ISSN: 0973-7510
E-ISSN: 2581-690X
The heterogeneous etiology of asthma makes its diagnosis complicated. Measurement of cytokine levels could be relevant in determining the asthma phenotype, predicting severity, and identifying the treatment type. Enzyme-linked immunosorbent assay (ELISA) is one of the most reliable methods, with high sensitivity and specificity. This study aimed to determine the accuracy and utility of interleukin (IL)-13 and IL-17 A in diagnosing children with asthma. A total of 74 asthmatic and 75 healthy children were enrolled in this case-control study between 10/2019 and 3/2021. Sera were collected and analyzed for IL-13 and IL-17A using ELISA. Diagnostic utility assessment was performed using receiver operating characteristic (ROC) analysis. The results showed that both cytokines had a significant capacity to differentiate patients with asthma from the control group. The sensitivity and specificity for IL-17A were 97.3% and 52.0%, respectively, whereas for IL-13 it was 81.1% and 52.0%, respectively. Positive predictive values (PPV) were 66.7% and 62.5% for IL-17A and IL-13, respectively. In conclusion, although both biomarkers had low specificity, IL-17A was more sensitive in differentiating children with asthma from those in the control group and had a higher sensitivity rate than IL-13.
IL-13, IL-17A, ELISA, Diagnostic Utility, Asthma
Asthma is a chronic inflammatory condition common in children. The underlying pathophysiology is associated with airway narrowing and inflammation that results in common symptoms such as dyspnea, cough, and wheezing.1
The mechanism or pathophysiological background of asthma has been studied extensively over the years, as they can affect disease prognosis and management protocols. Cytokines could be regarded as the core for the treatment like IL-5 and IL-13 antagonists.2,3 The cytokine pathways may vary significantly among patients with asthma. This cytokine variation can be used as a biomarker for discriminating asthma phenotypes.
The T-helper (Th)-2 pathway plays a major role in asthma by secreting cytokines, such as IL-4, IL-5, and IL-13. These cytokines are responsible for eosinophil and B-cell activation with subsequent immunoglobulin E (IgE) secretion.4 Another prominent cytokine pathway in asthma is Th17.5 This pathway is associated with IL-17 secretion, which is a proinflammatory cytokine that leads to the production of IL-8 and tumor necrosis factor alpha and could be associated with a more severe form of asthma.6 Both the aforementioned pathways (Th17 and Th2) are reciprocally regulated in asthma.7 When Th2 pathway is downregulated by corticosteroids, the Th17 pathway is activated.8,9
Medical diagnostic tests should have the ability to confirm the presence of a disease and the capacity to discriminate between those with disease and healthy individuals. Sensitivity and specificity with a single cutoff point are among the most widely used characteristics for diagnostic accuracy.10 However, sensitivity and specificity could be computed across different cutoff points with inverse correlation of sensitivity and specificity using the receiver operating characteristic (ROC) curve, which is a more comprehensive method.11 The area under the ROC curve (AUC) is a measure of specificity and sensitivity that provides valid diagnostic accuracy.12 When the ROC curve approaches the top-left corner of the graph, it indicates accurate and valid discrimination between the groups. An AUC value closer to 1 indicates a more accurate test that maximizes discrimination; a value of 0.5 means failure of the test in classifying the patient group.13
Different epidemiological studies have assessed the diagnostic utility of serum markers using ROC analysis.12,14,15
As aforementioned, the pathophysiology of asthma depends on different immune pathways with the participation of a wide range of cytokines; therefore, the diagnosis of different phenotypes is associated with the types of cytokines. The diagnosis of asthma based on these phenotypes remains controversial.
Measurement of serum cytokine levels could indicate the phenotype of asthma, highlighting the diagnostic utility of these cytokines in the diagnosis of asthma. Therefore, the present study aimed to determine the diagnostic utility of both IL-13 and IL-17A in children with asthma, using the ELISA technique and to compare the diagnostic accuracy of both the cytokines.
Study Design and Settings
This case-control study was conducted at the Department of Medical Microbiology/Kerbala College of Medicine between October 2019 and March 2021.
Subjects
The patient group consisted of 74 children who were diagnosed with asthma based on the guidelines of the American Thoracic Society (ATS) for asthma.16 The patients included 56 men and 18 women, with ages ranging from 1 to 15 years. The control group consisted of 75 healthy children with matching age to the patient group (50 men and 25 women). National Asthma Education and Prevention Program/Expert Panel Report 3 (NAEPP/EPR 3) guidelines were used to assess the severity of asthma.17 All patients were selected using convenience sampling (no duplicate sampling).
Inclusion and Exclusion Criteria
All patients who met the American Thoracic Society criteria for asthma16 were included in the study. The control group had no medical history of asthma, allergy, or inflammatory diseases.
Collecting blood sample
In both the groups, 5 ml of venous blood was first collected from each participant, and the serum was then separated by centrifugation and stored at -20 °C for further analysis.
Assays
ELISA kits for IL‑13 and IL-17A (CUSABIO, China) (Catalog number CSB-E04601h for IL-13 and CSB-E12819h for IL-17) were used. The detection range for IL‑13 was 62.5–4000 pg/ml and that for IL-17A was 6.25–400 pg/ml.
The Ethical approval
The ethical committee of Kerbala College of Medicine, in addition to the Karbala Health Directorate Committee, approved this study. Furthermore, verbal and written consent was obtained from each child’s parents before blood sampling and consent was obtained through an interview for data collection.
Statistical analysis
The Statistical Package for the Social Sciences, SPSS, version 20 (IBM, Chicago, Illinois, USA) was used for statistical analysis. The data are summarized in tables and figure. Frequencies and percentages were used to describe the demographic and clinical data of the patients. The Shapiro-Wilk test (P < 0.05), visual appearance of the histogram, normal Q-Q plots, and box plots showed that the data were approximately non-normally distributed for different variables of cases and controls. For comparison of mean and standard error of interleukins and demographic variables, the Mann-Whitney U test was used. Diagnostic utility was evaluated using the receiver operating characteristic (ROC) curve. In addition to sensitivity and specificity, both positive predictive value (PPV) and negative predictive value (NPV) were calculated for the study groups. Statistical significance was set at P < 0.05.
This study found a significant association between a positive history of allergic conjunctivitis and IL-17 A levels, and a significant association between a negative history of eczema and family history of smoking and IL-17 A levels as shown in Table 1. No significant difference was observed between the other variables and IL levels.
Table (1):
Differences in the concentration of IL-13, and IL-17A according to age and clinical variables among asthmatic group.
| Variable | N | Interleukin-13 | Interleukin 17-A | |||
|---|---|---|---|---|---|---|
| Mean ±SE | P-value | Mean ±SE | P-value | |||
| Gender | Male | 56 | 0.97±0.05 | 0.464 | 26.68±5.19 | 0.173 |
| Female | 18 | 1.08±0.12 | 19.68±8.54 | |||
| History of Eczema | Positive | 8 | 0.95±0.22 | 0.296 | 1.85±0.14 | 0.007** |
| Negative | 66 | 1.00±0.04 | 27.78±4.86 | |||
| History of Allergic rhinitis | Positive | 40 | 0.93±0.05 | 0.328 | 31.04±7.29 | 0.152 |
| Negative | 34 | 1.07±0.08 | 17.84±4.20 | |||
| History of Allergic conjunctivitis | Positive | 20 | 0.92±0.06 | 0.349 | 44.92±13.61 | 0.025* |
| Negative | 54 | 1.02±0.06 | 17.59±2.94 | |||
| Family history of eczema | Positive | 16 | 0.96±0.06 | 1 | 12.41±3.10 | 0.141 |
| Negative | 58 | 1.01±0.06 | 28.44±5.51 | |||
| Family history of asthma | Positive | 50 | 1.05±0.06 | 0.101 | 25.05±5.71 | 0.764 |
| Family history of allergic rhinitis | Negative | 24 | 0.87±0.07 | 24.82±6.87 | ||
| Positive | 58 | 1.00±0.05 | 0.979 | 22.65±3.68 | 0.141 | |
| Negative | 16 | 0.99±0.11 | 33.40±15.78 | |||
| Family history of smoking | Positive | 34 | 1.00±0.07 | 0.879 | 13.01±2.50 | 0.035* |
| Negative | 40 | 0.99±0.06 | 35.15±7.59 | |||
| Aggravation by flu | Positive | 50 | 1.00±0.06 | 0.781 | 29.21±6.23 | 0.380 |
| Negative | 24 | 0.98±0.07 | 16.16±3.85 | |||
| Aggravation by dust | Positive | 40 | 0.99±0.06 | 0.761 | 33.48±7.68 | 0.362 |
| Negative | 34 | 1.00±0.07 | 14.97±2.59 | |||
| Type of treatment | Montelukast | 34 | 0.91±0.07 | 0.467 | 23.77±4.80 | 0.249 |
| Corticosteroid# | 4 | 0.96±0.14 | 2.00±0.00 | |||
| Mixed | 2 | 0.96±0.00 | 1.90±0.00 | |||
| No treatment | 38 | 1.07±0.43 | 29.56±7.63 | |||
| Severity level | Mild | 62 | 0.98±0.05 | 0.639 | 26.15±5.21 | 0.681 |
| Moderate | 12 | 1.07±0.13 | 18.91±4.64 | |||
Mann Whitney U test, * significant p-value, ** highly significant p-value, SE=standard error, P=probability value, # inhaled corticosteroid.
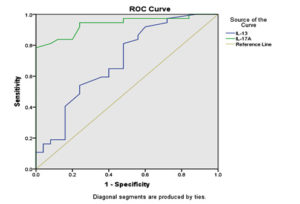
Evaluation of diagnostic accuracy for both IL-13 and IL-17A using ROC curve analysis revealed that the value of AUC was 0.697 and 0.936 for IL-13 and IL-17A, respectively, which are statistically highly significant for the diagnosis of asthma (shown in Table 2). In addition, the ROC curve was shifted to the left side away from the reference line, as shown in Figure, and the IL-17A curve was deviated to the left more than the IL-13 curve. The cutoff points for IL-13 and IL-17A were ≥ 0.6400 and ≥ 0.9435, respectively, as shown in Table 3. The sensitivity and specificity for IL-17A were 97.3% and 52.0%, respectively, while for IL-13 the sensitivity and specificity were 81.1% and 52.0%, respectively. The accuracy was 63.4% and 68.04% for IL-13 and IL-17A, respectively.
Table (2):
Diagnostic utility of area under the curve in Figure.
Test Result Variable(s) |
Area (AUC) |
95% Confidence Interval |
P-value |
|---|---|---|---|
IL-13 |
0.697 |
0.613-0.781 |
.0000 |
IL-17A |
0.936 |
0.896-0.977 |
0.000 |
Mann Whitney U test, AUC: area under curve, P: probability.
Table (3):
Sensitivity, specificity, positive, and negative predictive value for IL-13, and IL-17A.
Variable |
Cut-off value |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy% |
|---|---|---|---|---|---|---|
IL-13 pg/ml |
≥ 0.6400 |
81.10% |
52.00% |
62.50% |
73.60% |
63.40% |
IL-17A pg/ml |
≥ 0.9435 |
97.30% |
52.00% |
66.70% |
95.10% |
68.04% |
PPV: positive predictive value, NPV: negative predictive value.
Activation of both the Th2 and Th17 pathways in asthma represents an asthma phenotype.18 The role of IL-13 in pediatric asthma pathophysiology is very important and is associated with concomitant activation of other Th2 cytokines, such as IL-4 and IL-5 and the activation of eosinophils and plasma cells.19 In addition, IL-17A is a proinflammatory cytokine that could be associated with the severity of asthma.6
The role of IL-17A in allergic and atopic diseases is unclear. The current findings shown in Table 1 are in accordance with those of a previous study, which showed that activation of Th17 cells promoted Th2 activity and was associated with symptoms of allergy,20,21 such as allergic conjunctivitis and allergic rhinitis. This could be explained by the common pathophysiology of all atopic diseases such as asthma and allergic conjunctivitis.
The lower levels of IL-17A in patients with a family history of both smoking and a history of eczema may be due to the effect of sample size or because eczema was not in the active form. Previous studies have indicated that the level of IL-17 and infiltration of Th17 cells in eczematous dermatitis is dependent on the state of severity; therefore, in patients with no symptoms of atopic dermatitis, no elevation in serum levels is expected,22,23 which is in agreement with the findings of the current study. Another explanation for the unchanged level of IL-17A is that eczema is not similar to other immunological skin diseases such as psoriasis, which can affect serum levels of IL-17A.22 The effect of passive smoking is widely dependent on the number of cigarettes smoked and the proximity of smokers and children. Disease activity and control were one of the confounder limitations of the study because it required more sessions with patients to collect data.
Clinical reports depend on ROC analysis to assess the diagnostic utility of certain serum markers.12,14,15 The current data revealed a higher sensitivity for IL-17A than IL-13. In addition to low specificity for both markers, the PPV was 66.7% and 62.5% for IL-17A and IL-13, respectively. A previous study concluded that IL-13 was a relevant biomarker for the diagnosis of asthma superimposed by rhinovirus infection in children.24 The study by Akiki et al. showed that IL-13 is a serum cytokine that can be used as a predictor to control asthma.25 The current findings are in accordance with those of previous studies that have recorded the role of elevated IL-17 in airway inflammation in patients with asthma.26,27 In addition, another study reported an increase in IL-17 in pediatric asthma and concluded that this increment is age- and phenotype-limited, that is, the level of IL-17 differs according to the age of the patient and phenotype of asthma.28 Shabana et al. studied the diagnostic utility of baseline IL-17A/IL-10 ratio using ROC analysis and concluded that it can be regarded as a predictive biomarker in asthmatics with vitamin D deficiency with a cutoff point ≥ 2.66, sensitivity of 72.2%, and specificity of 83.3%.29 The lower specificity for both markers in this study could be related to the sample size, genetic background, or different ethnic groups compared to other studies.
Although IL-13 and IL-17A serum markers had low specificity, both showed a high sensitivity rate in the diagnosis of pediatric asthma; however, IL-17A was more sensitive than IL-13. Both the markers could be used together to determine the specific phenotype of asthma in children (Th2/Th17 dominant), but confirmation of this conclusion requires a larger study and inclusion of more Th2 and Th17 cytokines, other than IL-13 and IL-17A.
ACKNOWLEDGMENTS
The authors would like to thank participants and their families who contributed in this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both the authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Asthma. World Health Organization. 2022. https://www.who.int/news-room/fact-sheets/detail/asthma. Accessed 17 August 2021.
- Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: The next steps toward personalized care. J Allergy Clin Immunol. 2015;135(2):299-310.
Crossref - Hilvering B, Pavord I. What goes up must come down: biomarkers and novel biologicals in severe asthma. Clinical & Experimental Allergy. 2015;45(7):1162-1169.
Crossref - Wills-Karp M, Finkelman F. Untangling the Complex Web of IL-4- and IL-13-Mediated Signaling Pathways. Sci Signal. 2008;1(51):pe55.
Crossref - Pene J, Chevalier S, Preisser L, et al. Chronically Inflamed Human Tissues Are Infiltrated by Highly Differentiated Th17 Lymphocytes. J Immunol. 2008;180(11):7423-7430.
Crossref - Silvestri M, Bontempelli M, Giacomelli M, et al. High serum levels of tumour necrosis factor-? and interleukin-8 in severe asthma: markers of systemic inflammation? Clinical & Experimental Allergy. 2006;36(11):1373-1381.
Crossref - Choy DF, Hart KM, Borthwick LA, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7(301):301ra129.
Crossref - Massoud A, Charbonnier L, Lopez D, Pellegrini M, Phipatanakul W, Chatila T. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med. 2016;22(9):1013-1022.
Crossref - Menson K, Mank M, Reed L, et al. Therapeutic efficacy of IL-17A neutralization with corticosteroid treatment in a model of antigen-driven mixed-granulocytic asthma. Am J Physiol Lung Cell Mol Physiol. 2020;319(4):L693-L709.
Crossref - Mandrekar J. Simple Statistical Measures for Diagnostic Accuracy Assessment. J Thorac Oncol. 2010;5(6):763-764.
Crossref - Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013;4(2):627-635. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3755824/. Accessed 20 August 2021.
- Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011;48(4):277-287.
Crossref - Polo TCF, Miot HA. Use of ROC curves in clinical and experimental studies. J Vasc Bras. 2020;19:e20200186.
Crossref - Daubin C, Quentin C, Allouche S et al. Serum neuron-specific enolase as predictor of outcome in comatose cardiac-arrest survivors: a prospective cohort study. BMC Cardiovasc Disord. 2011;11(1):48.
Crossref - Darmon M, Vincent F, Dellamonica J, et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15(4):R178.
Crossref - Chung K, Wenzel S, Brozek J. Comment on: International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;44(1):267-268.
Crossref - Urbano F. Review of the NAEPP 2007 Expert Panel Report (EPR-3) on Asthma Diagnosis and Treatment Guidelines. J Manag Care Pharm. 2008;14(1):41-49.
Crossref - Irvin C, Zafar I, Good J, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 2014;134(5):1175-1186.e7.
Crossref - Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol. 2013;131(5):1267-1275.
Crossref - Meng X-T, Shi Y-Y, Zhang H, Zhou H-Y. The role of th17 cells and IL-17 in th2 immune responses of allergic conjunctivitis. J Ophthalmol. 2020;2020:1-9.
Crossref - Sheha D, El-Korashi L, AbdAllah AM, El Begermy MM, Elzoghby DM, Elmahdi A. Lipid Profile and IL-17A in Allergic Rhinitis: Correlation With Disease Severity and Quality of Life. J Asthma Allergy. 2021;14:109-117.
Crossref - Sugaya M. The Role of Th17-Related Cytokines in Atopic Dermatitis. Int J Mol Sci. 2020;21(4):1314.
Crossref - Hofmann MA, Fluhr JW, Ruwwe-Glosenkamp C, Stevanovic K, Bergmann KC, Zuberbier T. Role of IL-17 in atopy-A systematic review. Clin Transl Allergy. 2021;11(6):e12047.
Crossref - Nguyen-Thi-Dieu T, Le-Thi-Thu H, Le-Thi-Minh H, Pham-Nhat A, Duong-Quy S. Study of Clinical Characteristics and Cytokine Profiles of Asthmatic Children with Rhinovirus Infection during Acute Asthma Exacerbation at National Hospital of Pediatrics. Can Respir J. 2018;2018:9375967.
Crossref - Akiki Z, Rava M, Diaz Gil O, et al. Serum cytokine profiles as predictors of asthma control in adults from the EGEA study. Respir Med. 2017;125:57-64.
Crossref - Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma. Am J Respir Crit Care Med. 2005;171(3):224-230.
Crossref - Bullens DM, Truyen E, Coteur L, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7(1):135.
Crossref - Pukelsheim K, Stoeger T, Kutschke D, Ganguly K, Wjst M. Cytokine profiles in asthma families depend on age and phenotype. PLoS ONE. 2010;5(12):e14299.
Crossref - Shabana MA, Esawy MM, Ismail NA, Said AM. Predictive role of IL-17A/IL-10 ratio in persistent asthmatic patients on vitamin D supplement. Immunobiology. 2019;224(6):721-727.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.