ISSN: 0973-7510
E-ISSN: 2581-690X
Chamomile (Matricaria chamomilla L.) is one of the nine important medicinal plants found in the world. In this study, the effect of different concentrations of plant growth regulators and explants has been investigated on embryogenesis and rooting of German chamomile (Matricaria spp.). MS culture medium (Murashige Skoog., 1986) was the basal medium in this experiment. Factorial experiments were conducted in a Randomized Complete Block Design with three replications. In testing the effect of 1-Naphthaleneacetic acid (NAA) and Kinetin on the embryogenesis, factors included: (NAA) at four levels (0, 0.5, 1, 2 mg/l), Kinetin at four levels (0, 0.5, 1, 2 mg/l) and explants in three levels: Stem, Axillary buds and leaves. In investigating the effect of NAA and Kinetin on rooting, factors included NAA at four levels: (0, 0.5, 1, 2 mg/l), Kinetin at four levels: (0, 0.5, 1, 2 mg/l). Results showed that leaf explants in culture medium containing a treatment Al: (NAA: 1 +Kin: 1mg/l) and stem explants in culture medium containing a treatment A2: (NAA: 1 +Kin: 0.5 mg/l) and the Axillary bud explants in culture medium containing hormonal composition of A3: (NAA: 0.5 +Kin: 1mg/l) produced the highest embryogenic calli. In terms of rooting, the culture medium containing the treatment A4: (NAA: 1 +Kin: 0 mg/l) created the highest rooting.
Chamomile, Explants, Plant Growth Regulator, Embryogenesis, Rooting
Chamomile (Matricaria chamomilla L.) is one of the nine important medicinal plants in the world known by man (Salamon., 1992; Witchl., 1994). Research has shown that different parts of this plant flower (disc florets and ray florets), shoots and roots have certain chemical compounds (Pekic, Zekovic, Petrovic et al. 1999, Karami 2006, Szoke, Maday, Tyithak et al. 2004, Repcak, pastirova, Imrich et al. 2001). Today, this plant is used in Pharmaceutical, Cosmetics, Healthcare, Perfumery industries and also flavorings and preparing drinks (Omid Beygi 2006; Afzali, Shariatmadari, Abbasi et al. 2007; Della logia, Tubaro, Dri et al. 1986; Makay and Blumberg., 2006; Viola, Wasowski, Levi et al. 1995; Mehrin 2004, Samsamshariat 2007; Zaman 1997; Gardiner; 1999; Jaymand and Rezaei., 2002; Jaymand, Rezaei, Asgari et al. 2003). Reichling, Bisson and Becker (1984) have optimized Callus culture from sterilized stem segments of BK2 variety chamomile in MS medium containing 27.7 µM NAA and 11.9 µM Kinetin levels. Passamonti, Piccioni, Standardi et al (1998) reported callus culture and micropropagation in seeds of five chamomile varieties cultured on MS containing 0.05 µM NAA and 11.6 µM Kinetin levels. Sato, Lima, Affonso et al (2006) reported micropropagation of chamomile using shock treatment model with different growth regulators. Kintzios & Michaelakis (1999) investigated callus induction and somatic embryogenesis of flower end tissue explants in MS medium containing 26.8 µM NAA and 11.5 µM Kinetin levels. Moreover, the authors suggested that favorable results be obtained in studies of somatic embryogenesis of chamomile flower when the explants under study is the end tissue of the flower and all stages of tissue culture are performed in a 24-hour cycle of 16 hours of darkness and 8 hours of light. Gene transfer methods in plants for genetic modification of them is fully depended on the development of efficient methods for regeneration of alive and green stems from cultured tissues (Salmanian and Kahrizi., 2007). Without regeneration of plant, the process of producing transgenic plants in order to insert new genes, propagation of plant species and other tissue-culture related activities will not be efficient. (Abe and Futsuhara., 1989; Brisibe, Miyake, Taniguchi et al. 1992; Gholizadeh., 2005) The effect of different concentrations of growth regulators and the type of explants on embryogenesis and rooting of German chamomile (Matricaria spp) have been studied.
Plant material and disinfection
The experiments were performed in Saravan University, Iran in 2014. The Coral genotype (2n=4x=32) was used in this study. Before the flowering stage, when the plants are 10-15 cm height, young plants with stronger structure were selected and transferred to the laboratory for the experiments. MS Medium Culture (Murashige Skoog., 1986) was a basal medium in these experiments. The culture media were added with 30 g of sucrose per liter and the required regulators were added to it according to the type of experiment and PH was set on 5.8 with the help of caustic soda and HCl 1N. Finally, to make the solid culture medium, 8 grams of agar was added to a liter of culture medium and autoclaved for 20 minutes in order to disinfect it at atmospheric pressure and a temperature of 125 °C. After autoclave, it was distributed into 9 cm Sterile Petri Dishes so that 25 mL culture medium was put into each Petri Dish. Explants disinfection for testing was so that plants were placed under running water for primary disinfection for 30 minutes after primary washing with tap water. The final disinfection under Laminar Air Flow Hood was conducted by ethanol 70 percent for 60 seconds. Then they were placed in a solution of sodium hypochlorite 1percent for 10 minutes. After these stages, they were rinsed with sterilized distilled water four times and each time for 5 minutes. Finally, explants at levels: stem, Axillary buds, and leaves were cut with a sterile razor/scalpel as long as 0.5 cm and incubated on the culture media. The required tools were sterilized with distilled water and ethanol 96 percent and wrapped in an aluminum foil and placed in the oven at 200 °C for 2 hours for disinfection.
Embryogenesis and rooting tests of chamomile
With the aim of establishing sterilized explants in vitro, in order to callus production and evaluation of embryogenesis, 32 Hormonal Treatments were used in two separate tests with different concentrations and types. The experimental design used for both experiments was implemented as Factorial Experiments in Randomized Complete Block Design (RCBD) format with three replications. In testing the effect of NAA and Kinetin on the embryogenesis, factors included: (NAA) at four levels: (0, 0.5, 1, 2 mg/l, Kinetin at four levels: (0, 0.5, 1, 2 mg/l), explants at four levels: (Stem, Axillary bud and leaves). In investigating the effect of NAA and Kinetin on rooting, factors were NAA at four levels: (0, 0.5, 1, 2 mg/l) and Kinetin at four levels: (0, 0.5, 1, 2 mg/l).
The effect of different concentrations of growth regulators NAA and Kinetin on embryogenesis of chamomile (Matricaria chamomilla L.) explants:
After moving explants (Stem, Axillary buds, and leaves) to the culture medium containing different levels of growth regulators NAA and Kinetin to produce callus and evaluate embryogenesis and rooting percentage, the traits were noted and Means were compared using Duncan’s Multiple Range Test at the 5 percent level.
Embryogenesis and Rooting Percentage
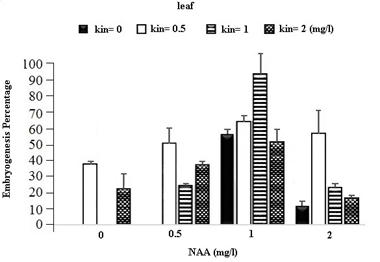
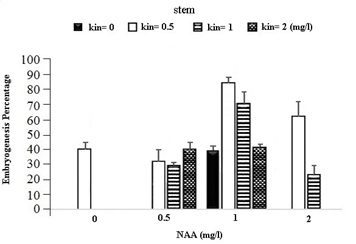
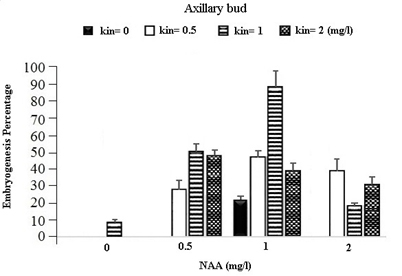
In the table for analysis of data variance for the Embryogenesis Percentage of calluses, it was observed that the effect of different concentrations of growth regulators (NAA) and Kinetin was significant on embryogenesis of calli obtained from different explants (Table 1). It was observed that leaf explants in media containing 1 mg/l (NAA) and 1 mg/l Kinetin have produced the most Embryogenesis Percentage with the mean of 95.93 percent while the most embryogenic calli in stem explants are provided by the medium containing 1 mg/l NAA and 0.5 mg/l Kinetin with a mean of 83.78 percent and in Axillary bud explants using the medium containing 1 mg/l NAA and 1 mg/l Kinetin with a mean of 87.63 percent (Figures 1).
Table (1):
Analysis of variance was conducted on the evaluated traits to study embryogenesis in different explants of chamomile at different levels of growth regulators (NAA) and kinetin.
Rooting percentage |
Embryogenesis percentage |
Degree of freedom |
Sources of variation |
|---|---|---|---|
649.14 |
81.98* |
2 |
Replication |
431.36 |
2133.91*** |
3 |
NAA |
8401.05*** |
5397.64*** |
3 |
Kinetin |
1927.78* |
936.11* |
2 |
Explant |
1571.52** |
1112.22*** |
9 |
NAA × Kinetin |
1194.11 |
522.12 |
6 |
NAA × Explant |
849.69 |
372.61 |
6 |
Kinetin × Explant |
269.89 |
972.65*** |
18 |
NAA×Kinetin×Explant |
568.92 |
258.31 |
94 |
Experimental Error |
*, ** and ***, indicate significant differences in the level of 5 percent, one percent and 0.1 percent.
Fig. 1. The effect of Different Concentrations of Growth Regulators (NAA) and Kinetin on Embryogenesis Percentage of Calli Obtained from Explants of Vegetative Parts of the Chamomile
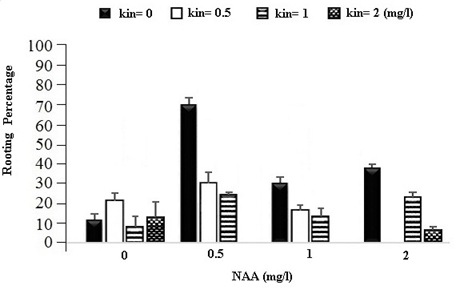
Moreover, in this experiment, it was observed that most calli have rooted at the same primary culture medium, so they have been investigated in terms of rooting traits percentage too and the variance analysis results of this trait are shown in Table 1. According to this table, Level 1 mg of NAA and control level (0) of Kinetin have the highest rooting rate with the average of 60.07 percent (Figure 2).
Fig. 2. The Effect of Different Concentrations of Growth Regulators (NAA) and Kinetin on the Rooting Percentage of Calli in Chamomile
The effect of different concentrations of growth regulators NAA herbicide and Kinetin on embryogenesis of explants obtained from the vegetative parts of chamomile: Analysis of variance and comparison of averages of these traits are shown in Tables 2 &3.
Table (2):
Analysis of variance for traits assessed in embryogenesis study of different explants of chamomile at different levels of growth regulators 2, 4-D herbicide and Kinetin.
Sources of Variation |
Degree of freedom |
Embryogenesis percentage |
Rooting percentage |
|---|---|---|---|
Replication |
2 |
6435.15*** |
101.99 |
NAA |
3 |
6500.01*** |
55.89 |
Kinetin |
3 |
4349.12** |
22.94 |
Explants |
2 |
1626.13 |
16.03 |
NAA × Kinetin |
9 |
657.11 |
45.06 |
NAA × Explants |
6 |
1324.20 |
54.13 |
Kinetin × Explants |
6 |
1467.10 |
46.99 |
NAA × Kinetin × Explants |
18 |
983.03 |
42.92 |
Experimental Error |
94 |
774.11 |
46.02 |
*, ** and ***, indicate significant differences in the level of 5 percent, 1 percent and 0.1 percent.
Table (3):
Comparing means of different levels of growth regulators NAA, Kinetin and explants on traits evaluated to investigate embryogenesis in chamomile.
| Case factors of study | Levels of factors | Embryogenesis percentage |
|---|---|---|
| NAA (mg/l) | 0 | 3.51 ± 16.06b |
| 0.5 | 5.27 ± a 45.02 | |
| 1 | 5.07 ± a 42.96 | |
| 2 | 5.62 ± a 34.08 | |
| Kinetin (mg/l) | 0 | 4.31 ± b 20.04 |
| 0.5 | 39.12a± 4.94 | |
| 1 | 46.97a± 5.31 | |
| 2 | 35.11a± 5.14 | |
| Explants | Stem | 4.71 ± a 41.64 |
| Axiliary bud | 4.54 ± a 33.01 | |
| Axis of leaf | 5.02 ± a 31.06 |
*Numerical data with the similar alphabetical indices, based on Duncan’s Compare Mean at the probability level of 5 percent are not significantly different.
Analysis of data variance for the embryogenic callus percentage showed that growth regulators NAA and Kinetin had significant impact on embryogenesis of calli (Table 2). So that the highest percentage of embryogenesis was achieved for NAA at level of 1mg/l with the Mean of 45.02 percent. Besides, in different levels of Kinetin, the highest percentage of embryogenic calli with the mean of 46.97 percent belongs to level of 1mg/l. Similar to previous experiment; it was observed that most calli produced roots at the same primary culture medium. Analysis of variance results for this trait is also listed in Table 2. It is observed that the studied factors did not impact on this trait significantly, so the data of this trait were not analyzed. In rooting with growth regulator NAA after 14 days of culture, Axillary bud explants with 1mg/l NAA were thickened and produced thick root-like organs. It was observed in experiments that each of explants: (stem, Axiliary buds and leaves) in various hormonal compounds induce the highest rate of embryogenic calli. So that the leaf explants in culture medium containing treatment A1: (NAA:1 + Kin: 1mg/l) (equally NAA to kinetin ratio), and stem explants in culture medium containing the treatment A2: (NAA:1 + Kin: 0.5 mg/l) (NAA/Kinetin ratio: 2) and axiliary bud explants in culture medium containing hormonal composition A3: (NAA:1 +Kin: 1mg/l) (equally NAA to kinetin ratio) produced the highest embryogenic calli. In terms of rooting, the medium containing the treatment A4: (NAA: 0.5 + Kin :0 mg/l) created the highest rooting.
Callus production of explants at levels: stem, Axillary bud and leaf of Chamomile in modified Murashige and Skoog (MS) culture medium has also been reported by other researchers (Reichling, Bisson and Becker., 1984; Reichling and Becker., 1976; Passamonti, Piccioni, Standardi et al. 1998). According to the reports, it is found that NAA and Cytokinin Kinetin are efficacious hormones in callogenesis of explants taken from the vegetative parts of Chamomile and these results correspond to our research. According to importance of embryogenic calli for the regeneration and studies of somaclonal variation and use in suspension cultures, this trait has also been investigated. In these experiments, it was found that the highest rate of embryogenesis is obtained in the presence of high concentrations of NAA and low concentration of Cytokinin Kinetin. Similar results have been reported by Kintzios and Michaelakis (1999). They could perform a successful somatic embryogenesis using NAA/kinetin approximate ratio: 2, (NAA :26.8 :Kin µM +Kin:11.5 µM). The presence of NNA for rooting in other medicinal plants is also approved. Tavares, Pimento and Goncalves (1999) reported that NNa is necessary for rooting of Axiliary bud explants from Mellisa officinalis. Leaf explants far ahead of stem and bud explants began callogenesis which is probably related to the effect of explants source. Although, high proportion of Cytokinin typically promotes rooting (Skoog and Miller., 1957) but it is observed that high proportion of Auxin to Cytokinin induces shoot regeneration in some varieties. Considering the importance of the role of genetic control of responding to Invitro Culture in Plant Breed projects established based on tissue culture, it is recommended to use Goral genotype that is one of the most important cultivars of Chamomile as parent in crosses. Moreover, genetic studies have been conducted for identifying how to inherit genes responsible for callogenesis and regeneration and transfer them to corresponding gens if required.
ACKNOWLEDGMENTS
None.
Conflict of Interest
The authors declare that there is no conflict of interest.
- Abe. T and Y. Futsuhara. Selection of higher regeneration callus and changein isozyme pattrern in rice (oriza sativa L.). Theor. Appl. Genet, 1989; 78: 648-652.
- Abe. T., M. Kudo., Y. Oka., J. Yamaguchi and T. Sasahara. Changes in a-amilase activity during plant regeneration from rice calli. J. plant PHysiol, 1996; 146: 592-598.
- Afzali, S., Shariatmadari, H., Abbasi, M., Moattar, F, The effect of salinity and drought stresses on performance of flower and flavonol-o-glycosides amount) (Matricaria chamomilla L.) Journal of Medicinal and Aromatic Plants Research, 2007; 23: 382-390.
- Alvandi, T, Effect of hormonal composition on Astragalus root and leaves. Master’s Thesis of Plant Science– Physiology, University of Tabriz, Tabriz 2007.
- Brisibe. E.A., H. Miyake., T. Taniguchi and E. Meada. Callus formation and scanning electron microscopy of plantlet regeneration in Africa rice (Oriza glaberrima Sleud). Plant Sci., 1992; 83: 217-224.
- Della logia. R., A. Tubaro., P. Dri., C. Zilli and P. Del Negro. The role of flavonoids in the antiiflamatory activity of Chamomilla recutita. In: Plant Flavonoids in Medicine: Biochemical, PHarmacological and Structure-Activity Relation ship. A.R.Liss, New York, 1986; 650 Pp.
- Gardiner. P. Chamomile (Matricaria recutita, Anthemis nobilis). Longwood Herbal Task Force Press: 1999; p: 1-21.
- Gholizadeh, F. Effects of hormonal treatments on the growth and development of root and leaf explants of Pegaum harmala. Master’s Thesis of Plant Sciences – Cellular-Developmental Major. Tabriz University 2005.
- Heutteman. C. and G. E. Preece. Thiadiazuron: Apotent cytoKINin for wood plang tissue culture. Plante Cell Tiss Org. Cult. 1993; 33: 105-119.
- Jaymand, K., Rezaei, B., Evaluation of Medicinal chamomile essential oil compounds in Tehran areas, Hamedan, Kazerun 2002.
- Jaymand, K., Rezaei, B., Asgari, F., Meshkizadeh, S, Chemical components of essential oilsof Chamomile (Matricaria Chamomilla L.) Researches of medicinal and aromatic plants., 2001; 10: 105-125.
- Kakani. A., G. Li and Z. Peng. Role of Aux1 in the control of organ identity during in vitro organogenesis and in mediating tissue specific auxinand cytoKINin interaction in Arabidopsis. Planta, 2009; 229: 645-657.
- Karami, A. 2006. Effects of nitrogen, phosphor and potassium on yield and qualitative characteristics of wild and domesticated populations of German chamomile (Matricaria chamomilla L.) Master’s thesis in the field of Horticulture. University of Shiraz
- Kintzios. S and A. Michaelakis. Induction of somatic embryogenesis and in vitro flowering from inflorescences of chamomile (Chamomilla recutita L.). Plant Cell Reports, 1999; 18: 684–690.
- Makay. D. L and J. B. Blumberg. A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L). PHytotherapy Research. 2006; 20: 519-530.
- Mehrin, M. Principles of natural treatment (herbal medicine), Ganjineh Publication 2004.
- Omid Beigi hope R. production and processing of medicinal plants, Astan Quds Razavi 2006.
- Passamonti. F., E. Piccioni., A. Standardi and F. Veronesi. Micropropagation of Chamomilla recutita (L.) Rauschert. Acta Hortic, 1998; 457: 303-309.
- Pekic. B., Z. Zekovic., L. Petrovic and D. Adamovic. Essential oil of chamomile ligulate and tubular flowers. J. Essent. Oil Res. 1999; 11: 16-18.
- Rahimi Kolamrudi, H. Botany, Cultivation of Diplotid and tetraploid species of Chamomile and evaluating essential oil composition and comparing with the essential oils in Iran. Doctoral thesis Faculty of Pharmacy, Isfahan University of Medical Sciences. Research Institute of Forests and Rangelands, 305: 11-24.
- Reichling. J and H. Becker. Callusculturen von Matricaria chamomilla. Planta Med. 1976; 30: 258–268.
- Reichling. J., W. Bisson and H. Becker. Vergleichende Untersuchungen zur Bildung und Akkumulation von etherischem Öl in der intakten Pflanze und in der Calluskultur von Matricaria chamomilla. Planta Med. 1984; 56: 334–337.
- Repcak. J., A. pastirova. J. Imrich., V. Svehlikova and P. Martonfi. The variability of (z) – and (E)-2 -D-glucopyranosyloxy-4-metoxycinnamic acids ad apigenin glucosides in diploid and tetraploid Chamomilla recutita (L) Rauschert. Plant Breed. 2001; 120: 188-190.
- Salamon, I. Chamomile production in ezecho-slog atria. Foccus on Herb., 1992; 10: 1-8.
- Salmanian, H., Kahrizi, D. The effect of genotype and type of explants on shoot regeneration of canola seedlings (Brassica napus L.). Iranian Journal of Biology, 2007; 3: 171-179.
- Samsamshariat H, Analysis and identification of herbal medicinal products to the microscopic and macroscopic approaches. First Edition, Mash’al-i Isfahan, 1989.
- Sato. A., S. S. Lima., V. R. Affonso., M. A. Esquibel and C. L. S Lage. Micropropagation of Chamomilla recutita (L.) Rauschert: A shock treatment model with growth regulators. Scientia Horticulturae. 2006; 109: 160–164.
- Skoog. F and CO. Miller. Chemical regulation of growth and organ formation in Plant tissues Cultured in Vitro. Sympsoc Exp Biol., 1957; 54: 118-130.
- Stang. C., D. Prehn and M. Gebauer. Optimization of in vitro culture conditions for pinus radiat embryos and histological characterization of regeneration shoots. Bio Res. 1999; 32: 19-28.
- Szoke. E., E. Maday., E. Tyithak., I. N. KuzovKINa and E. Lemberkovis. New terpenoids in cultivation and wild chamomile (In vivo and in vitro). J. Chromatogr. 2004; 800: 231-238.
- Tavares. AC., MC. Pimento and MT. Goncalves. Micropropagation of Melissa Officinalis L. through proliferation of axillary shoots. Plant Cell Rep. 1996; 15: 441-444.
- Viola. H., C. Wasowski., M. Levi., C. Wolfman., R. Silveria., F. Dajas., j. h. Medina and A. C. Paladina. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Medica. 1995; 61: 213-216.
- Volag, Zh., Zhari, A. Translated by: Saed Zaman. Medicinal plants. Qoqnoos Publishing 1997.
- Witchl, M. 1994. Herbal drugs and phytopharmaceuticals. Boca Raton. FL CRC. Press, pp. 322-325.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.