ISSN: 0973-7510
E-ISSN: 2581-690X
This study aimed to identify the diversity of the alkali protease producers, thermophilic obligate Bacillus spp., from Sungai Tutung Hot Spring in Kerinci, Jambi, Indonesia. The use of modified methods in exploring biodiversity from an extreme environment was useful as a tool for the discovery of more specific and thermostable enzymes from new sources. Based on the sequence analysis of the DNA isolate, there were six species with 14 strains identified from the hot spring. All strains grew well in thermophilic Bacillus medium at 60 0C with pH 8.0. They are also potentially able to produce alkali protease with the proteolytic index in the range of 0.25-6.15. Although characteristics of each strain were diverse, generally the color of the strains was from white to dark white and the shape was round with flat edges and raised elevations. Gram-positive bacteria with rod shape, central endospore, and purple color were observed under the microscope. Biochemical observation showed that all strains exhibited positive result from the catalase test, did not produce gas and sulfide, had positive mortality and to the sugar series test showed positive and negative variations. The identified species were: Bacillus sonorensis, Bacillus lichenmiformis, Brevibacillus borstelensis, Paenobacillus, Bacillus aerius, dan Fictibacillus gelatini.
Diversity, Bacillus, thermophilic obligate, protease alkali, Sungai Tutung.
Protease is an important enzyme, 60% of the industrial production of enzymes in the world is a protease, and 25% of them are thermostable. The sale value for protease throughout 2013-2014 was equal to two-thirds of the world’s enzyme market value. Demand for proteases is increasing, especially alkaline proteases from microbial species. In Indonesia, the need for a protease is also increasing and to fulfill it, this country still depends on imported products. The most appropriate strategies to anticipate the import of this enzyme are by increasing production of enzymes from microbes, exploring new bacterial sources from Indonesia’s natural terrain, and increasing the isolation and screening of alkaline protease producing Bacillus in selective medium1-3.
The thermophilic Bacillus spp. that produce alkaline protease are; B. amyloliquefaciens, B. alcalophilus, B. brevis, B. clausii, B. cereus, B. halodurans B. licheniformis, B. lentus, B. subtilis and B. stearothermophillus. This species is widely used in biotechnology, such as in food industry, medicine, chemical, silk and leather 3,4. Special for detergent industries, they need thermostable protease, and it is usually produced by thermophilic obligate Bacillus5. Thermophilic obligate Bacillus spp. is widespread in extreme and high-temperature habitats, such as in hot springs, volcanic craters, geothermal, hot mud, mining, composting, lakes, and deep seas at temperatures between 60-800C, optimally at 700C1,6.
Based on the optimum temperature, the thermophilic microbes are divided into three groups: thermophile with optimum temperatures between 500C and 640C and maximum at 700C, extremes with optimum temperatures between 650C and 800C, and hyperthermophile with optimum temperatures above 800C and maximum above 900C. Bacillus thermophilic obligate cannot survive at room temperature more than seven days, and most of the bacterial colonies from hot water survive at temperatures above 700C 7,8.
Bacillus gains popularity in biotechnology because it is relatively easy to isolate from various environments and can grow in a synthetic medium7. The fermentation ability of different types of Bacillus differs at various pH and temperature. The combination of alkaline pH and thermophilic properties in the Bacillus genus are indications that this genus is potential to be developed for producing stable enzymes at high temperatures4. Benefits of using thermophilic Bacillus spp. Are: the bacteria can be isolated from thermal environments with high cell growth rates, the contamination risk can be minimized, volatile compounds can be easily separated, the viscosity of the fermentation solutions is low, reaction rates are relatively fast, and the catalyzing reactions are more efficient and eco-friendly without byproducts9.
Hot springs are widespread in Indonesia because this country had 22 active volcanoes10. This hydrothermal condition contains abundant and diverse thermophilic bacteria. However, up until now, there has been little attempt to isolate and characterize the thermophilic bacteria from hot springs7. The hot spring of Sungai Tutung is located in one of the volcanoes of Mount Kerinci, in Kecamatan Air Hangat, Kerinci District, Jambi Province, Indonesia (Fig 1). This hot water is unique because it has alkaline pH. The hot springs with alkaline water are rare, both in Indonesia and in the world. The alkaline hot springs usually contain a high amount of base mineral, which makes microbial populations are more diverse than in the acidic hot springs11,12. Therefore, this study aimed to determine the diversity of thermophilic obligate Bacillus spp. that could produce alkaline protease in Sungai Tutung.
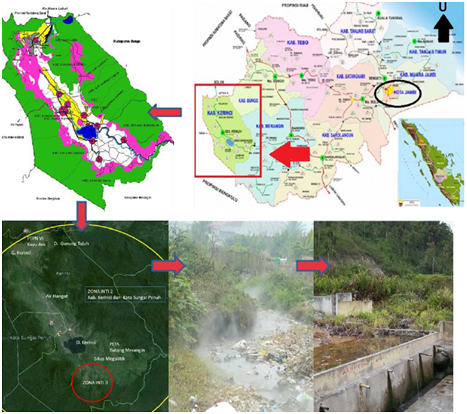
Fig. 1 Sungai Tutung in Kecamatan Air Hangat, Kerinci, Jambi, Indonesia
Experimental section
Hot water sampling
The sampling of hot water at the studied location was done using a modified method of Agustien, Yetria7 and Panda, Sahu8. The samples were taken 10cm below the surface of the water with a 100mL sterile bottle and 500mL flask from ponds and streams of Sungai Tutung (Fig 2). There were four sampling points with temperatures of 70, 75, 80 and 850C. The temperature of the hot water samples was kept stable during the transportation to the laboratory by placing the sample containers in a box containing hot water. The samples were isolated from a medium of thermophilic Bacillus within 24 hours of the sample collection. Temperature, pH, and metal content of the water were analyzed. Microorganisms that presented at the edges and in the streams of the hot springs were also identified.

Fig. 2 Hot spring water sampling.
Isolasion of thermophillic obligate Bacillus
Thermophilic obligate Bacillus spp. isolation was carried out as follows: 1mL of the hot water sample was transferred into a petri dish and 15mL of TB medium (Thermophilic Bacillus) pH 8.0 was added13. The petri dish was wrapped and incubated at 600C for 24 hours. The Bacillus spp. colonies can be identified by the round shape and white color. The thermophilic Bacillus spp. colonies were purified on tilted TB medium and labeled as Bacillus spp. stock7,14,15.
Screening of the thermophilic obligate Bacillus spp. that produced alkaline protease
Screening of the thermophilic obligate Bacillus spp. that produced alkaline protease was done following the method of Irfan, Safdar 16 with modification. Each pure thermophilic Bacillus spp stock was inoculated into a skim milk agar medium (SMA) pH 8.013 and incubated at 600C for 24 hours. Bacterial colonies and clear zones formed around the colony were the indications of the proteolytic activity. The colonies were observed and the diameter was measured to determine its proteolytic index (PI) using the formula:
PI=(∅ clear zone-∅bacterial colony)/(∅bacterial colony)
Indentification of the thermophillic obligate Bacillus spp. that produced alkali protease
Bacillus sp. isolates living in alkaline and high-temperature conditions were identified macroscopically, microscopically, biochemically, and molecularly using a 16S r RNA method. The steps are as follows: 1) the bacteria colony morphology was macroscopically observed from the agar plate under the age of 24 hours; 2) microscopic observation was done by coloring the pure cell and spore isolates; 3) biochemical observations were carried out by testing the catalase oxidase, gelatin, amylase hydrolysis, gas formation, and motility11,14; 4) the bacteria molecular was analyzed following the method from8 with modification. The molecular analysis was initiated by isolating isolate DNA from TB medium grown at 600C, pH 8.0, for 8 hours using lysozyme. The DNA from polymerase chain reaction (PCR) product was electrophoresed on 1% agarose gel and purified with QIA quick PCR (Qiagen). The measurement was done with A260 / A280 spectrophotometry, 16S rDNA isolate sequence amplification with Big Dye ABI PRISM Chemistry, Biosystems, USA, with 1492R primers (5′-TACTACGGYCTTGTTACGACT-3 ‘) and 27F (5’-AGAGTTGAT CMTGGCTCAG-3′) on a PCR machine. The PCR protocol was performed with one denaturation cycle at 94 0C for 1 minute, annealing at 480C for 1 minute, 30 cycles of elongation at 720C for 2 minutes per cycle, and an additional elongation for 10 minutes after the amplification process 17. The pure mixture of sequence reaction was electrophoresed with automatic DNA sequencer Biosystems Model 310 (Perkin Elmer, USA). PCR fragments were analyzed by a Macrogen sequencer machine and the sequence results were then compared with Gen Bank database using Blast N program. The analysis of phylogenetic isolates was done with the Molecular Evolutionary Genetic Analysis (MEGA) version 7.018. The phylogenetic tree was constructed based on genetic kinship distance by neighbor-joining (NJ) method19. The strength of phylogenetic trees was tested using bootstrap with 1000 repetitions20.
The hot spring of Sungai Tutung is part of the Kerinci Seblat National Park area under the Mount Kerinci (Jambi Local Government Data). This river is located on 20 ‘S 02 02’ 19.4 “and E 101 26 ’37.9″, 812 M above sea level. The temperature of the river is in the range of 70-85oC with alkaline pH (8.4). The hot spring is alkaline (pH 7,5 – 14), rich in minerals, and contains sodium carbonate, which causes a diversity of its biotas. Wang, Xu3 and Logares, Lindström 21 stated that the environment affects community development in extreme habitats and special habitat is more responsive to the environmental selection. The water pH increases as it flows to the surface because it dissolves minerals and CO2. Even though H2S sometimes present in the water, sulfur oxidation on the surface does not affect the pH, as it stays constant in the range of 9-10 due to water abundance. Mothe and Sultanpuram 15 reported that the optimum pH and temperature for protease activity were at 8.0 and 60oC, respectively.
The composition of some minerals in the hot springs of Sungai Tutung was: 2.8mg/L NO3, 0.37 ppm Fe, and 0.54ppm Ca. Trashes that were found at around the ponds and streams (Fig.1) contained various biotic compounds: 23 plant species were identified from 15 families. This environmental condition was favorable for the development of different types of thermophilic and alkaliphilic Bacillus. All isolated bacterial strains produced alkaline proteases with their proteolytic modulations ranging from 0.25 to 6.15 (Table 1 and Fig. 3).
Table (1):
Proteolytic index of thermophilic obligate Bacillus spp. from hot spring in the Sungai Tutung.
Code Isolates |
Proteolytic Index |
|---|---|
ST-1 |
0.78 |
ST-2 |
0.64 |
ST-3 |
0.25 |
ST-4 |
0.76 |
ST-5 |
1.91 |
ST-6 |
0.68 |
ST-7 |
0.92 |
ST-8 |
6.15 |
ST-9 |
0.59 |
ST-10 |
0.90 |
ST-11 |
0.32 |
ST-12 |
0.46 |
ST-13 |
1.48 |
ST-14 |
2.06 |

Fig. 3. Screening of the thermophilic obligate Bacillusspp.
Biological and physicochemical interactions play a role in shaping the conditions of microbial compositions in hot springs. In addition to the temperature, pH, geochemical composition and geographic distance determine the colony structure and bacterial populations. The presence of particular strains contributes significantly to habitat composition and is correlated with the geochemical properties of hot springs 12.
Identified isolates from the hot springs of Sungai Tutung are shown in phylogenetic trees in Fig. 4. Six species of thermophilic Bacillus with 14 strains were successfully identified. They were: Bacillus sonorensis (1 strain), Bacillus lichenmiformis (6 strain), Paenobacillus residui (1 strain), Brevibacillus borstelensis (2 strain), Bacillus aerius (3 strain), and Fictibacillus gelatini (1 strain). Some of these species have also been discovered by Habibie, Wardani 22 in hot mud Lapindo (temperature 45-700C, pH 7, 14 isolates), by Aanniz, Ouadghiri 23 in hot springs in Morocco (temperature 39-570C, pH 7-7.4), by Panda, Sahu 8 in the Tarabalo hot spring in India (temperature 50-900C, 22 isolates). Sungai Medang hot springs; 39 isolates 24. One Genus of Fictibacillus has also been found in the Chorao Island marine sediments, Goa Provence, India 25.
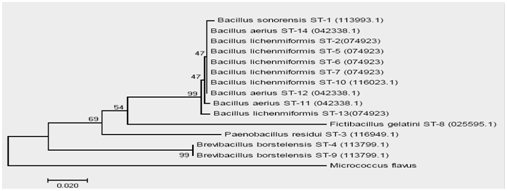
Fig. 4 The phylogenic tree of Bacillus thermophilicobligate that produce protease alkali isolate from SAP. Sungai Tutung
Only Fictibacillus gelatini had not been found in any hot springs, but according to Glaeser, Dott26 this species has a growth high temperature. The discovery of a new strain of the thermophilic Bacillus at this hot spring water of Sungai Tutung was most likely because of the uniqueness of this hot water. As explained before, this hot spring had not only high-temperature water but also had alkaline pH, contained various minerals, and the origin of the source sluice is also different.
The characteristics of the thermophilic obligate Bacillus spp. strains that produced alkali protease from Sungai Tutung are listed in Table 2. The color of the colonies of Bacillus spp. was white and dark white. The shape, edges, and elevation of colonies were varied. Most of the strains had rounded colonies with flat edges and showed raised elevation (Fig. 5). According to Pandey, Dhakar11, the thermophilic Bacillus spp colony is characterised by a round shape flat edge and white color. Based on this morphological observation, nine types of strains were identified. Five types of thermophilic and alkaliphilic Bacillus were identified from the microscope observation. All of these five types bacteria were gram-positive, which characterized with a purple color. The cell shape varied from polymorphic, diplobacillus, and half-shaped bacillus. The spores of the thermophilic obligate Bacillus spp. were mostly located in the center of the cell, partially located almost to the end of the cell, and spores of one strain located at the end of the cell (Fig. 5). Based on the biochemical characters (Table 2), the whole strains showed the characteristics of thermophilic obligate Bacillus spp. that generated protease. The bacteria could be grouped into 13 strains based on the differences in biochemical characters, especially in sugar series reaction 8,17,27.
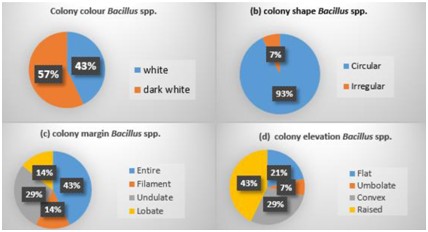
Fig. 5. Colony morphology Bacillus spp. obligatethermophillic (a. color; b. shape; c. margin; d. elevation)
Table (2):
The identification of Bacillus spp. Thermophilic obligate that produced alkaline protease isolates from hot springs Sungai Tutung Kerinci Jambi Indonesia
| Isolate Code | Colony morphology (color, shape, edge, surface) | microscopic (cell shape, Gram, spore location) | Biochemistry | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalase | Oxydase | Gas | Sulfide | TSIA | Mortility | Indol | Urea | Citrate | Lactose | Glucose | Sucrose | Mannitol | MR | VP | OF | Arabinose | Xylose | Nitrate | Gelatin | |||
| ST-1 | White, circular, entire, flat | Diplobacillus, G+, subterminal | + | – | – | – | M/K | + | – | – | – | – | – | + | – | + | + | – | + | – | + | + |
| ST-2 | Dark white, circular, entire, convex | Bacillus , G+ , subterminal | + | – | – | – | M/K | + | – | – | – | – | + | + | + | – | + | – | – | – | + | + |
| ST-3 | Dark white, circular filament, flat | Polimorpis , G+ , terminal | + | + | – | – | M/K | + | – | – | – | – | + | + | + | – | + | – | – | – | – | + |
| ST-4 | White, circular entire, flat | Diplobacillus , G+ , central | + | – | – | – | M/K | – | – | – | – | – | – | + | – | + | + | – | – | – | + | + |
| ST-5 | Dark white, circular, undulate, raised | Diplobacillus , G+ , central | + | – | – | – | M/K | + | – | – | – | – | + | + | + | + | + | – | + | – | + | + |
| ST-6 | White, circular, undulate, raised | Bacillus , G+ , subterminal | + | – | – | – | M/K | + | – | – | – | – | – | + | + | – | + | – | + | – | – | + |
| ST-7 | Dark white, circular, entire, convex | Bacillus , G+ , central | + | – | – | – | M/K | + | – | – | – | – | – | + | + | – | + | – | + | – | + | + |
| ST-8 | Dark white, irregular, lobate, raised | Bacillus , G+ , central | + | – | – | – | K/K | + | – | – | – | – | – | + | + | – | + | – | – | – | + | + |
| ST-9 | Dark white, circular, undulate, raised | Diplobacillus , G+ , central | + | – | – | – | M/K | + | – | – | – | – | – | – | – | – | – | – | – | – | + | + |
| ST-10 | Dark white, circular, undulate, raised | Diplobacillus , G+ , central | + | – | – | – | M/K | + | – | – | – | – | + | + | + | + | + | – | + | – | – | + |
| ST-11 | White, circular, lobate, raised | Bacillus , G+ , central | + | – | – | – | M/K | + | – | – | – | – | – | + | – | – | + | – | + | – | + | + |
| ST-12 | Dark white, circular, entire, convex | Bacillus , G+ , subterminal | + | – | – | – | M/K | + | – | – | – | – | – | + | + | – | + | – | + | – | + | + |
| ST-13 | White, circular, entire, convex | Bacillus , G+ , central | + | – | – | – | M/K | + | – | + | – | – | + | + | + | – | + | – | + | – | – | + |
| ST-14 | White, circular, filament, undulate | Diplobacillus , G+ , central | + | – | – | – | M/M | + | – | – | – | – | – | + | + | – | + | – | – | – | + | + |
The cell shape of thermophilic Bacillus spp. consisted of 50% bacillus, 36% diplobacillus and 14% polymorphism (Fig 6a). About 57% of Bacillus spp. had spore located in the middle of the cell, 36% located in the subterminal of the cell, and 7% located at the end of the cell (terminal) (Figure 6 b). Gram staining showed that the cells were purple with the shape of short bacillus, which indicated a Gram-positive bacteria that form endospores.
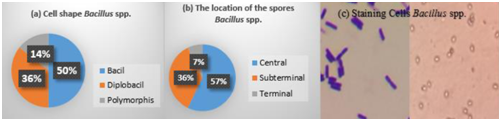
Fig. 6. Microscopic observations Bacillus spp. obligate thermophilic (a. cell shape, b. the location of the spores, c. staining cells)
The hot spring of Sungai Tutung in Kerinci, Jambi, Indonesia contained varieties of the thermophilic obligate Bacillus. Six species with 14 different strains were identified from the analysis of the DNA sequences. All of these strains had the potential of producing alkali proteases with a proteolytic index ranging from 0.25 to 6.15. Each strain showed different morphological, microscopical, and biochemical properties.
- Badoei-Dalfard A, Karami Z, Ravan H. Purification and characterization of a thermo-and organic solvent-tolerant alkaline protease from Bacillus sp. JER02. Preparative Biochemistry and Biotechnology 2015; 45(2): 128-43.
- Dewan S. Enzymes in industrial applications: Global markets. Market Research Repor t Wellesley, MA: BCC Research 2011.
- Wang J, Xu A, Wan Y, Li Q. Purification and characterization of a new metallo-neutral protease for beer brewing from Bacillus amyloliquefaciens SYB-001. Applied biochemistry and biotechnology 2013; 170(8): 2021-33.
- Ghareib M, Fawzi EM, Aldossary NA. Thermostable alkaline protease from Thermomyces lanuginosus: optimization, purification and characterization. Annals of microbiology 2014; 64(2): 859-67.
- Ebrahimpour A, Kariminik A. Isolation, characterization and molecular identification of protease producing bacteria from Tashkooh mountain located in Ahvaz, Iran. International Journal of Life Sciences 2015; 9(2): 39-42.
- Canganella F, Wiegel J. Anaerobic thermophiles. Life 2014; 4(1): 77-104.
- Agustien A, Yetria R, Arzita, Yunofrizal. Catalytic activity and conditions of extracellular protease alkaline thermosstable Bacillus sp. SR-09. Journal of Chemical and Pharmaceutical Research 2015; 7(11): 417-21.
- Panda MK, Sahu MK, Tayung K. Isolation and characterization of a thermophilic Bacillus sp. with protease activity isolated from hot spring of Tarabalo, Odisha, India. Iranian journal of microbiology 2013; 5(2): 159.
- Sinha R, Khare SK. Thermostable proteases. Thermophilic Microbes in Environmental and Industrial Biotechnology: Springer; 2013: 859-80.
- Khoirunnas AL. Lempeng Tektonik Indonesia. Wahana Keilmuan Geospasial 2012.
- Pandey A, Dhakar K, Sharma A, Priti P, Sati P, Kumar B. Thermophilic bacteria that tolerate a wide temperature and pH range colonize the Soldhar (95° C) and Ringigad (80° C) hot springs of Uttarakhand, India. Annals of microbiology 2015; 65(2): 809-16.
- Valverde A, Tuffin M, Cowan DA. Biogeography of bacterial communities in hot springs: a focus on the actinobacteria. Extremophiles 2012; 16(4): 669-79.
- Agustien A. Protease Bakteri Termofilik. Universitas Padjajaran Bandung 2010.
- Al-Qodah Z, Daghistani H, Alananbeh K. Isolation and characterization of thermostable protease producing Bacillus pumilus from thermal spring in Jordan. African Journal of Microbiology Research 2013; 7(29): 3711-9.
- Mothe T, Sultanpuram VR. Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacilluscaseinilyticus. 3 Biotech 2016; 6(1): 53.
- Irfan M, Safdar A, Syed Q, Nadeem M. Isolation and screening of cellulolytic bacteria from soil and optimization of cellulase production and activity. Turkish Journal of Biochemistry/Turk Biyokimya Dergisi 2012; 37(4).
- Selim S, Sherif ME, El-Alfy S, Hagagy N. Genetic Diversity among Thermophilic Bacteria Isolated from Geothermal Sites by Using Two PCR Typing Methods. Geomicrobiology Journal 2014; 31(2): 161-70.
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular biology and evolution 2016; 33(7): 1870-4.
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences of the United States of America 2004; 101(30): 11030-5.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 1985; 39(4): 783-91.
- Logares R, Lindström ES, Langenheder S, et al. Biogeography of bacterial communities exposed to progressive long-term environmental change. The ISME journal 2013; 7(5): 937.
- Habibie FM, Wardani AK, Nurcholis M. Isolasi dan Identifikasi Molekuler Mikroorganisme Termofilik Penghasil Xilanase dari Lumpur Panas Lapindo [In Press Oktober 2014]. Jurnal Pangan dan Agroindustri 2014; 2(4): 231-8.
- Aanniz T, Ouadghiri M, Melloul M, et al. Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Brazilian Journal of Microbiology 2015; 46(2): 443-53.
- Wahyuna D, Agustien A. Isolasi Dan Karakterisasi Bakteri Termo-Proteolitik Sumber Air Panas Sungai Medang, Sungai Penuh, Jambi. Jurnal Biologi Unand 2012; 1(2).
- Dastager SG, Mawlankar R, Srinivasan K, et al. Fictibacillus enclensis sp. nov., isolated from marine sediment. Antonie van Leeuwenhoek 2014; 105(3): 461-9.
- Glaeser SP, Dott W, Busse H-J, Kämpfer P. Fictibacillus phosphorivorans gen. nov., sp. nov. and proposal to reclassify Bacillus arsenicus, Bacillus barbaricus, Bacillus macauensis, Bacillus nanhaiensis, Bacillus rigui, Bacillus solisalsi and Bacillus gelatini in the genus Fictibacillus. International journal of systematic and evolutionary microbiology 2013; 63(8): 2934-44.
- Sharma N, Vyas G, Pathania S. Culturable diversity of thermophilic microorganisms found in hot springs of Northern Himalayas and to explore their potential for production of industrially important enzymes. Scholars Academic Journal of Biosciences 2013; 1(5): 165-78.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


