ISSN: 0973-7510
E-ISSN: 2581-690X
In the present study, three polycentric chytrid species of the genus Cladochytrium, specifically Cladochytrium replicatum, Cladochytrium tenue and Cladochytrium setigerum are described briefly herein and accompanied by photographs illustrating their morpho-taxonomical characteristics. All the recorded species serve as the first record for the North Indian mycobiota. Further detailed information regarding the occurrence and geographical distribution of these Cladochytrium species are presented herein.
Chytridiomycota, Freshwater, Morpho-taxonomical Characteristics, Polycentric, Systematics, Zoosporic-true Fungi
The members belonging to the phylum Chytridiomycota Doweld within the kingdom Mycota (Fungi) are colloquially referred to as chytrid (Greek for “little pot”). Ten classes constitute Chytridiomycota: Caulochytriomycetes, Cladochytriomycetes, Chytridiomycetes, Lobulomycetes, Mesochytriomycetes, Polychytriomycetes, Rhizophlyctidomycetes, Rhizophydiomycetes, Spizellomycetes and Synchytriomycetes.1,2 Within these, the class Cladochytriomycetes comprised of a single order: Cladochytriales Mozley-Standridge.1 Within these, the class Cladochytriomycetes is represented by a single order: Cladochytriales Mozley-Standridge.1 Hitherto, this class is considered among the most understudied orders of chytrid, despite the recognition of their genera and species for longer than many other taxa. The order Cladochytriales comprised five families: Cladochytriaceae Schröt., Catenochytridiaceae Doweld, Endochytriaceae Sparrow ex Barr, Nowakowskiellaceae Sparrow ex Mozley-Standridge and Septochytriaceae Mozley-Standridge with Cladochytriaceae as type family.3,4 Further, most of the taxa within these mentioned families have often been frequently observed as saprobic on a variety of substrates (decomposed/decaying plant material such as vegetable debris, moribund algae, insect exuviae and other organic materials) in freshwater and terrestrial ecosystems. The members in this clade show a broad range of distribution and have been commonly isolated and reported from a variety of cellulosic substrates such as cellophane, straw/leaves of corn (Zea mays L), fibrin film, filter paper, grass leaves, hemp seed or fibre, lens paper and the epidermis of Allium cepa L.3,5-8 These members are mostly involved in the degradation of lignocellulose in woody material and cellulose in leaves through which they play a primary role in maintaining the balance of soil fertilization process and ecosystems through decomposition and mineralization of organic matters in mostly lentic (lakes, peat swamps, pools, ponds), lotic (brooks, creeks, rivers, streams) and terrestrial habitats.4,9
The family Cladochytriaceae at present consist of a sole genus Cladochytrium Nowak. represented by C. tenue Nowak. as the type species.10 The presence of polycentric thallus with septate intercalary swellings (turbinate cells) in the rhizomycelium, and inoperculate zoosporangia (reproductive bodies) that produce posteriorly uniflagellate zoospores through a discharge pore; resting spores apparently asexually formed, with a thickened smooth or spiny wall and borne-like the zoosporangia on the thallus are the main general features of this genus.5 Based on the Index Fungorum11 database, under this generic concept, around 51 species have been described but over the years, most of them were gradually transferred to the plant parasitic genus, Physoderma under the phylum Blastocladiomycota based on the molecular studies.12 As a consequence, currently the genus comprises 15 legitimate species (Cladochytrium aneurae, Cladochytrium aureum, Cladochytrium aurantiacum, Cladochytrium cornutum, Cladochytrium crassum, Cladochytrium granulatum, Cladochytrium hyalinum, Cladochytrium irregular, Cladochytrium menyanthis, Cladochytrium novoguineense, Cladochytrium replicatum, Cladochytrium salsuginosum, Cladochytrium setigerum, Cladochytrium taianum and Cladochytrium tenue). and has very wide range of geographical distribution, reported to occur in over six continents.13 The genus is primarily composed of saprophytic spp. and is commonly found on cellulose-rich substrates (particularly cellophane bait) in freshwater and terrestrial ecosystems with one exception: Cladochytrium menyanthis (de Bary) Debray ex Pat. that was reported parasitic on leaves of Menyanthes trifoliata L.14,15 According to the extensive scrutiny of the literature, out of 15 valid Cladochytrium spp., the molecular data is available for merely 2 species, specifically, C. replicatum and C. tenue which have molecular sequence data from collections throughout the world. Due to these constraints, the systematics of the genus is uncertain and have been in need of taxonomic revision.6,16
During our continuing exploration of zoosporic true-fungi diversity in North India, some interesting species pertaining to the genus Cladochytrium mainly associated with plant cellulosic waste materials were discovered. Based on morpho-taxonomical identification, these Cladochytrium species were determined to represent undescribed species from North Indian states. Therefore, this study aims to enhance the knowledge of the genus Cladochytrium, by describing and illustrating these North Indian records along with their distributional range and habitat associations.
Isolation and identification
Fresh samples of water and soil were collected at random from various locations in North India at seasonal intervals in 2014, with the aid of a sterile whirl-top bag and transported to the laboratory. Thereafter, each sample was placed in triplicates by introducing an appropriate amount in a sterile Petri dish and flooding it with 40 mL of sterile deionized water. Afterwards, all the Petri dishes were multiply baited by following the standard method described previously5 for the recovery of Cladochytrium spp. using plant-based cellulosic organic substrates such as onion skin, corn husk, cellophane, corn leaves, lens paper and grass leaf followed by incubation at 20°C temperature in the dark for two weeks.
In order to study the morpho-taxonomical features, the colonized substrates (baits) in the water culture were examined for developmental stages of the isolates and photographed periodically using a Dewinter microscope. The developmental pattern of the isolates was examined on the bait to assess the range and variation in thallus structural features, including types of zoosporangia, their morphometric characterization, features of zoospore, resting spores, possession of rhizomycelia and morphology of rhizoidal system. Afterwards, the isolates were identified after matching with standard descriptions provided by Sparrow,5 Karling,17 original descriptions of the species and other relevant taxonomic literatures containing original descriptions of taxa. After identification, the examined specimens were incorporated into the Laboratory of Mycopathology and Microbial Technology, Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi, India, with a specific accession number.
Description of the species
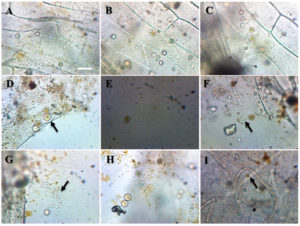
Cladochytrium replicatum Karling (Figure 1).
Figure 1. Cladochytrium replicatum A-B. Developing thallus of Cladochytrium replicatum C-F. Varying shape of sporangia G. Sporangial discharge H. Empty sporangium I. Golden brown resting spores. Bar = 50 µm
Morphology
Thallus eucarpic, polycentric, extra-intramatrical. Extensive, delicate, rhizoidal system consisting of more or less hyaline, narrow to wide, much branched, fine filaments with one to several fusiform, or spindle-shaped, frequently septate, intercalated turbinate cells of diverse size, 11-22 long x 5-7 wide µm in diameter, seldom directly transformed into zoosporangia, or resting bodies. Zoosporangia smooth, inoperculate, hyaline, thin-walled, non-apophysate, usually terminal, or rarely intercalary on short lateral branches, proliferating, spherical or ovoid, elongated or pyriformed, irregular or symmetrical, occasionally spindle, 8-18 μm in diameter with a prominent short single narrowly cylindrical discharge tube or exit-tube or neck or papilla, varying in length, 5-12 μm long, with beaded appearance. Zoospores, microscopic, spherical, 5-7.5 μm in diameter, varying in number, ranging from 6-12 per zoosporangium, with a conspicuous single large orange or golden-brown lipid globule; emerging in a gelatinous vesicle at the apex or orifice or tip or a papilla, temporary quiescent in group for about 30 sec before escaping from the vesicle, becoming amoeboid or swimming away. Resting spores predominantly spherical or ovoid, 9-21 µm in diameter, borne like the zoosporangia, wall smooth or spiny, with a typical large distinct orange or golden-brown lipid globule; germination not observed.
Specimen examined
Isolated from water and soil samples on onion skin, corn husk, cellophane, and grass leaf. Collection sites: Prayagraj (25.4358° N, 81.8463° E), Varanasi (25.3176° N, 82.9739° E), Chandauli (25.2605° N, 83.2645° E), Nainital (29.3924° N, 79.4534° E). Accession number MMT-BOT 294.
Comments
This saprophytic species primarily occurs on a wide variety of cellulose-rich substrates in both aquatic and soil habitats, however, Karling18 isolated it as a weak parasite from several aquatic plants. C. replicatum has been mostly obtained from permanently submerged lake muds by using cellulose-type baits. It is most ubiquitous, very abundant and frequent in distribution throughout most of the sampling sites. The species is readily distinguished from the less common C. tenue by brilliantly orange or golden-brown colored lipid droplet due to the presence of carotenoids in zoospores and resistance spores. The coloration makes its appearance early in the development of the sporangium. Evidence obtained so far suggests that C. replicatum is an aquatic species.19
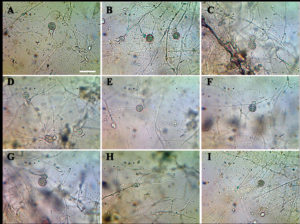
Cladochytrium tenue Nowakowski (Figure 2).
Figure 2. Cladochytrium tenue A-I. Delicate rhizomycelium of C. tenue with proliferating intercalary swellings and zoosporangia. Bar = 50 µm
Morphology
Thallus eucarpic, polycentric, extra-intramatrical. Rhizomycelium extensive, delicate, narrow to wide, richly and finely branched, with numerous elongate cells, fusiform or oval, spherical or sub-spherical, spindle-shaped, 10-26 μm long × 7-12 μm wide; hyaline, intercalated turbinated cells, transversely septate in two or more divisions, 10-18 μm in diameter; either swelling in the rhizoid or enlargement of a segment of septate intercalary swelling directly transformed into zoosporangia. Zoosporangia proliferating, inoperculate, smooth, thin-walled, hyaline, non-apophysate, terminal, somewhat pyriform, 15-25 μm in diameter, or spherical, 12-18 μm in diameter, broadly oval or slightly citriform, 12-20 μm in diameter; secondary sporangia smaller, with a single short discharge tubes or exit-tube or neck, usually 5-12 μm long, with beaded appearance. Zoospore microscopic, spherical, 5-6 μm in diameter, with a single large hyaline refractive lipid droplet (globule) and a posteriorly directed long flagellum; escaping in a temporary globular mass imbedded in “slime” after tip/orifice of discharge tube deliquesces, briefly quiescent before becoming amoeboid or swimming away. Resting spores hyaline, smooth, without spines, generally spherical or oval, or broadly fusiform, 10-18 μm in diameter, moderately thick-walled, intercalary at rhizomycelium; germination not seen.
Material examined
Water and soil samples on onion epidermis and lens paper. Collection sites: Varanasi (25.3176° N, 82.9739° E), Chandauli (25.2605° N, 83.2645° E) and Nainital (29.3924° N, 79.4534° E). Accession number MMT-BOT 213.
Comments
C. tenue is readily distinguished from other representatives of its genus by the presence of smooth-walled zoosporangia, septate turbinated cells or septate swellings and the formation of extensively branched rhizomycelium.
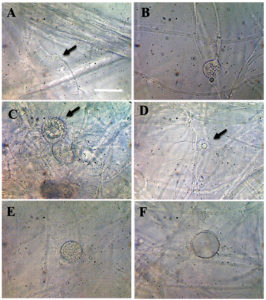
Cladochytrium setigerum Karling (Figure 3).
Figure 3. Cladochytrium setigerum A. Delicate rhizomycelium B. Developing sporangium C-D. Inoperculate setigerous zoosporangia which bear numerous simple or branched setae or hair-like appendages E. Zoosporangium before discharge F. Empty zoosporangium. Bar = 50 µm
Morphology
Thallus eucarpic, polycentric, epibiotic and endobiotic. Zoosporangia inoperculate, hyaline, generally intercalary, occasionally terminal, usually spherical, 12-26 µm in diameter, or oval, 10-35 µm in diameter, sometimes elongate and constricted, occasionally two zoosporangia occur adjacent to each other, with an intervening cross-wall, often with funnel-shaped elongate apophysate, occasionally bear setae like zoosporangia; wall bearing 10-50 simple or branched setae, or hair-like appendages, 5-25 µm long x 1.5-2.5 µm in diameter, generally with single conical, or hemispherical papilla or a short discharge tube. Rhizomycelium extensive, very delicate, fine, slender, much branched, 1-2.5 µm in diameter, thin-walled; very few non-septate, intercalary swellings or enlargements, or turbinate cells, usually fusiform or elongate, quite irregular, 5-10 µm wide x 10-16 µm long, occasionally setae may also occur on the tenuous portion of the rhizomycelium near the zoosporangia; usually form zoosporangia either from swelling in the rhizoid or enlargement of a segment of septate intercalary swelling while the other segment either remains at the base or may also develop into a zoosporangium. At maturity, zoospores released when the tip of the short discharge tube/exit papilla deliquesces, forming a temporary globular mass at the exit orifice before gradually separating and swimming away, remaining usually emerge singly and swim directly away. Zoospore minute, spherical, 3-3.5 µm in diameter, with a single small hyaline refractive lipid droplet, 0.5-1.3 µm in diameter and a posteriorly long flagellum. Resting spores unknown.
Material examined
Saprophytic on lens paper from the soil samples. Collection sites: Prayagraj (25.4358° N, 81.8463° E) and Mussorie (30.4598° N, 78.0644° E) 2 and 10. Accession number MMT-BOT 212.
Comments
This species is characterized by the presence of a very delicate rhizomycelium system and inoperculate setigerous zoosporangia bearing numerous simple or branched setae or hair-like appendages which distinguish it sharply from the other known species of the genus, and for this reason, it is named C. setigerum. Further, the species is characterized by minute zoospores which are considerably smaller than those of C. tenue, C. hyalinum, and C. replicatum.
As part of an ongoing study of zoosporic true-fungi in North Indian ecosystems,20-22 taxonomic descriptions of some chytrid species belonging to the genus Cladochytrium are presented herein. The taxonomic studies revealed these Cladochytrium species new to North India. Previous studies indicate that this genus is widely distributed in India, and at present represented by five species, namely, C. aneurae, C. hyalinum, C. replicatum, C. setigerum and C. tenue.23 This limited number of reported species is perhaps due to the intensive exploitation of Indian ecosystems for agricultural expansion purposes, however, in such biomes, the diversity of zoosporic true-fungi remains largely known. Despite being a ubiquitous genus in India, it is also important to notice that all these species were reported from central and south India, generally facilitated with ecological, morphological, and systematic studies.23 Associatedly, there have been no reports in India regarding the isolation, culture, and inclusion of these species in molecular phylogenies, while all were described based on solely on gross cultural features, like microscopic morphological features, thereby keeping their interspecific relationships and monophyly of the generic concept, indeed uncertain. To preclude this problem more efforts to find and isolate, or directly amplify DNA from these Indian taxa will be necessary to evaluate their Indian diversity.
The present study provided a better understanding towards the abundance of Cladochytrium species in the underexplored Indian ecosystem to ensure their conservation for future biochemical, genetic and molecular studies. The occurrence and distribution of many zoosporic true-fungal species are still inadequately known in India, especially in northeast India. Thus, there are still large gaps in current knowledge of their ecology, biogeography, substrata and habitat requirements. The specific reasons behind this are very few research specialists in India, unstable taxonomy, challenges in species identification, time-consuming sampling and narrow ecological niches (substratum). More intensive surveys of underexplored ecosystems in India harbouring a large mycobiota diversity near freshwater sources will certainly lead to major advances in our knowledge of these zoosporic true-fungal species and perhaps, using phylogenetic methods, new endemic lineages within it.24,25 Identification of these species depicts the current status of the ecosystem, information that is necessary to identify and characterize and in turn guides to follow future conservation measures.
In the present study, three Cladochytrium species were investigated based on their critical morphological characteristics. Descriptions of the isolates are accompanied by illustrations and a discussion of related taxa are presented herewith. These three species possess unique taxonomical features that differ from other allied species in Cladochytrium, and therefore, they are described as a new record for North India. Further, this study shed light on the current status of the genus Cladochytrium in India. Overall, this study contributes to the knowledge of the zoosporic true-fungi diversity present in North India, demonstrating the importance of exploring other new habitats in India during mycological surveys.
ACKNOWLEDGMENTS
The authors are grateful to the Centre of Advanced Study in Botany, Banaras Hindu University, Varanasi, UP, India, for the fellowship and for providing laboratory facilities for this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MKD conceptualized, designed, executed all the research activities and wrote the manuscript. RSU supervised, reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Tedersoo L, Sanchez-Ramirez S, Koljalg U, et al. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018;90:135-159.
Crossref - Wijayawardene NN, Hyde KD, Mikhailov KV, et al. Classes and phyla of the kingdom Fungi. Fungal Divers. 2024;15:1-65.
Crossref - Mozley-Standridge SE, Letcher PM, Longcore JE, Porter D, Simmons DR. Cladochytriales: A new order in Chytridiomycota. Mycol Res. 2009;113(Pt 4):498-507.
Crossref - Calabon MS, Hyde KD, Jones EBG, et al. www. freshwaterfungi.org, an online platform for the taxonomic classification of freshwater fungi. Asian J Mycol. 2020;3(1):419-445.
Crossref - Sparrow FK. Aquatic Phycomycetes. 2 ed. Ann Arbor, University of Michigan Press. 1960.
Crossref - Mozley SE. Taxonomic status of genera in the ‘Nowakowskiella‘ clade (kingdom Fungi, phylum Chytridiomycota): phylogenetic analysis of molecular characteristics with a review of described species, PhD thesis, University of Georgia, Athens, GA. 2005
- Marano AV, Steciow MM, Arellano ML, Arambarri AM, Sierra MV. The genus Nowakowskiella (Cladochytriaceae, Chytridiomycota) in environments of the Province of Buenos Aires (Argentina): Taxonomy, frequency and abundance of the species found. Bol. Soc. Argent. Bot. 2007;42:13-24.
- Marano AV, Steciow MM. Frequency and abundance of zoosporic fungi in some lotic environments of Buenos Aires province (Argentina). Agric Technol. 2006;2:17-28.
- Shearer CA, Descals E, Kohlmeyer B, et al. Fungal biodiversity in aquatic habitats. Biodivers Conservation. 2007;16:49-67.
Crossref - Clements FE, Shear CL. The Genera of Fungi. New York: H.W. Wilson. 1931.
- Index Fungorum. 2023. http://www.indexfungorum.org/Names/Names.asp. Assessed on 7 September 2023.
- James TY, Letcher PM, Longcore JE, et al. A molecular phylogeny of the flagellated fungi (Chytridiomycota) and the description of a new phylum (Blastocladiomycota). Mycologia. 2006;98(6):860-871.
Crossref - Calabon MS, Hyde KD, Jones EBG, et al. Freshwater fungal numbers. Fungal Divers. 2022;114(1):3-235.
Crossref - Sparrow FK. Phycomycetes from the Douglas Lake region of northern Michigan. Mycologia. 1952;44(6):759-772.
Crossref - Sparrow FK. Observations on chytridiaceous parasites of phanerogams. VII. A Physoderma on Lycopus americanus. Am J Bot. 1957;44(8):661-665.
Crossref - Simmons DR, Bonds AE, Castillo BT, et al. The collection of zoosporic eufungi at the University of Michigan (CZEUM): introducing a new repository of barcoded Chytridiomyceta and Blastocladiomycota cultures. IMA Fungus. 2020;11:20.
Crossref - Karling JS. Chytridiomycetarum Iconorgraphia. Leberecht and Cramer, Monticello, New York, USA. 1977.
- Karling JS. Studies in the Chytridiales VI. The occurrence and life history of a new species of Cladochytrium in cells of Eriocaulon septangulare. Am J Bot. 1931;18(7):526-557.
Crossref - Willoughby LG. The ecology of some lower fungi at Esthwaite Water. Trans the Br Mycol Soc. 1961;44(3):305-332.
Crossref - Dubey MK, Gajbhiye MH, Upadhyay RS. New records, rare and noteworthy species of the genus Nowakowskiella (Nowakowskiellaceae, Chytridiomycota) from India. Curr Sci. 2022;123(12):1462-1472.
Crossref - Dubey MK, Zehra A, Meena M, Upadhyay RS. Taxonomic notes on Allomyces neomoniliformis (Blastocladiaceae) isolated from Nanital lake, Uttarakhand, India. Vegetos. 2016;29(2):1-7.
Crossref - Dubey MK, Zehra A, Samal S, Upadhyay RS. First report on Obelidium megarhizum (Chytridiaceae) from India. Vegetos. 2019;32:643-646.
Crossref - Dayal R. Chytrids of India. M.D. Publications Pvt. Ltd., New Delhi. 1997.
- Hyde KD, Abdel-Wahab MA, Abdollahzadeh J, Abeywickrama PD, Absalan S, Afshari N, et al. Global consortium for the classification of fungi and fungus-like taxa. Mycosphere. 2023;14(1):1960–2012.
Crossref - Dubey MK, Upadhyay RS. Morpho-taxonomical notes on some Rhizophydium species (Rhizophydiaceae, Rhizophydiales) of North India. Plant Sci. Today. 2025;12(2):1-10.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.