ISSN: 0973-7510
E-ISSN: 2581-690X
Rhizobacteria (PGPR) that promote the plant growth are essential component of sustainable agriculture. Pea (Pisum sativum L.) root nodule Rhizobium leguminosarum bv. viciae ten strains were cultured at two different temperatures (28°C and 45°C). Out of eight strains screened the three N25, N30 and N40 were temperature tolerant while only one strain (N40) showed tolerance to pH11. The growth of Rhizobium strain N40 at 45 °C was 96.8 percent as compared to the growth of the at 28°C. The temperature tolerant strain N40 produced maximum IAA and solubilized insoluble tri calcium phosphate compared to other strains and thus can be used microbial inoculant in biofertilizer technology.
Rhizobium leguminosarum bv. viciae, indole acetic production, phosphate solubilization, temperature, pH tolerance
Pea (Pisum sativum L.), an important leguminous crop belongs to family Fabaceae. China, India, Canada, Russia, France and the United States of America are the main pea countries (Food and Agriculture Organization, 2012). Plants are cultivated in temperate regions and during winter season in tropical countries. In India, the area under pea cultivation is about 275 thousand hectares which accounts 4.5 per cent global area under pea cultivation. Pea is an important nutritional pulse and the green peas contain a form of omega-3 fats alpha- linolenic acid (ALA). Rhizobia are the single largest contributor to the biological N2 fixation (Jason et al., 2012). Legume-Rhizobium interaction is a key model for understanding the molecular basis of communication which represents most studied one but not fully understood symbiosis (Sachs et al., 2013). Plant growth promoting rhizobacteria (PGPR) as a microbial inoculant provide an alternative to chemical fertilizers and pesticides. In recent years, the use of PGPR has increased the productivity tremendously worldwide (Asghar et al., 2002). Combination of PGPR and efficient strains of Rhizobium in integrated biofertilizer technology has enhanced the growth of pea by inducing nitrogen fixation and efficient symbiosis (Kaur and Khan, 2013). Inoculation of Rhizobium strains with high temperature tolerance and effective nodulation to enhance plant growth would be required to improve crop productivity under climate change scenario (Singh et al.,2013, Gray and Smith,2005).
High soil temperature poses a major problem for cultivation of legume crop. Temperature of the surface soil (5-10 cm) layer reaches up to 40-50°C in the arid zone of North West India in the summer months and the microbial inoculants are often exposed to high temperatures which adversely affect the survival of rhizobia strains in the soil and establishment rhizobia-legume symbiosis. The prolonged exposure to high temperature reduces the saprophytic survival of rhizobia in soil and effective nodulation (Kiran et al., 2007). Temperature affects Rhizobium-legume symbiosis in various ways such as poor infection of root hairs, differentiation of bacteroids in nodule and nitrogen fixation. As an important a biotic factor, temperature influences the plant growth, metabolic enzymes activity and uptake of nutrients and indirectly on the solute solubility, transport of ions, membranes permeability, density and many colloidal properties of matter in soil-water interface (Sardesai and Babu, 2001; Xie et al., 2013). Rhizobium is a mesophilic bacterium and the growth optimum temperature ranges from 25°C to 31°C (Somasegaran and Hoben, 1994) with the upper temperature limits ranges between 32 – 47°C (Munevar and Wollum, 1988). The climate of Varanasi is humid subtropical with high temperature ranges between 32 – 46°C in summer and 5 – 15°C during winter (Singh et al., 2012). In the present communication, isolation and screening of high temperature and pH tolerant strains of Rhizobium isolated from pea nodules have been described. Further, the growth promoting parameters such as IAA production and solubilization of phosphate were also investigated in selected strains.
Rhizobium strains
Healthy nodules from Pea (Pisum sativum L.) roots were collected from the 20-25 days old plants growing at different location of Eastern Utter Pradesh, India. Healthy determinate nodules were washed several times thoroughly with tap water and transferred to sterile water. Flask was shaken on a shaker thoroughly. Surface sterilized nodules with 95% ethanol for 1 min, followed by treatment with 0.1% HgCl2 for 5 min and cleaned with sterile distilled water (Chaintreuil et al., 2000). A loop ful crushed surface sterilized nodule was inoculated on yeast-extract mannitol (YEM) agar plates and incubated in a BOD incubator at 28°C for 2-3 days. The pure colony were selected and incubated on Bakelite screw cap tube slants in same medium at 4°C for maintenance.
The strains were identified by cross inoculation test and based on 16s rRNA gene sequence phylogeny following the standard protocol.
Temperature tolerance
Temperature tolerance of the strains was investigated by growing the strains at 28°C and 45°C in 10 ml YEM broth with an inoculum of 108 CFU/ml. The growth was measured in terms of absorbance at 420 nm in a Spectrophotometer.
Pea plant seeds were inoculated with R. leguminosarum bv. viciae strains and nodulation was checked on slant culture at 45°C in a Growth Chamber. Also, the bacterized seed were grown in the sterilized soil in earthen pot to observed the nodule formation and growth of the host plant as compared to control.
Effect of high pH
Isolated Rhizobium strains were grown at pH 9 and 11 on YEMA plates to observe their pH tolerance. The pH was maintained with sodium hydroxide (NaOH) and stabilized with Tris-HCl buffer (10mM). The plates were incubated in a BOD incubator at 28°C.
Indole acetic acid (IAA) production
IAA produced by Rhizobium strains was determined in cultures supplemented with 100 µg
/ml filter sterilized L-tryptophan at 28°C with continuous shaking for 48 hours. Cell biomass was centrifuged at 10,000 x g and the IAA produced (ml-1 culture) was measured by mixing 2 ml culture supernatant with 4 ml of Salkowski reagent (1 ml 0.5 M FeCl3 in 50 ml of 35% perchloric acid). The absorbance of color developed was measured at 530 nm after 30 min (Gordon and Weber, 1951). The amount of IAA produced was determined with the help of standard curve of IAA (10- 100 µg/ml) and calculated by equation, y = mx + c (in µg /ml)
where:
y = O.D. of Rhizobium culture. m = O.D. of blank solution
x = amount of IAA produces by Rhizobium isolates c = Zero (constant)
Phosphate solubilization
Phosphate solubilization of Rhizobium strains was determined on Pikovskaya nutrient agar plates (Pikovskaya, 1948). The plates were inoculated as spots with 10µl of exponentially grown Rhizobium strains (Yanni et al., 2001). The radius of the clear zone including colony was measured and phosphate solubilization activity was determined in mm per unit time. The phosphate solubilization index (SI) was determined using the following formula by measuring diameters of halo (clear zone) and colony.
SI=Colony diameter +halo zone diameter/Colony diameter
The pea nodulating symbiotic bacterial strain was identified as Rhizobium leguminosarum bv viciae as revealed by 16s rDNA gene sequence phylogeny and the accession number of the strain is KT150239.1 which has been submitted in NCBI.
Temperature tolerance of Rhizobium leguminosarum bv. viciae strains
Ten clones of Rhizobium leguminosarum bv. viciae were isolated from the pea nodules collected from North-Eastern districts of UP, India. Based on their growth in YEM broth and agar plates at elevated temperature and cross inoculation test these were numbered as N13, N16 (temperature sensitive), N24, N25, N29, N30, N39, N40 and N42 (temperature tolerant) selected for further studies. The strains were grown at two different temperatures viz 28°C and 45°C in YEM broth to observe their temperature tolerance presented in Table 1.
Table (1):
Growth and survival of Rhizobium leguminosarum bv. viciae strains at 28°C and 45°C temperatures.
| Strain | Absorbance (420 nm) of cultures grown | Percent growth at 45°C as compared to 28°C | |
|---|---|---|---|
| at 28 °C | at 45 °C | ||
| 4 DAI | 4 DAI | ||
| P09 | 2.664 | 1.987 | 74.6 |
| N13 | 1.457 | ND* | ND |
| N16 | 1.875 | ND* | ND |
| N24 | 2.473 | 1.476 | 59.68 |
| N25 | 2.785 | 2.465 | 88.5 |
| N29 | 2.598 | 1.463 | 56.31 |
| N39 | 2.645 | 1.534 | 60.54 |
| N30 | 2.975 | 2.398 | 80.60 |
| N40 | 2.987 | 2.875 | 96.25 |
| N42 | 2.527 | 1.364 | 57.98 |
*ND= Not detectable, DAI= Day after incubation
The growth of all the strains retorted at 45°C as compared to at 28°C, however, some of them could survive and grow well at 45°C. The growth recorded at 45°C was in decreasing order of N40>N25>N30>N39>N29>N42>N24 which indicated that strains N40 could be considered as the best temperature tolerant among the strains. The strains N13 and N16 failed to grow at 45°C (Table 1).
A nodulation test whether strain N40 is efficient for effective and competent symbiosis at 45°C was performed. The pea seed inoculated with strain N40 formed pink color nodules on slant culture in growth chamber at this temperature. Also, the bacterized pea seed formed effective nodules in sterilized soil in earthen pot and the growth of host plant was better as compared to control. A photograph showing nodulation on slant culture has been presented in Fig. 1
pH tolerance of Rhizobium leguminosarum bv. viciae strains
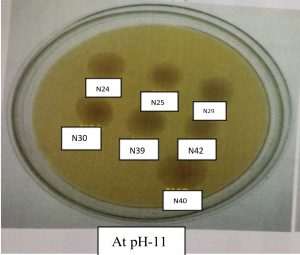
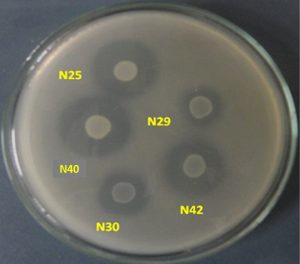
All the Rhizobium strains (N13, N16, N24, N25, N29, N30, N39, N40, and N42) grew well at pH 9. The maximum growth of Rhizobium strains at pH 9.0 was in N40, followed by N42, N24, N29, N30, N25, N13 and N16. At pH 11, strain N40 showed maximum growth, followed by N30, N24, N42, N29, N39, N25, N13 and N16. Growth pattern of Rhizobium levels. The isolate N40 exhibited maximum phosphate solubilization activity (Figs. 4 and 5).
Fig. 4. Phosphate solubilization by some R. leguminosarum bv. viciae strains indicated by clear zone formation on nutrient agar plate containing tricalcium phosphate
Rhizobium is a mesophilic bacterium which forms determinate (pink) nodules at soil temperature between 15-30°C. Global warming and climate change have necessitated microbiologist to isolate such strains of rhizobia which could tolerate high temperature with additional characteristics of growth promotion. Growth and survival of three Rhizobium strains N25, N30, and N40 at 45°C higher than the other strains and the performance of strain N40 was best. Rhizobium strains AER, ANR, ASR, ATR, ANR nodulating legume trees in South Riyad exhibited grow that elevated temperatures of 35°C and 45°C (Shetta et al.,2011). Fifteen Pseudomonas fluorescence and Rhizobium rhizospheric strains were isolated from Hyderabad soils with a view to screen temperature tolerant isolates(Manasa et al.,2017). Two Rhizobium isolates RR-1, GNR-1 and Pseudomonas fluorescence isolates GGP-1 were temperature tolerant with temperature optima of the growth for most isolates at 40°C. Fast growing Rhizobium isolates generally have less temperature tolerance as compared to slow growing (Singh et al., 2001). Isolate NE19 reduced the pH of the medium significantly and exhibited acid phosphatase activity. The strains exhibited different capacity to solubilize P. These strains require test to assess their field performance.
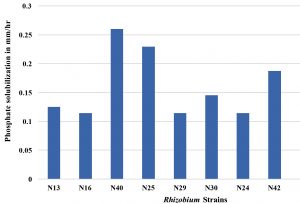
In this present study all the Rhizobium strains showed an appreciable amount of phosphate solubilization activity. The maximum phosphate solubilization activity was in N40 (0.260mm/hr). Actinobacteria solubilized insoluble tricalcium phosphate or aluminium phosphate as sole sources of phosphorus. The rhizospheric bacterial strains T1C, T1H, T3A, T3C, P3E, F1A, F2A and V2B expressed phosphate solubilization in the quantitative assay which could be used for further studies to evaluate plant growth promotion activities (Salcedo et al.,2014). Although P-solubilization was not evaluated in N40 strain at 45°C but on the basis of its growth at 45°C it can be speculated that this strain may be efficient P-solubilizer at this temperature.
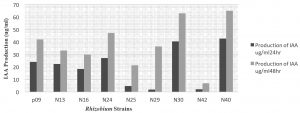
All the Rhizobium strains present study produced IAA using tryptophan as substrate which was maximum (65.13µg/ml) by strain N40. The rhizospheric PGPR from Tunjung isolates, UPMB19, endophytic PGPR from Besut, Terengganu UPMB20, rhizobia from soybean UPMR 30 and from Mimosa UPMR31 were screened for plant inoculation trials alongwith a oil palm PGPR isolate UPMB 10 as the reference strain (Tan et al., 2014).
Strain UPMB19 significantly stimulated the seedling (7 days after transplanting) growth which could beat tribute to the higher N2– fixation and IAA production rates. Karnwal (2012) isolated Rhizobium strains from maize and wheat plant which were able to produce different amount of IAA in a range of 0.6 to 2.7 µg/ml. Rhizobium leguminosarun bv. viciae N40 formed effective pink color nodulation on pea plant and in slant the pod cultures. The growth and nodule formation were superior under both the conditions.
Global warming and frequent climate change are posing serious threat to the farmers world-wide. The farmers need such varieties which could tolerate high temperature and some time temperature and drought both. Thus, the microbial inoculant Rhizobium should also possess the temperature tolerance capacity. Our temperature tolerant R. leguminosarum bv. viceae strain N40 could tolerate 45°C temperature with a slight decrease in growth compared to mesophilic growth condition. Also, this strain has additional PGPR traits activity such as phosphate solubilization and IAA production. However, it is necessary to test the performance of strain N40 for PGPR activity under field condition before it is recommended for use as microbial inoculant. Among the R. leguminosarum strains, N40 strain could tolerate the performance to pH tolerance was high. The results suggested that the strain tolerant to one adverse condition may be also tolerant to other stresses. Under the climate change condition CO2 fertilization is expected to enhance the tremendous growth of the crop plants while the elevated temperature level may have adverse effect. The temperature tolerance strain under these conditions will be most fevoured choice for the farmers.
ACKNOWLEDGMENTS
The authors are thankful to Director, Institute of Agricultural Sciences and Head, Department of Botany, Institute of Science for providing the facility.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Every author has significantly contributed in experimentation and preparation of manuscript.
FUNDING
The research work was supported from the growth of Indian Council of Agricultural Research, University of Grant Commission(Centre of Advanced Study in Botany) New Delhi. Mr. AK Patel gratefully acknowledge to Department of Biotechnology (DBT, New Delhi) for Junior Research and Senior Research fellowship.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
- Asghar HN, Zahir ZA, Arshad M, Khaliq A. Relationship between in vitro production of auxins by rhizobacteria and their growth promoting activities in Brassica juncea L. Biol Fert Soils. 2002;(35):231-237.
Crossref - Chaintreuil C, Giraud E, Prin Y, et al. Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl Environ Microbiol. 2000;66:5437-5447.
Crossref - Edi-Premono M, Moawad AM, Vlek PLG. Effect of phosphate-solubilization Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J Crop Sci. 1996;11:13–23.
- Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant physiology. 1951;26(1):192.
Crossref - Gray EJ, Smith DL. Intracellular and extracellular PGPR. commonalities and distinctions in the plant-bacterium signalling processes. Soil Biology and Biochemistry. 2005;37:395-412.
Crossref - Jason CH, Jeffrey HD, Daniel JF, McMahan LG, Genggeng Q, Christopher WJ. Designing Adsorbents for CO2 Capture from Flue Gas- Hyperbranched Aminosilicas Capable of Capturing CO2 Reversibly. J Am Chem Soc. 2012;10(130):2902-2903.
- Karnwal A. Screening of plant growth promoting rhizobactera from maize (Zea mays L.) and wheat (Tritium aestivum L.). African J food Agric Nutrition Dev. 2012;12:6170-6185.
- Kaur S, Khanna V. Effect of temperature-tolerant rhizobial isolates as PGPR on nodulation, growth and yield of Pigeon pea [ Cajanus cajan (L) Milsp.]. Journal of Food Legumes. 2013;26(3 & 4):80-83.
- Kiran D, Krishnamoorthy U. Rumen fermentation kinetics and nitrogen degradability of commonly used ruminant feeds tuffs in vitro. Anim Nutr Feed Technol. 2007;7(1):63-71.
- Manasa K, Subhash RR, Trivenu S. In vitro screening of temperature stress tolerance of Rhizobial and Pseudomonas fluorescence isolates. J Pharmaco Phytochem. 2017;6(5):764-67.
- Manivannan M, Ganesh P, Kumar SR, Tharmaraj K, Ramya SB. Isolation, Screening, Characterization and Antagonism Assay of PGPR Isolates from Rhizosphere of Rice Plants in Cuddalore District. Int J Pharm Biol Arch. 2012;3:179-185.
- Munevar FAG, Wollum II. Response of soybean plants to high temperature as affected by plant cultivar and Rhizobium strain. Agronomy Journal. 1982;74:138- 142.
Crossref - Rai R, Dash PK, Mohapatra T, Singh A. Phenotypic and molecular characterization of indigenous rhizobia nodulating chickpea in India. Indian J Exp Biol. 2012;50:340-350.
- Sachs JL, Gano A, Hollowell AC, Regus JU. The Legume-Rhizobium Symbiosis. Ecology. 2013;54:435-446.
Crossref - Salcedo LDP, Prieto C, Correa FM. Screening phosphate solubilizing actinobacteria isolated from the rhizosphere of wild plants from the Eastern Cordillera of the Colombian Andes. Biol Fertil Soils. 2014;56:765-773.
- Sardesai N, Babu CR. Poly-β-hydroxybutyrate metabolism is affected by changes in respiratory enzymatic activities due to cold stress in two psychrotrophic strains of Rhizobium. Current Microbiology. 2001;42(1):53-58.
Crossref - ShettaND, Al-Shaharani TS,Abdel-Aal M. Identification and Characterization of Rhizobium Associated with Woody Legume Trees Grown under Saudi Arabia Condition. Am Eurasian J Agric Environ Sci. 2011;10:410-418.
- Singh RK. Genetics of thermotolerance in Ciprofloxaein resistance mutant of Rhizobium leguminosarum bv. Phasesl. Ind J Expl Boil. 2001;39:818-820.
- Singh RK, Malik N, Singh S. Impact of rhizobial inoculation and nitrogen utilization in plant growth promotion of maize(Zea mays L.). Bioscience. 2013;5:8- 14.
Crossref - Somasegaran P, Hoben HJ. Quantifying the growth of rhizobia. In Handbook for Rhizobia (pp. 47-57). Springer, New York, 1994.
Crossref - Tan KZ, Radziah halimi MS, Khairuddin AR, Cabib SH, Khamsuddin ZH. Isolation and characterization of rhizobia and plant growth-promoting rhizobacteria and the ireffects on growth of rice seedlings. Am J Agric Biol Sci. 2014;9(3):342-360.
Crossref - Xie B, Bishop S, Stessman D, Wright D, Spalding MD, Halverson LJ. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME Journal (online). 2013;7:1544-1555.
Crossref - Yanni YG, Rizk RY, El-Fattah FKA, Squartini A. The beneficial plant growth-promoting association of Rhizobium leguminosarum bv trifolii with rice roots. Aust J Plant Physiol. 2001;28:845-870.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.