ISSN: 0973-7510
E-ISSN: 2581-690X
Medical entomology involves the study of medically important insects, such as cockroaches and mosquitoes, which have a dangerous role as transmitters for deadly diseases, such as Malaria, Leishmaniasis, and Dengue fever, which are responsible for many deaths among humans. Huge concern about the use of chemicals insecticides encourages the development of alternative methods for insect control, and due to the harmful effects of these chemicals, new strategies are being developed to replace or reduce the use of synthesized insecticides. Therefore, chitinolytic enzymes produced by microorganisms have a significant effect as biocontrol agents and will be more critical than synthetic pesticides for control. This study was primarily aimed to study the impact of various isolated bacteria using chitinolytic and spraying assays against adult stages of Periplaneta americana and Aedes aegypti as biological controls. Eight species of bacteria were isolated, and only Chryseomonas luteola was used against adult insects because of its high chitinolytic activity by spraying assay. Our results showed that the LC50 values of C. luteola against P. americana were 22.04% and 17.21% after 24 and 48 h, respectively. For A. aegypti adult stages, LC50 values of C. luteola were 2.78% and 2.12% after 24 and 48 h, respectively. Based on the results of this investigation, it is reasonable to say that using microbial insecticides may be an effective strategy to control the adult stages of P. americana and A. aegypti.
Insecticides, Periplaneta americana, Aedes aegypti, Chitinolytic Activity, Biological Control
Medical entomology involves the study of medically important insects, such as cockroaches and mosquitoes, which have a dangerous role as transmitters for deadly diseases, such as Malaria, Leishmaniasis, and Dengue fever, which are responsible for many deaths among humans. As these diseases increase in communities in developing countries, the environmental contamination caused by increasing the use of synthesized insecticides and alternative methods for controlling insect populations have become important research subjects to increase the use of biological or ecological control methods to limit the destructive impacts of insect populations.1,2 Numerous pathogens, including bacteria, fungi, nematodes, and viruses that are antagonistic to insects, are used as biological insecticides to control them by dissolving and digesting chitin in the cell wall by making different hydrolytic enzymes, the most important of which are chitinases.3,4 Because the cuticle of insects is composed of a vast amount of chitin, it was assumed that chitinase produced by these microorganisms could be involved in insect control by causing severe damage and even death, thereby exhibiting insecticidal activity.5 Recently, much attention has been paid to the production of chitinases for insect control, which has also received widespread attention for its biotechnological applications in certain insects.6,7 Many researchers have investigated the degrading effect of chitinase enzymes produced by bacterial species on insect chitin (aerobic and anaerobic bacteria), which can be found in a wide range of habitats;5 these chitinolytic bacteria can be found in soil, marine, lake, or chitinous wastes, such as industrial shrimp waste.8 Cody9 pointed out that chitinases are the constituents of several bacterial species, including Aeromonas, Serratia, Vibrio, Streptomyces, and Bacillus. Biological insecticides and their toxins can be utilized in the form of conventional insecticidal sprays, dust, liquid drenches, liquid concentrates, wettable powders, and granules.10 Paulitz et al.11 found that Streptomyces, Bacillus, and Pseudomonas species could be used as insecticides. The effect of chitinase enzymes on insect growth has been investigated and lead to death if insects are in contact with chitinases.12,13 Chitinases are one of the most significant biocatalysts with the potential to dissolve chitin in several phytopathogenic fungi and the integument of insects.14 Microbial chitinases have become promising candidates for controlling plant pests. These enzymes can be used directly as biocontrol agents and in combination with chemical pesticides or other biopesticides.15 In 1978, Brandt et al.16 demonstrated that chitinases destroy the peritrophic matrix in Orgyia pseudotsugata, and this effect was also observed in vivo in Spodoptera littoralis and Escherichia coli expressing the endochitinase ChiAII from Serratia marescens.17 For many decades, Bacillus thuringiensis has been a well-known biological insecticide, and many strains express chitinase.18 Hollensteiner et al.19 and Prasanna et al.20 found that Brevibacillus laterosporus possesses chitinase enzymes with hydrolytic effects. Based on previous research, many agricultural fields have been ruined due to insects, which unfortunately depend on the use of chemical pesticides to reduce insect abundance as a consequence.21 A recent study by Sharawi et al.22 found that isolated bacteria can be used as biological control agents against the ootheca of P. americana due to the pathogenic action of their toxins through the cuticle of the egg case. Huge concerns about the use of these chemicals encourages the development of alternative methods for insect control, and because of the harmful effects of the traditionally used chemicals, new strategies are being developed to replace or reduce synthesized insecticides.23,12 The use of chitinase as a biocontrol agent is an attractive and environmentally safe strategy.24 In this context, chitinolytic enzymes produced by microorganisms, such as bacteria, have a significant effect as biological control agents and are more effective than synthetic pesticides.5 This study aimed to determine the effect of isolated bacteria from soil samples against adult stages of Periplaneta americana and Aedes aegypti as biological controls using a spraying method.
Bacterial isolation from soil samples
One g of soil sample was mixed with 2 ml of distilled water in a Falcon tube and shaken for 2 min. The supernatant (100 µL) was spread using a spreader on nutrient agar after many dilutions. In nutrient broth, a single colony was selected and inoculated into 50 mL nutrient broth solution and placed in a shaking incubator for two days at 28°C. The sample was centrifuged and filtered through a mini-pore (0.2 mm). Bacterial isolates were identified using an Analytical Profile Index test (API-20E test strip).
Adulticidal bioassays of bacterial isolates against A. aegypti
Lab strains of adult A. aegypti were collected from the Dengue Mosquito Research Station at King Abdulaziz University, Jeddah (Saudi Arabia). To determine the toxicity of isolated bacteria against A. aegypti, five concentrations were prepared (0.5%, 1%, 1.5%, 2%, and 3%) using sterilized water. A commercial hand-sparing product and cylindrical cages (12 cm long and 80 cm in diameter) made of a wire screen were used in this study. The control group was maintained without exposure to isolated bacteria. Twenty adults were used for a single replicate, and the experiment was repeated three times. Mortality was recorded after 24 and 48 h of exposure.
Adulticidal bioassays of bacterial isolates against P. americana
The field strain of P. americana was used in this study and collected from dark, damp places like sewers in Jeddah Province, Saudi Arabia, using food jars surrounded with a dark cloth as traps. Jars from the upper inside surface (3 cm) were lightly grassed with petroleum jelly to prevent cockroaches from escaping, and a piece of bread soaked in a small amount of juice was placed in the collecting jars to attract cockroaches.25
The traps were placed in sewers, and cockroaches were collected every two days and maintained in glass containers. The collected adults were separated in glass containers (30 × 60 × 30 cm). The containers were glued 2 cm from the top with petroleum jelly to prevent cockroaches from escaping and supplied with water, dry dog pellets, and cardboard as shelter. The cultures were maintained in the laboratory at 25 ± 3 °C and 75 ± 5% Relative humidity (R.H). After two weeks, adult cockroaches were used in the experiments. Five concentrations of isolated bacteria were prepared using sterilized water (5%, 10%, 15%, 20%, and 30%). Water alone was used for the control group. Spraying bioassays were performed according to Baggio et al.26 with some modifications to the adult stages of cockroaches. Spraying bioassays were conducted using plastic boxes, and 1 mL of each concentration was applied using a hand sprayer. Ten adult insects were used as a single replicate, and the experiment was repeated three times. Mortality was recorded after 24 and 48 h of exposure.
Statistical analysis
The experimental design was randomized, and the mortality percentage was calculated using SAS. Determination of lethal concentrations (LC50) was performed by probit analysis using LDP line software with the lower and upper confidence limits, the inclination of the toxicity line, and chi-square.27
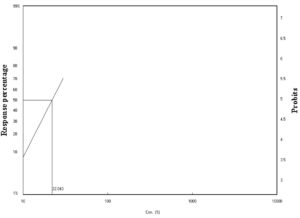
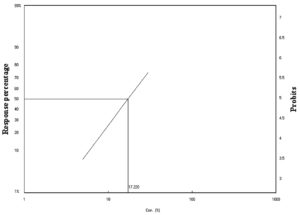
In the present study, different types of bacteria were isolated from soil samples and tested against adult stages of P. americana and A. aegypti as biological controls after 24 and 48 h using the spraying method. Eight species of bacteria were isolated (Enterobacter agglomerans, Bacillus subtills, Xantho maltophilia, Enterobacter amnigenus, Chryseomonas luteola, and Pseudomonas spp.). In this study, C. luteola was chosen because of its high chitinolytic activity. The mortality percentage of C. luteola filtrate against the adult stages of P. americana and A. aegypti was used. Our findings are in agreement with those of Paulsen et al.28 who also found that 11 types of isolated bacteria cause chitin degradation. Many studies have described the chitinase activity of bacterial species (Enterobacter agglomerans, Bacillus subtills, Vibrio, Aeromonas, Pseudoalteromonas, Chitiniphilus, Nocardiopsis, and Burkholderia).29 The mode of action of chitinolytic bacteria depends on the production of chitinase and hydrolysis of chitin to produce monomers via enzymatic reactions.30 The highest concentration (30%) caused higher mortality in P. americana after 24 (70%) and 48 h (80%) (Table 1). As shown in Figures 1 and 2, the lethal concentration (LC50) was determined (22.04%, 17.21%) after 24 and 48 h, respectively. Our findings agree with many studies. Lacey et al.31 pointed out that some bacterial species were developed as insect pest control, such as Bt sub-species, Lysini bacillus sphaericus, Paenibacillus spp., and Serratia entomophila. Bt sub-species kurstaki was the most used insect pest control in agricultural fields, and Bt sub-species israelensis and L. sphaericus were used as medical pest control. Bt toxins have minimal negative environmental impacts and hold more than 50% of the market share. Lacey et al. 31 found that bacteria and fungi such as Beauveria bassiana have a long history as biological control agents for various pests. Schnepf et al.32 investigated that toxins present in Bt var. israelensis were used to control cockroaches. Payne et al.33 reported that some bacterial species could be an effective agent in controlling cockroaches and reported that isolated Bt induced cockroach mortality.
Table (1):
Mortality effect of C. luteola against adult stages of P. americana after exposure times.
| Con. (%) | Exposure time | |
|---|---|---|
| After 24 h. | After 48 h. | |
| 5 | 0 % | 10 % |
| 10 | 6.66 % | 23.33 % |
| 15 | 26. 66 % | 36.66 % |
| 20 | 43.33 % | 56.66 % |
| 30 | 70 % | 80 % |
| Control | 0 % | 0 % |
| LC 50 (Lower limit – Upper limit) | 22.04 20.37 – 24.20 |
17.21 15.57 – 19.22 |
| Slope | 4.11 | 2.77 |
| Tubulated (Chi)2 | 6 | 7.8 |
| Calculated (Chi)2 | 0.48 | 5.16 |
When tabulated (Chi)2 larger than calculated at 0.05 level of significance indicates the homogeneity of results.
Figure 1. Laboratory toxicity line of C. luteola against adult stages of P. americana after 24 h of exposure
Figure 2. Laboratory toxicity line of C. luteola against adult stages of P. americana after 48 h of exposure
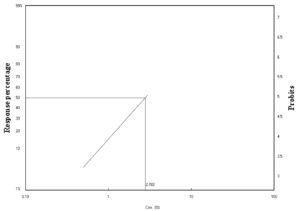
Figure 3. Laboratory toxicity line of C. luteola against adult stages of A. aegypti after 24 h of exposure
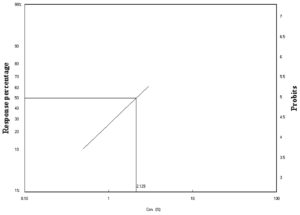
Figure 4. Laboratory toxicity line of C. luteola against adult stages of A. aegypti after 48 h of exposur
Table (2):
Mortality effect of C. luteola against adult stages of A. aegypti after exposure times.
| Con. (%) | Exposure time | |
|---|---|---|
| After 24 h. | After 48 h. | |
| 0.5 | 6.66 % | 15 % |
| 1.0 | 10 % | 18 % |
| 1.5 | 25 % | 35 % |
| 2.0 | 38.33 % | 50 % |
| 3.0 | 55 % | 65 % |
| Control | 0 % | 0 % |
| LC 50 (Lower limit – upper limit) |
2.78 2.38 – 3.45 |
2.12 1.84 – 2.56 |
| Slope | 2.33 | 2.00 |
| Tubulated (Chi)2 | 7.8 | 7.8 |
| Calculated (Chi)2 | 4.04 | 6.34 |
When tabulated (Chi)2 larger than calculated at 0.05 level of significance indicates the homogeneity of results.
Table 2 shows that the highest concentration (3%) caused higher mortality of A. aegypti after 48 (55%) and 72 h (65%). As shown in Figures 3 and 4, the lethal concentration (LC50) was determined (2.78%, 2.12%) after 24 and 48 h, respectively. For many decades, many bacteria have been used for controlling mosquitos. For example, Wolbachia spp. has been used as a population control method for the Aedes mosquito.34 Recently, Silva-Filha et al.35 investigated the microbial insecticide Bacillus thuringiensis var. israelensis (Bti), and Lysinibacillus sphaericus were active in controlling dipteran insects without harming non-target organisms. Bti was the first bacterium to be used against Diptera larvae.36 Lysinibacillus sphaericus was also used against Culicidae larvae.37 Therefore, insecticides composed of Bti are considered effective microbial insecticides for controlling mosquito larvae and black flies.31 Bti and L. sphaericus showed high and selective larvicidal activity against some Diptera species, such as Aedes, Anopheles, Culex, and Simulium.31
This study shows that bacterial insecticides can be used for biological control by causing severe damage and even death of P. americana and A. aegypti. LC50 values of C. luteola against P. americana were 22.04% and 17.21% after 24 and 48 h, respectively. For adult stages of A. aegypti, LC50 values of C. luteola were 2.78% and 2.12% after 24 and 48 h, respectively. The use of bacterial insecticides may be an effective strategy to control the adult stages of P. americana and A. aegypti. However, biological control is only significant as a laboratory method. The current challenge is to use microbial pesticides in the environment to control targeted insects and limit their population.
ACKNOWLEDGMENTS
None.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hokkanen HMT, Lynch JM. Biological Control: Benefits and Risks. Cambridge University Press. New York. 1995.
Crossref - Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8(5):1402-19.
Crossref - Clarke PH, Tracy MV. The Occurrence of Chitinase in some Bacteria. J Gen Microbiol. 1956;14(1):188-196.
Crossref - Joe S, Sarojini S. An Efficient Method of Production of Colloidal Chitin for Enumeration of Chitinase Producing Bacteria. Mapana Journal of Sciences. 2017; 16(4):37- 45.
Crossref - Edreva A. Pathogenesis-related proteins: research progress in the last 15 years. Gen Appl Plant Physiol. 2005;31:105-124.
- Cohen E. Chitin Biochemistry: Synthesis, Hydrolysis and Inhibition. Advances in Insect Physiology, 1st Edn. 2010;38:5-74.
Crossref - Henriques BS, Garcia ES, Azambuja P, Genta FA. Determination of Chitin Content in Insects: An Alternate Method Based on Calcofluor Staining. Front Physiol. 2020;18:11:117.
Crossref - Jabeen F, Qazi JI. Isolation of Chitinase Yielding Bacillus cereus JF68 from Soil Employing an Edible Crab Shell Chitin. Journal of Science and Industrial Research. 2014;73(12):771-776.
- Cody RM. Distribution of chitinase and chitobiase in bacillus. Curr Microbiol. 1989;19(4):201-205.
Crossref - Patel S, Rahul SN. Role of Microbial Insecticides in Insect Pest Management. Popular Kheti. 2020;8(3):88- 92.
- Paulitz TC, Belanger RR. Biological control in greenhouse systems. Annu Rev Phytopathol. 2001;39:103-133.
Crossref - Veliz EAP, Hirsch AM. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 2017;43(3):689- 705.
Crossref - Carozzi NB, Koziel M. (Eds.). Advances In Insect Control: The Role Of Transgenic Plants; 1st Edn. 1997. CRC Press.
Crossref - Gursharan S, Shailendra KA. Antifungal and insecticidal potential of chitinases: A credible choice for the eco-friendly farming. Biocatal Agric Biotechnol. 2019;20:101289.
Crossref - Berini F, Casartelli M, Montali A, Reguzzoni M, Tettamanti G, Marinelli F. Metagenome-Sourced Microbial Chitinases as Potential Insecticide Proteins. Front Microbiol. 2019 18;10:1358.
Crossref - Brandt CR, Adang MJ, Spence KD. The peritrophic membrane: ultrastructural analysis and function as a mechanical barrier to microbial infection in Orgyia pseudotsugata. J Invertebr Pathol. 1978;32:12-24.
Crossref - Regev A, Keller M, Strizhov N, et al. Synergistic activity of a Bacillus thuringiensis delta-endotoxin and a bacterial endochitinase against Spodoptera littoralis larvae. Appl Environ Microbiol. 1996;62:3581-3586.
Crossref - Chen YL, Lu W, Chen YH, Xiao L, Cai J. Cloning, expression, and sequence analysis of chiA, chiB in Bacillus thuringiensis subsp. colmeri 15A3. Wei Sheng Wu Xue Bao. 2007;47(5):843-848.
- Hollensteiner J, Wemheuer F, Harting R. Bacillus thuringiensis and Bacillus weihenstephanensis inhibit the growth of phytopathogenic Verticillium Species. Front Microbiol. 2017;7:2171.
Crossref - Prasanna L, Eijsink, V, Meadow R, Gåseidnes S. A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Applied microbiology and biotechnology. 2013;97:1601-1611.
Crossref - Oerke EC, Dehne HW, Schonbeck F, Weber A. Crop production and crop protection: estimated losses in major food and cash crops. Amsterdam, Netherlands: Elsevier. 1994.
- Sharawi S, Mahyoub J, Ullah I, Alssagaf A. Effect of Isolated Bacteria and Their Supernatant from American cockroach’s Ootheca on Its Hatching as a Safety Method for Control in Jeddah Governorate. GSC Biol. Pharm. Sci. 2019; 6: 036-044.
Crossref - Russell PE. The development of commercial disease control. Plant Pathol. 2006;55(5):585-594.
Crossref - Okongo RN, Puri AK, Wang Z, Singh S, Permaul K. Comparative biocontrol ability of chitinases from bacteria and recombinant chitinases from the thermophilic fungus Thermomyces lanuginosus. J Biosci Bioeng. 2019;127(6):663-671.
Crossref - Wang C, Bennett GW. Comparative study of integrated pest management and baiting for German cockroach management in public housing. J Econ Entomol. 2006;99(3):879-885.
Crossref - Baggio MV, Farreia MC, Monteiro AC, Juior WM, Lemos M. Pathogenicity of Aspergillus westerdikiae to females and ootheca of Periplanta americana. Crop Protection. 2016;46(1):20-25.
Crossref - Finney DJ. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve. 7th Edition, Cambridge University Press, Cambridge. 1972;33.
- Paulsen SS, Andersen B, Gram L, Machado H. Biological Potential of Chitinolytic Marine Bacteria. Marine Drugs. 2016; 14(12):230.
Crossref - Hyesuk Kong, Makoto Shimosaka, Yasuo Ando, Kouji Nishiyama, Takeshi Fujii, Kiyotaka Miyashita, Species-specific distribution of a modular family 19 chitinase gene in Burkholderia gladioli, FEMS Microbiology Ecology, 2001;37(2): 135–141.
Crossref - Al-Ahmadi KJ, Yazdi MT, Najafi MF, et al. Optimization of Medium and Cultivation Conditions for Chitinase Production by the Newly Isolated: Aeromonas sp. Biotechnology. 2008;7(2):266-272.
Crossref - Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc. 2007;23(sp2):133-163.
Crossref - Schnepf E, Crickmore N, van Rie J, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol. 1998;62(3):775-806.
Crossref - Payne JM, Kennedy KM, Randall JB, Brower DO. Bacillus thuringiensis isolates active against cockroaches and genes encoding cockroach-active toxins. U. S. Patent No. 5302387. 1994.
- Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C, Bourtzis K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc Natl Acad Sci U S A. 2004; 101(42):15042-15045.
Crossref - Silva-Filha MHNL, Romao TP, Rezende TMT, et al. Bacterial Toxins Active against Mosquitoes: Mode of Action and Resistance. Toxins (Basel). 2021;13(8):523.
Crossref - De Barjac H. A new variety of Bacillus thuringiensis very toxic to mosquitoes: B. thuringiensis var. israelensis serotype 14. Comptes Rendus Seances Hebd. l’Academ. Sci Ser D. 1978;286:797-800.
- Kellen WR, Clark TB, Lindegren JE, Ho BC, Rogoff MH, Singer S. Bacillus sphaericus Neide as a pathogen of mosquitoes. J Invertebr Pathol. 1965;7(4):442-448.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.