ISSN: 0973-7510
E-ISSN: 2581-690X
Streptococcus mutans is a bacterium involved in the formation of caries. Red ginger essential oil is extracted from rhizomes, has a content of natural compounds, and is used in medicine for antibacterial, anti-inflammatory, and anticancer. To prove the effect of red ginger essential oil on Streptococcus mutans bacteria. The agar diffusion method is performed to test antimicrobial activity and determine the Minimum Inhibitory Concentration (MIC) against Streptococcus mutans. Furthermore, the adherence test of Streptococcus mutans bacteria was carried out using a spectrophotometer l = 570nm. MIC red ginger essential oil against Streptococcus mutans bacteria at concentrations of 0.78% and 1.56%. In the 0.78% concentrate, an adherence value of 2.12 was obtained and in the 1.56% concentrate, an adherence value of 1.93 was obtained and 3.125% concentrate obtained an adherence value of 1.78. Red ginger essential oil has potential as an antimicrobial agent by inhibiting the adherence of Streptococcus mutans bacteria.
Adherence, Fluorosis, Red Ginger Essential Oil, Streptococcus mutans
Dental caries poses a significant oral health challenge in Indonesia, as indicated by findings from the 2018 Basic Health Research (RISKESDAS). As reported in the same study, the prevalence of caries in Indonesia, reaching 88.8%, surpassed the target set by the World Health Organization (WHO).1,2 Dental caries is a condition where teeth are demineralized due to bacteria that produce acids as a result of carbohydrate fermentation and will colonize to form biofilms.1,3,4-7 Streptococcus mutans, a gram-positive bacterium, is identified as the primary culprit behind dental caries development in humans. Foods containing carbohydrates, especially sucrose, can accelerate the attachment of Streptococcus mutans to the enamel surface and will become dental plaque. These bacteria produce acids to lower the pH of the host or its surroundings.8-11 A biofilm is a collection of microbes attached to the surface of a tooth and encased in a polysaccharide matrix. Bacterial biofilms consist of bacterial enzymes such as GTF, FTF polysaccharides, nucleic acids, proteins, bacterially secreted compounds, salivary components (including salivary enzymes and food debris), and other host constituents. Mechanical cleaning, such as brushing and flossing, can serve to control bacterial biofilms on the surface of teeth.1,11-15 Caries prevention measures to date are carried out with the administration of effective ingredients such as fluoride.1,13-19 Excessive use of fluoride has side effects of fluorosis.1,13 Herbal medicines today are developed and receive greater attention because they have many properties, are safer to cure diseases, and are well tolerated.20 Natural ingredients such as herbal plants that can be used to prevent caries are red ginger because it has various health benefits. Red ginger has benefits as an antibacterial, anti-inflammatory, and anticancer.21-24 Red ginger essential oil, as a small aromatic, organic, and molecular natural metabolic product, is a volatile compound of red ginger that plays an important role in therapeutic activity.23,25 Red ginger essential oil is a natural ingredient that shows the best antibacterial activity against gram-positive bacteria such as Streptococcus mutans bacteria.23,24,26-29 Some researchers have attributed the antibacterial activity of red ginger essential oil to key active ingredients such as zingiberene, α-farnesene, 6-gingerol, and α-curcumene. Zingiberene has the highest content of all components, followed by α-curcumene.22-24,30 Previous research has been conducted on red ginger essential oil on Staphylococcus aureus as an antibacterial, obtaining effective concentrations of MIC of 2.0 and 1.0 μL/mL.22 The process of colonization of Streptococcus mutans bacteria on tooth surfaces, known as biofilm formation, involves physicochemical and molecular interactions that begin with the process of bacterial adherence.24,29,30 Bacterial adherence is the first step in the formation of caries, which involves the interaction between the tooth surface.9,29,30 One of the most important pathogenic mechanisms of bacteria is its adherence capacity through electrostatic and chemical interactions on the surface of teeth and other bacteria in the complex extracellular matrix.14,29,31,32
The purpose of this study is to assess the optimal concentration of red ginger essential oil for its antibacterial efficacy against Streptococcus mutans and to substantiate its effectiveness in hindering the adherence of Streptococcus mutans bacteria.
The first phase is the preliminary stage, which involves the production of bacterial culture and ginger essential oil. Bacterial culture was used to multiply S. mutans Serotype C stock obtained from Airlangga University’s Research Center, by inoculating one dose of pure S. mutans culture into Tryptone Soya Broth (TSB) media and incubation at 37°C for 24 hours. The steam dilution is then used to create ginger essential oil. Red ginger was obtained from the Herbal Material Medika Laboratory, Malang. After that, the ginger was cleaned with distilled water to remove the microbes on the red ginger, then it was sliced thin and then put in steam distillation for five hours until the essential oil was obtained. To analyze the compounds present in essential oils, an examination was carried out using Gas Chromatography-Mass Spectrometry (GC-MS). The acquired essential oil was preserved in a yellow container at a temperature of 4°C until its utilization. After completing the preparation stage, test the MIC and MBC first. Microdilution tests were performed on concentrations of 100%, 50%, 25%, 12.5%, 6.25%, 3.125%, 1.56%, and 0.78%. In each test tube, 0.05 ml of the bacterial culture with the McFarland 0.5 standard was cultivated in BHIB medium in each test tube with a varied concentration. This is known as the treatment group. Following that, the positive control 0.05 ml of BHIB media and Streptococcus mutans bacterial suspension (with McFarland standard 0.5) were prepared without the addition of red ginger essential oil. Negative controls were created using BHIB media with sucrose or red ginger essential oil. The test tubes were then all incubated. The biofilm adherence test of Streptococcus mutans bacteria was cultivated in a test tube for 24 hours in Brain Heart Infusion Broth (BHIB) media with a slope of 30°C at 37°C. Tube K0 contains cultures cultured in Brain Heart Infusion Broth (BHIB), tube K1 contains cultures cultured in BHIB with 5% sucrose as the negative control group, and tubes P1, P2, and P3 contain cultures cultured in BHIB with 5% sucrose and ginger essential oil. The culture is then slowly drained out of the test tube. Cells adhered to the tube’s surface were removed with 0.5 M NaOH and centrifuged. The cells were then cleaned and submerged in a saline solution. Their adherence was observed using a spectrophotometer (BIORAD SmartSpec Plus) with a wavelength of 570 nm.
The ginger essential oil obtained consists of 1,4 Cineol, Alpha-Curcumen, Alpha-Zingiberene, (E)-farnesene, Beta-Bisabolene. MIC results were found in the treatment consisting of BHIB with Streptococcus mutans, 5% sucrose, and 0.78% red ginger essential oil, and BHIB with Streptococcus mutans, 5% sucrose, and 1.56% red ginger essential oil. MBC results on treatment consisting of BHIB with Streptococcus mutans, 5% sucrose, and 3.125% red ginger essential oil, are shown in Table.
Table:
Average and standard deviation of adherence of Streptococcus mutans bacteria after administration of red ginger essential oil
No. |
Groups |
N |
Mean |
SD |
Maximum |
Minimum |
|---|---|---|---|---|---|---|
1 |
Streptococcus mutans + BHIB |
4 |
1.68 |
0.037 |
1.63 |
1.72 |
2 |
Streptococcus mutans + BHIB + Sukrosa 5% |
4 |
2.32 |
0.086 |
2.21 |
2.41 |
3 |
Streptococcus mutans + BHIB + Sukrosa 5% + essential oil 0.78% |
4 |
2.12 |
0.021 |
2.1 |
2.15 |
4 |
Streptococcus mutans + BHIB + Sukrosa 5% + essential oil 1.56% |
4 |
1.93 |
0.031 |
1.89 |
1.96 |
5 |
Streptococcus mutans + BHIB + Sukrosa 5% + essential oil 3.125% |
4 |
1.78 |
0.034 |
1.75 |
1.8 |
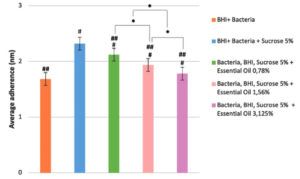
The findings indicated that, subsequent to exposure to ginger essential oil, the average adherence of Streptococcus mutans exhibited a decline when compared to the negative control group, wherein Streptococcus mutans was cultivated in BHIB + 5% sucrose media. Notably, as the concentration of ginger essential oil increased, the average adhesion of the Streptococcus mutans bacterium decreased. This substantiates the correlation that a heightened concentration of ginger essential oil corresponds to a diminished adhesion of the Streptococcus mutans bacterium (Figure).
Figure. Bar table of the average adherence concentration of bacteria for each group
*: Significant difference value (P<0.05) between treatment groups
#: Value of significance difference (P<0.05) between certain groups with group 1
##: The value of the difference in significance (P<0.05) between certain groups with group 2
Data analysis was performed using Kolmogorov-Smirnov to see the normality of the data distribution. Then, the homogeneity between the data variances was tested using Levene’s Test. The Oneway-ANOVA and Duncan tests are carried out to determine the significance of the differences between groups. As per the outcomes of the Kolmogorov-Smirnov normality test conducted through the SPSS (Statistical Product and Service Solution) software, it was determined that all groups exhibited normal distribution, with a p-value of 0.790 (p < 0.05). Subsequently, a test for homogeneity of sample variances was executed utilizing Levene’s Test to assess whether variance homogeneity was satisfied. The value of p = 0.123 (p > 0.05) was obtained, which means that the data between variances are homogeneous. However, in this study, there was a group whose normality still needed to be fulfilled. Consequently, the decision was made to employ the parametric test, specifically Oneway ANOVA, resulting in a probability value of 0.000. This probability value is below the 0.05 significance level, signifying the rejection of the null hypothesis (H0). Hence, it can be inferred that a statistically significant difference exists between the groups.
Streptococcus mutans is a pathogenic bacterium found in the human body, mainly located on the surface of the teeth which contributes to the development of caries.22-24,30 The karyogenic potential of Streptococcus mutans is attributed to its ability to form biofilms on tooth surfaces.10,11,28,33-35 The prevalence of dental caries in the population in developing countries is currently still high, so research is needed related to efforts to prevent dental caries. Currently, the therapy used in the prevention of dental caries is to use fluoride both systemically and topically.1, 17-20 The topical use of fluoride that children often use is toothpaste. Children who tend not to understand the use of toothpaste often swallow paste when brushing their teeth, so fluorosis will be at risk.1,17 The exact pathomechanism of fluorosis remains incompletely understood; however, it is highly probable that the process occurred at the time of biomineralization resulting in mineralization disorders. The clinical manifestations of fluorosis manifest as hypomineralization and increased porosity of enamel, potentially causing significant hypoplasia of enamel. Enamel fluorosis is clinically evident through stains and varying degrees of discoloration on the enamel.17 With the toxicity of fluoride, herbal ingredients were developed that have antibacterial benefits. This study used essential oil from red ginger because it has high antibacterial activity compared to other types of ginger.36 Essential oils can be obtained from a wide variety of plants, one of which is red ginger. Parts of the plant such as leaves, flowers, fruits, seeds, stems and rhizomes can be processed into essential oils. The rhizome or part we often know as the “root” is most commonly processed because it contains the highest concentration of essential oils. The results of this research are the same as previous research, the chemical compounds of red ginger essential oil that has antibacterial activity include zingiberene, α-farnesene, 6-gingerol, and α-curcumene. The highest content of essential oil from ginger is zingiberene.23,31,32 The mechanism of action of red ginger essential oil as an antibacterial is carried out by damaging cell membranes, destroying cell membrane structures, and denaturing proteins, causing bacterial cell death. Growth metabolites such as enzymes, proteins, water, carbohydrates, and organic ions are bound by the hydrophobic compounds of red ginger essential oil so that the survival needs of bacterial cells are not met. Essential oils have also been shown to interfere with bacterial metabolism by interfering with protein expression in bacteria, especially some large molecular-weight proteins.7,27 From the MiC and MBC tests, the MIC concentration of red ginger essential oil against Streptococcus mutans bacteria was 0.78% and 1.56%, and the MBC concentration was 3.125%. The results will be used for further research on the adherence test of red ginger essential oil against Streptococcus mutans bacteria using 0.78%, 1.56%, and 3.125% concentrates. Processes of bacterial colonization on tooth surfaces, known as biofilm formation, begin with the process of bacterial adherence.10,16,28,34 The adherence of Streptococcus mutans bacteria works through the enzyme glucosyltransferase, which converts sucrose into glucan.1,12,15,28 These glucans play a role in bacterial adherence, which will then form biofilms, which can increase the adherence of Streptococcus mutans to the tooth surface.1 Besides attaching bacteria through the adherence of specific bonds between bacteria and hosts, bacteria can also attach to tooth surfaces with non-specific physical bonds such as van der Waals forces, electrostatic forces, and hydrophobic interaction.36 In classical DLVO theory, the process of bacterial adherence is produced from the Lifshitz-Van der Waals force, namely the process of electrostatic bilayer interaction and acid-base binding.37 In bacteria, there are fimbriae that contribute to the initial adherence of the tooth surface and the adherence between bacteria. Fimbriae are proteins from the EPS matrix (extracellular polysaccharide) that play a role in the formation of biofilms and increase the development of EPS.35,38,39 The second adherence process is irreversible through bacterial cell surface hydrophobicity, hydrogen bonds, covalent bonds, ionic bonds, and dipole-dipole interactions.35
The red ginger essential oil can act as an antibacterial Streptococcus mutans. Essential oils can decrease the adherence of bacterial biofilms as antibacterial Streptococcus mutans. The increase in the concentration of essential oils by 0.78%, 1.56%, and 3.125% lowers the adherence of Streptococcus mutans.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PN conceptualized the study. AAP performed data collection. PN and DPW applied methodology and performed validation. PN wrote original draft. DPW, KL, AAP, PN wrote the manuscript. KL reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Universitas Airlangga Faculty of Dental Medicine Health Research, Indonesia, with reference number 921/HRECC.FODM/VIII/2023
- Chen J, Kong L, Peng X, et al. Core microbiota promotes the development of dental caries. Applied Sciences. 2021;11(8):3638.
Crossref - Alshammary FL, Mobarki AA, Alrashidi NF, Madfa AA. Association between different behavioral factors and dental caries among children attending the dental clinics in a sample from Saudi Arabia. BMC Oral Health. 2023;23(1):198.
Crossref - Heersema LA, Smyth HDC. A multispecies biofilm in vitro screening model of dental caries for high-throughput susceptibility testing. High-Throughput. 2019;8(2):14.
Crossref - Khalid GS, Hamrah MH, Ghafary ES, Hosseini S, Almasi F. Antibacterial and antimicrobial effects of xanthorrhizol in the prevention of dental caries: A systematic review. Drug Des Devel Ther. 2021;15:1149-1156.
Crossref - Singh P, Sehgal P. G.V Black dental caries classification and preparation technique using optimal CNN-LSTM classifier. Multimedia Tools and Applications. 2021;80(4):5255-5272.
Crossref - Shirato M, Nakamura K, Tenkumo T, et al. Inhibition of tooth demineralization caused by Streptococcus mutans biofilm via antimicrobial treatment using hydrogen peroxide photolysis. Clin Oral Investig. 2023;27(2):739-750.
Crossref - Zhang Z, Ji Y, Liu D, et al. Heat Shock Protein Inhibitors Show Synergistic Antibacterial Effects with Photodynamic Therapy on Caries-Related Streptococci In Vitro and In Vivo . mSphere. 2023;8(2):e0067922.
Crossref - Maasi G, Stsepetova J, Joesaar M, Olak J, Mandar R. Different Patterns of Virulence Genes in Streptococcus mutans and Streptococcus sobrinus Originating from Estonian Toddlers-Mothers Cohort. Microbiol Res. 2022;13(4):928-936.
Crossref - Lemos JA, Palmer SR, Zeng L, et al. The Biology of Streptococcus mutans . Microbiol Spectr. 2019;7(1):10.
Crossref - Liu Y, Han L, Yang H, Liu S, Huang C. Effect of apigenin on surface-associated characteristics and adherence of Streptococcus mutans. Dent Mater J. 2020;39(6):933-940.
Crossref - Nguyen LTP, Liu BH. In-situ investigation on nanoscopic biomechanics of streptococcus mutans at low ph citric acid environments using an afm fluid cell. Int J Mol Sci. 2020;21(24):9481.
Crossref - Schneider-Rayman M, Steinberg D, Sionov RV, Friedman M, Shalish M. Effect of epigallocatechin gallate on dental biofilm of Streptococcus mutans: An in vitro study. BMC Oral Health. 202121(1):1-11.
Crossref - Takenaka S, Ohsumi T, Noiri Y. Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn Dent Sci Rev. 2019;55(1):33-40.
Crossref - Khoury ZH, Vila T, Puthran TR, et al. The Role of Candida albicans Secreted Polysaccharides in Augmenting Streptococcus mutans Adherence and Mixed Biofilm Formation: In vitro and in vivo Studies. Front Microbiol. 2020;11:1-14.
Crossref - Karnjana K, Jewboonchu J, Niyomtham N, et al. The potency of herbal extracts and its green synthesized nanoparticle formulation as antibacterial agents against Streptococcus mutans associated biofilms. Biotechnol Rep (Amst). 2023;37:e00777.
Crossref - Arango-Santander S, Martinez C, Bedoya-Correa C, Sanchez-Garzon J, Franco J. Assessment of polydopamine to reduce streptococcus mutans adhesion to a dental polymer. Pathogens. 2023;12(10):1223.
Crossref - Lubojanski A, Piesiak-Panczyszyn D, Zakrzewski W, et al. The Safety of Fluoride Compounds and Their Effect on the Human Body-A Narrative Review. Materials. 2023;16(3):1242.
Crossref - Elkhodary HM, Abdelnabi MH, Swelem AA, et al. Individual, familial and country-level factors associated with oral hygiene practices in children: an international survey. BMC Oral Health. 2023;23(1):50.
Crossref - Tomazevic T, Drevensek M, Kosem R. Evaluation of fluoride varnish treatment of postorthodontic white spot lesions by visual inspection and laser fluorescence-A randomized controlled study. Clin Exp Dent Res. 2022;8(4):931-938.
Crossref - Xie Z, Yu L, Li S, Li J, Liu Y. Comparison of therapies of white spot lesions: a systematic review and network meta-analysis. BMC Oral Health. 2023;23(1):1-19.
Crossref - Pundir H, Pathak R, Pant T, Pant M, Chandra S, Tamta S. In Silico Screening of Phytochemicals Targeting SmdCD of Streptococcus mutans using Molecular Docking Approach. Trends Sci. 2023;20(6):6036.
Crossref - Mao QQ, Xu XY, Cao SY, et al. Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe). Foods. 2019;8(6):185.
Crossref - Wang X, Shen Y, Thakur K, et al. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules. 2020a;25(17):3955.
Crossref - Zhang S, Kou X, Zhao H, Mak KK, Balijepalli MK, Pichika MR. Zingiber officinale var. rubrum: Red Ginger’s Medicinal Uses. Molecules. 2022;27(3):775.
Crossref - Juariah S, Bakar FIA, Bakar MFA, Endrini S, Kartini S, Ningrum RS. Antibacterial Activity and Inhibition Mechanism of Red Ginger (Zingiber officinale var. rubrum) Ethanol Extract Against Pathogenic Bacteria. J Adv Res Appl Sci Eng Technol. 2023;30(1):145-157.
Crossref - Abers M, Schroeder S, Goelz L, et al. Antimicrobial activity of the volatile substances from essential oils. BMC Complement Med Ther. 2021;21(1):124.
Crossref - Lopez-Romero JC, Gonzalez-Rios H, Borges A, Simoes M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid Based Complement Alternat Med. 2015;795435.
Crossref - Park SY, Raka RN, Hui XL, et al. Six Spain Thymus essential oils composition analysis and their in vitro and in silico study against Streptococcus mutans. BMC Complement Med Ther. 2023;23(1):106.
Crossref - Mahboubi M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clinical Phytoscience. 2019;5(1):1-13.
Crossref - dos Santos Reis N, de Santana NB, de Carvalho Tavares IM, et al. Enzyme extraction by lab-scale hydrodistillation of ginger essential oil (Zingiber officinale Roscoe): Chromatographic and micromorphological analyses. Industrial Crops and Products. 2020;146:112210.
Crossref - Shukla A, Naik SN, Goud VV, Das C. Supercritical CO 2 extraction and online fractionation of dry ginger for production of high-quality volatile oil and gingerols enriched oleoresin. Industrial Crops and Products. 2019;130:352-362.
Crossref - Pertiwi KSS, Purnomo DS, Pujiasmanto B. The Use of ZA and SP 36 Fertilizer on Growth and Yield of Red Ginger (Zingiber officinale var. Rubrum). IOP Conference Series: Earth and Environmental Science. 2023;1162(1):012013.
Crossref - Nafarrate-Valdez RA, Martinez-Martinez RE, Zaragoza-Contreras EA, et al. Anti-Adherence and Antimicrobial Activities of Silver Nanoparticles against Serotypes C and K of Streptococcus mutans on Orthodontic Appliances. Medicina. (Lithuania). 2022;58(7):877.
Crossref - Qu Y, McGiffin D, Sanchez LD, et al. Anti-infective characteristics of a new Carbothane ventricular assist device driveline. Biofilm. 2023;5:100124.
Crossref - Zhao A, Sun J, Liu Y. Understanding bacterial biofilms: From definition to treatment strategies. Front Cell Infect Microbiol. 2023;13:1-23.
Crossref - Hefni D, Herdalina Y, Suharti N. Activity of Red Ginger Extract (Zingiber Officinale Var. Rubrum) Against Interleukin-6. Int J Appl Pharm. 2023;15(Spl Issue 1):21-23.
Crossref - Carniello V, Peterson BW, van der Mei HC, Busscher HJ. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv Colloid Interface Sci. 2018;261:1-14.
Crossref - Jakubovics NS, Goodman SD, Mashburn-Warren L, Stafford GP, Cieplik F. The dental plaque biofilm matrix. Periodontology. 2021;86(1):32-56.
Crossref - Joshi AS, Singh P, Mijakovic I. Interactions of gold and silver nanoparticles with bacterial biofilms: Molecular interactions behind inhibition and resistance. Int J Mol Sci. 2020;21(20):7658.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.