temperature, and Ag ion concentration play an important role in AgNP synthesis.13
In the present study, alkaline pH was used for the biosynthesis of AgNPs. Similarly, a study stated that stable and small nanoparticles were formed at alkaline pH. The nucleation of the nanoparticles was caused due to lower pH while at higher pH, electrostatic repulsion could be seen among nanoparticles and that will lead to formation of smaller nanoparticles.23
Silver nitrate (AgNO3) concentration can also affect the size and morphology of the AgNPs. In our study, 0.4 g/l concentration of AgNO3 was used and the same concentration was also used by other studies and they have observed, that at this concentration of AgNO3, there was an increase in the silver ions reduction rate to AgNPs. At the concentration below 0.4 g/l, AgNPs synthesis was reduced and at higher concentrations of AgNO3, aggregation of nanoparticles can be seen.12,13
Generally, the conversion rate of AgNO3 to AgNPs can be increased at higher temperatures. Higher concentration of AgNPs was clearly observed with increasing temperature, and when the temperature increases, smaller particles would be produced.13
Similar to the present study, previous research studies,12,13 have also used Ps.aeruginosa for the biosynthesis of AgNPs. A study24 has stated that this bacterium secretes NADH and NADH-dependent nitrate reductase enzyme which reduces silver ions to AgNPs. Nanoparticles synthesized from Ps.aeruginosa are more stable and when compared with the other bacterial nanoparticles Pseudomonas nanoparticles exhibit more antibacterial activity.12
In Figure 2, 24.187 ± 8.019 nm was the mean size of spherical AgNPs under scanning electron microscope. Similar to the present study, some other studies 12, 13 also found the spherical AgNPs synthesized from Pseudomonas spp. and the size varied from 20-60 nm.
The antibacterial property of AgNPs against UPEC (N=130) by agar well diffusion assay, was noted at 20 µg/ml of AgNPs and above (Figure 3).
Similar to our results, other studies also,25,26 reported the antibacterial property of AgNPs against E.coli at 20 µg/ml of AgNPs and 10 µg/ml of AgNPs, respectively. According to another study,17 antibacterial property of AgNPs against gram negative organisms was observed at 20 µg/ml to 40 µg/ml of AgNPs.
There are multiple theories for the antibacterial activity of AgNPs.18,27,28 According to the first theory, AgNPs bind to the functional groups of the bacterial cell wall and lead to the disturbance of the respiratory enzyme. Secondly, AgNPs penetrate through the bacterial cell wall and there they release silver ions and thus cause inhibition of DNA replication and cell division causing the death of bacterial cell.
The bactericidal effect of AgNPs is due to the attachment of AgNPs to the cell membrane surface. It is reported that the positive charge of silver ions can attract the negatively charged bacterial cell membrane by the electrostatic interaction,26,29 and change the physicochemical properties of the cell membranes which lead to disturbance in permeability, osmoregulation, electron transport and respiration.28
Among the various methods for biofilm detection, Tissue Culture Plate method (TCPM) is considered as the gold standard method.30
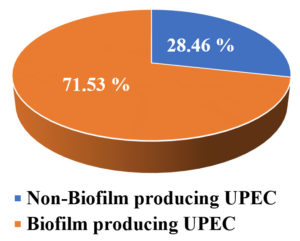
Among 130 isolated UPEC, by TCPM, 71.53 % of uropathogens were biofilm producers (Figure 4). Similar results were found in various studies,31-33 where the prevalence of biofilm producing UPEC was ranging from 54%-67%. Uropathogens have some virulence factors which help bacteria to sustain on to the uroepithelium and that leads to the biofilm formation.34
UPEC biofilms are formed on biotic and abiotic surfaces like uroepithelium and urinary catheters by means of flagella, fimbriae, curli, and conjugative pili, all these factors are involved in the adhesion of UPEC to biotic and abiotic surfaces. There are some proteins called autotransporter proteins, which help bacteria to aggregate and adhere on surfaces and produce exopolysaccharides (such as cellulose) which is the main element of the biofilm matrix.35
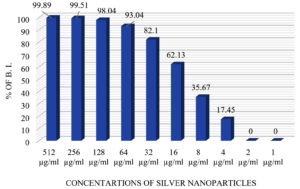
In this study, anti-biofilm activity of AgNPs was evaluated in terms of percentage of inhibition by TCPM (Figure 5). Anti-biofilm potential of AgNPs was noted at 4 µg/ml of AgNPs and above and at 512 µg/ml of AgNPs, 99.89 ± 0.45 % of biofilm inhibition was seen in all the tested UPEC (Figure 6).
Figure 5. showing antibiofilm activity of AgNPs against biofilm forming UPEC (Tissue culture plate method)
Similar results were stated by other studies,22,36 where at 0.5 – 64 µg/ml of AgNPs, 60-80 % biofilm inhibition was seen in E.coli. Some studies 21,37 have stated that anti-biofilm activity of AgNPs is due to the water channels which are present within the biofilm. These channels are mainly for nutrient transportation, but AgNPs find their way through these channels for diffusion and exhibit antimicrobial activity. Another study also stated38, that AgNPs neutralize the adhesive compounds required for biofilm formation and eventually quench the quorum-sensing property of biofilm-forming bacteria.
This study shows the potential use of AgNPs as an antibacterial as well as anti-biofilm agent to treat UTIs caused by biofilm producing UPEC. In future, AgNPs can be coated on medical devices to control antibiotic resistant biofilm but to determine the clinical relevance of these data, many in-vivo studies and clinical trials are needed.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PAJ performed conceptualization, data curation, original draft writing and Investigation.
SAG performed supervision, data validation, reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethical Committee, Bharati Vidyapeeth (Deemed to be University) Medical College & Hospital, Sangli, with reference number BV(DU)/MCH/2146/20-21 dated 28/01/2021.
- Kot B. Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol J Microbiol. 2019;68(4):403-415.
Crossref - Verma Y, Shah K, Hemachander SS. Increasing antibiotic resistance amongst Uropathogenic E. coli isolated from inpatients with symptomatic UTI – a three-year study. Trop J Pathol Microbiol. 2017;3(3):298-304.
Crossref - Elgendy MY, Ali SE, Abbas WT, Algammal AM, Abdelsalam M. Th role of marine pollution on the emergence of fish bacterial diseases. Chemosphere. 2023;344:140366.
Crossref - Davidova-Gerzova L, Lausova J, Sukkar I, et al. Hospital and community wastewater as a source of multidrug-resistant ESBL-producing Escherichia coli. Front Cell Infect Microbiol. 2023;13:1184081.
Crossref - Raya S, Belbase A, Dhakal L, Prajapati KG, Baidya R, Bimali N. In-Vitro Biofilm Formation and Antimicrobial Resistance of Escherichia coli in Diabetic and Nondiabetic Patients. Hindawi BioMed Res Int. 2019;1474578.
Crossref - Karigoudar RM, Karigoudar MH, Wavare SM, Mangalgi SS. Detection of biofilm among uropathogenic Escherichia coli and its correlation with antibiotic resistance pattern. J Lab Physicians. 2019;11(1):17-22.
Crossref - Dawadi P, Khanal S, Joshi TP, et al. Antibiotic Resistance, Biofilm Formation and Sub-Inhibitory Hydrogen Peroxide Stimulation in Uropathogenic Escherichia coli. Microbiol Insights. 2022;15:1-9.
Crossref - Kudinha T, Kong F. Antibiotic Susceptibility Patterns and Biofilm Production by Uropathogenic Escherichia coli from Reproductive Age Women in a Region of NSW. J Infect Dis Epidemiol. 2022;8(10):280.
Crossref - Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. molecules. 2016;21(7):836.
Crossref - Selem E, Asmaa F, Mekky, Wesam A, Hassanein , Reday FM, Selim YA. Antibacterial and antibiofilm effects of silver nanoparticles against the uropathogen Escherichia coli U12. Saudi J Biol Sci. 2022;29(11):103457.
Crossref - Nashat E, El Nagdy MM, Weefky GF, Nabiel Y. Effect of Silver Nanoparticles on Biofilm Producing Multi Drug Resistant Uro-pathogenic E. coli isolated from Catheterized Patients in Mansoura University Hospital. Egypt J Med Microbiol. 2022;31(1):63-68.
Crossref - Peiris MMK, Fernando SSN, Jayaweera PM, Arachchi NDH, Guansekara TDCP. Comparison of antimicrobial properties of silver nanoparticles synthesized from selected bacteria. Indian J Microbiol. 2018;58(3):301-311.
Crossref - Peiris MK, Gunasekara CP, Jayaweera P, Dharachchi N. Biosynthesized silver nanoparticles: are they effective antimicrobials? MemInst Oswaldo Cruz, Rio DE Janeiro. 2017;112(8):537-543.
Crossref - Keshari AK, Shrivastava R, Singh P, Yadav V. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integr Med. 2020;11(1):37-44.
Crossref - Soman S, Ray JG. Silver nanoparticles synthesized using leaf extract of ziziphus oenoplia (l.) mill: Characterization and assessment of antibacterial activity. Photochem PhotobiolB. 2016;163:391-402.
Crossref - Koneman E, allen SD, janda WM, Schreckenberger PC. Colour atlas and textbook of diagnostics microbiology, 5 thedn, san fransisco, lippincot 2006:624-671
- Mondal AH, Yadav D, Ali A, Khan N, Jin JO. Antibacterial and anticandidal activity of silver nanoparticles biosynthesized using Citrobacter species MS5 culture supernatant. Biomolecules. 2020;10(6):944.
Crossref - Manivasagan P, Venkatesan J, Senthikumar K, Sivakumar K. Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. Biomed Res Int. 2013;287638.
Crossref - Christensen GD, Simpson WA, Younger JJ, L M Baddour, Barrett FF, Melton DM, Beachey EH. Adherence of coagulase negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996-1006.
Crossref - Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis. 2011;15(4):305-311.
Crossref - Kalishwaralal K, Barathmaniknath S, Pandian SR, Deepak V. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B Biointerfaces. 2010;79(2):340-344.
Crossref - Barapatre A, Aadil KR, Jha H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Biosour Bioprocess. 2016;3:8.
Crossref - Maria BS, Devadiga A, Shettykodialbail V, Saidutta MB. Synthesis of silver nanoparticles using medicinal ziziphus xylopyrus bark extract. Applied Nanoscience.2015:5:755-762.
Crossref - Yanga J, Wangb Q, Wangc C, et al. Pseudomonas aeruginosa synthesized silver nanoparticles inhibit cell proliferation and induce ROS mediated apoptosis in thyroid cancer cell line (TPC1). Artif Cells Nanomed Biotechnol. 2020;48(1):800-809.
Crossref - Maiti S, Krishnan D, Barman G, Ghosh SK, Laha JK. Antimicrobial activities of silver nanoparticles synthesized from Lycopersiconesculentum extract. J Anal Sci Technol. 2014:5(1):1-7.
Crossref - Chandrakanth RK, Ashajyoti C, Oli AK, Prabhurajeshwar C. Potential bactericidal effect of silver nanoparticles synthesised from Enterococcus species. Orient J Chem. 2014; 30(3).
Crossref - Zafar S, Zafar A. Biosynthesis and characterization of silver nanoparticles using Phoenix Dactylifera Fruits extract and their In Vitro antimicrobial and cytotoxic effects. Open Biotechnol J. 2019;13:37-43.
Crossref - Ibrahim HMM. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. Journal of radiation research and applied sciences. 2015;8(3):265-275.
Crossref - Kesithevar M, Saravavan M, Prakash P, et al. Green synthesis of silver nanoparticles using Alysicarpusmonilifer leaf extract and its antibacterial activity against MRSA and CONs isolated in HIV patients. J Interdiscip Nanomed. 2017;2(2):131-141.
Crossref - Asati S, Chaudhary U. Comparison of Tissue culture plate and modified tissue culture plate method for biofilm detection in members of family enterobacteriaceae. J Clin Diagn Res. 2018;12(7): DC20 – DC23.
Crossref - Shrestha R, Khanal S, Poudel P, et al. Extended spectrum ג-lactamase producing uropathogenic Escherichia coli and the correlation of bioflm with antibiotics resistance in Nepal. Ann Clin Microbiol Antimicrob. 2019;18(1):42.
Crossref - Verma S, Patil SS. In Vitro biofilm formation by uropathogenic bacteria and their antibiotic susceptibility pattern. JKIMSU. 2016;5(3).
- Niveditha S, Pramodhini S, Umadevi S, Kumar S, Stephen S. The Isolation and the Biofilm Formation of Uropathogens in the Patients with Catheter Associated Urinary Tract Infections (UTIs). J Clin Diagn Res. 2012;6(9):1478-1482.
Crossref - Shanmugam K, Thyagarajan R, Katragadda R, Vajravelu L, Jayachandran AL. Biofilm formation and Extended Spectrum Beta Lactamases (ESBL) producers among the gram-negative bacteria causing Urinary tract infections. Int J Med Microbiol Trop Dis. 2017;3(3):86-90.
Crossref - Naziri Z, Kilegolan JA, Moezzi MS, Derakhshandeh A. Biofilm formation by uropathogenic Escherichia coli: a complicating factor for treatment and recurrence of urinary tract infections. J Hosp Infect. 2021;117:9-16.
Crossref - Akkther T, Ranjani S, Hemlatha S. Nanoparticles engineered from endophytic fungi (Botryosphaeria rhodina) against ESBL producing pathogenic multidrug resistant E.coli. Environ Sci Eur. 2021;33:83.
Crossref - Ansari MA, Khan HM, Khan AA, Cameotra SS, Pal R. Antibiofilm efficacy of silver nanoparticles against biofilm of Extended spectrum Beta lactamase isolates of E. coli and Klebsiella pneumoniae. Applied Nanoscience. 2014;4:859-868.
Crossref - Alzahrani RR, Alkhulaifi MM, Alenazi NM, et al. Characterization and biological investigation of silver nanoparticles biosynthesized from Galaxaur arugosa against multidrug resistant bacteria. J Taibahuniv Sci. 2020;14(1):1651-1659.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.