ISSN: 0973-7510
E-ISSN: 2581-690X
Traditional antibiotic abuse caused increased the incidence of bacteria resistance to treatment. Strigolactones (SLs) are phytohormones that play a vital role in the plant growing root, shoot and flowering. We previously reported that, SLs analogues exert pro-apoptotic effects on HepG2 cell lines. The current study investigated the antimicrobial activity of selected SLs analogue TIT3 against different strains of bacteria including gram positive and gram negative bacteria: Staphylococcus aureus, Salmonella typhimuriu, Escherichia coli, Klebsiella pneumoni and B. subtilis. The results obtained were compared with 100 µg amoxicillin. Results obtained showed that, TIT3 inhibited the growth of S. cereus (50 µg), Salmonella typhimuriu (20 µg), Escherichia coli (30µg), Klebsiella pneumoni (50 µg) and B. subtilis (50 µg) with mean inhibition zones diameter being 12.1 mm , 13.2 mm, 12.5, 8.9 and 12.9 mm respectively. It was concluded that, TIT3 is promising effective antibacterial agents compared with amoxicillin for different strains of bacteria that resistant to different antibiotics.
Strigolactons analogues, TIT3, antibacterial activity
Traditional antibiotic abuse caused increased the incidence of bacteria resistance to treatment .Bacteria that resist to major antibiotics have ability to grow and transform to dangerous species. Control of infections induced by multidrug-resistant species is a challenge obstacle for hospitals1. In Clinics, patients suffered from these bacteria need more time and much cost for relief2. Development of a novel antibacterial compounds with high efficacy from medicinal plants are suitable alternative sources. Microbial resistance to chemotherapeutic by antibiotics is considered as one of the most problems in medicine3. This resistance is an event of the adaption of pathogenic bacteria to antibiotics used in hospital4,5. Some microbes have developed resistance to all types of antibiotics and these multidrug-resistant microbes are dangerous to invade different tissues6. In order to design a novel antibiotic with different mechanism of action, plants are considered as a main sources for new bioactive compounds with high efficacy. Phytochemical are vital sources of compounds against diverse organisms including fungi, yeasts and bacteria7. It exerts its activity by inhibiting cell membrane synthesis, modify bacterial permeability and sensitivity.
However, only 35 percent of the microbial infection have been treated using synthetic or derivatives products. This is due to resistant of pathogens for prolong use of antibiotics [8]. The resistance to antibiotics increased dramatically due to mutation of microbial genome. To decrease the microbial resistance to antibiotics is by explore a novel antibiotic resistance inhibitors produced from plant origin9. It was found that, plants possess ability to protect themselves against different pathogens by producing a variety of phytohormones. Medicinal plants are widely used as complementary medicine. Due to their safety to human phytomedicines are used as therapeutic index for the exploring novel drugs. It can be used as anticancer, antioxidants and anti-inflammatory10.
Strigolactones (SLs) are a novel class of phytohormones synthesized by different plant species10. The SLs structure consists fused-ring system connected via an enol ether bridge11. Previous study reported that, SLs exert its effect by regulating different parts in plant development including shoot and roots12. It has been reported that, SLs analogs stimulate cell cycle phases arrest and programme cell death in different types of human cancer13. It was found that, growth inhibition of normal cells treated with SLs analogs. In other study, inhibition was observed in some cancer cells cell lines14.
Moreover, SLs showed as new anti-cancer agent in breast cancer15. SLs analogs showed down-regulation of proteins involved in cell cycle (cyclin)16. These facts proposed that SLs are potent and promising anti-cancer activity. SLs enhance induction of G2 arrest, stimulate apoptosis and suppress viability17. The bacteria is protected by thick cell wall that composed of layers of peptidoglycan, cross-linked covalently to form polymer of peptide-linked β-(1–4)-N-acetyl glucosamines. The mechanical strength of the cell wall is important for bacterium’s ability to survive under vigorous environmental conditions as osmotic pressures. The degree of cross-linking can be correlated with the structural integrity of the cell. It was reported that, transglycosylase and transpeptidase enzymes, which add disaccharide pentapeptides contribute to peptidoglycan formation and cell wall integrity18. In order to contribute to explore a novel SLs analogues (TIT3) as antimicrobial activity against different pathogenic bacteria. This study investigated the antimicrobial activity of TIT3 against different specis of bacteria as Staphylococcus aureus, Salmonella typhimuriu, Escherichia coli, Klebsiella pneumoni and B. subtilis.
Synthesis of Strigolactons
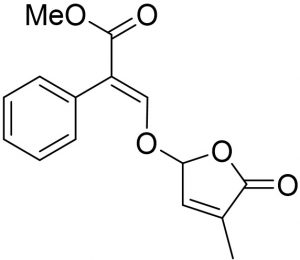
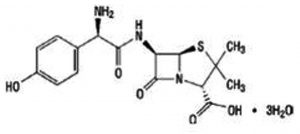
The SLs (TIT3) was synthesized and identified by Prof. Tadao Asami (University of Tokyo, Japan). We previously mentioned the details of synthesis and published recently19. Working standard dilutions to 10 µM was done using DMSO, the concentration of DMSO was maintained at 0.1- 0.5 %. The structure of tested SLs, as TIT3 with their molecular weights was shown in (Fig. 1) compared with amoxicillin as positive control (Fig. 1b).
Determination of MIC
Antimicrobial activity of the TIT3 at different concentrations were tested against different bacterial strains including Staphylococcus aureus, Salmonella typhimuriu, Escherichia coli, Klebsiella pneumoni and B. subtilis. The bacteria samples were obtained from Microbiology Department, Faculty of Science, KAU . Growth standard media was used (Per one liter media,1 g glucose, 15 g peptone, 6 g Sodium chloride) at pH 7.0. The antimicrobial activity of TIT3 were tested by using 10 μl of each type was added to sterile test tube contain 10 ml nutrient broth. Blank and negative control samples were done without microbe. After 24 h, growth was visually assessed by changes in turbidity and quantified by measuring O.D at 600 nm (Shimadzu spectrophotometer). Amoxiciilin (100 µg/ml) was used as a positive control. The diameters of growth inhibition zones was measured in mm. The initial screen to determine MIC of TIT3 by adding different concentration (0 –100 μg). Each concentration was incubated an overnight with media. After 24 h, growth was visually assessed as mentioned above.
Bioassay the cytotoxicity of TIT3by using brine shrimp lethality test
The toxicity of TIT3 was determined according to Michael et al.17 at different concentrations from (10- 1000 μg/mL in seawater containing 2% DMSO (v/v). Ten shrimps larvae was used in each test, three replica each test. A positive control from potassium dichromate solution (LC50 = 20-50 μg) was tested compared to blank test. The survivor count was recorded after 24 hours by using microscope and the mortality percent was calculated. Cytotoxicity was significant if the LC50 < 200 μg/ml.
Table (1):
Bioassay of lethality test (mortality %).
| compounds | Mortality % at different concentrations µg/ml. | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 30 | 100 | 200 | 400 | 500 | ||
| TIT3 | 1 | 90 | 6 | 16 | 18 | 30 | Non toxic |
| Pot.dichromate | 3 | 5.5 | 5 | 30 | 70 | 90 | Toxic |
| DMSO | 4 | 13.2 | 4.2 | 8.7 | 9 | 5.5 | Non toxic |
The values of triplicate experiments (mean ± SE).; No of shrimp per test was 10.; Scores for LC50.; Highly toxic < 20 μg/ml.; Toxic up to 200 μg/ml.; Non toxic > 500 μg/ml.
Bioassy lethality activity of the TIT3 was shown in Table 1. It was found that 30% mortality at 500 μg/ml concentration and its LC50 was 400 μg/ml which was considered moderately toxic. The Standard potassium dichromate revealed LC50 was 200 μg/ml. However DMSO showed no mortility.
Table (2):
Inhibation activity of TIT3 at different concentration (mm).
| Pathogen | S. aureus | Salmonella typhimurium | Escherichia coli | Klebsiella pneumonia | Bacillus cereus |
|---|---|---|---|---|---|
| TIT3 (µg/ml) | |||||
| 0 | — | — | — | — | —- |
| 10 | 3 | — | — | 5 | 7 |
| 20 | 4 | 13.2 | 4.2 | 8.7 | 9 |
| 30 | 8 | 10 | 12.5 | 5 | 7 |
| 40 | 10 | 6 | 7 | 3 | – |
| 50 | 12.1 | 5.5 | 6.6 | 8.9 | 12.6 |
| 60 | 8.5 | 7.6 | 7.3 | 6.6 | 7.6 |
| 70 | 9.1 | 7.3 | 8.2 | 6.2 | 9.2 |
| 80 | 8.3 | 6.9 | 7.1 | 5.8 | 7.8 |
| 90 | 7.9 | 8.1 | 7.5 | 3.9 | 6.4 |
| 100 | 8.9 | 5.2 | 4.1 | 6.3 | 7.2 |
| Amoxicillin 100 µg/ml |
11.9 | 14.5 | 13.6 | 10.5 | 14.2 |
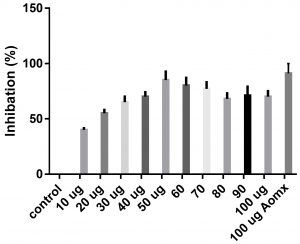
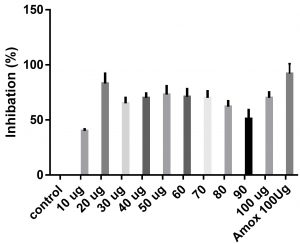
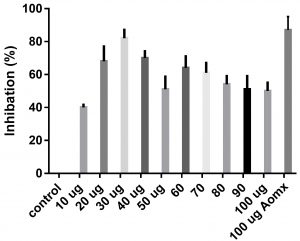
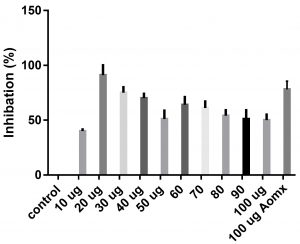
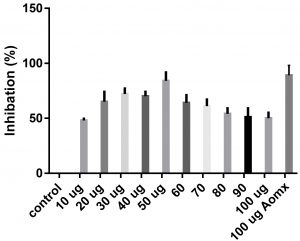
Data obtained (Table 2) indicated that TIT3 had variable activity against different species. It was found that, the maximum activity against S. aureus at 50 µg/ml, Salmonella typhimurium at 20 µg /ml, Escherichia coli at 30 µg /ml and Bacillus cereusat 50 µg /ml.
We screened different concentrations of three SLs analogues TIT3, (0-100 µg). Results obtained in Fig.s (2-6) showed that, TIT3 at concentrations 50 and 20 µg showed the most effective inhibitory effect on s.auresu and B. subtilis respectively with inhibitory effect against E. coli >80% and 77% inhibition for B. subtilis respectively. It is clear that, above these concentrations showed decreased activity.
The cytotoxicity of TIT3 was investigated on shrimp larvae. The data obtained in (Table 1) showed that by at very high concentration and exposure the toxicity increased. Recent published work by our research team Hasan et al.19 indicated that, TIT3 was effective on HepG2 cell line but more save of normal cell caused early apoptotic cell. For that, TIT3 is more save for eukaryotic cells. The MIC was similar to ampicilline for both Gram-positive and Gram-negative bacteria.
To examine the antimicrobial potential of the different concentrations of TIT3 . Overall, the results obtained revealed that TIT3 induced inhibitory effects against different species of bacteria with different efficacy. We found that by increasing the concentrations of TIT3, in making it more efficient in inhibiting efficacy. This effect is most pronounced in the case of S. aureus at, where the higher concentration is clearly associated with higher inhibition. The antibacterial properties of the TIT3 may be due to interference with cell wall synthesis of bacteria, so inhibiting its growth. TIT3 showed a better antibacterial activity through inhibition of bacterial several enzymes, microbial adhesion, and cell envelope transport proteins20. The antibacterial potential of TIT3 was found to be maximum against Salmonella typhimurium at low concentration. This antimicrobial susceptibility reported promising new vision for development and production of a novel agents against different pathogens. Thus MIC determined reflecting that TIT3 has antibacterial potential and that it gives real concentration required to inhibit bacterial growth. In conclusion, the TIT3 was found to be more effective against B.subtilis and S. cerevisiae. The potent activity may be due to the penetrating power of TIT3 to bacterial cell.
ACKNOWLEDGMENTS
The authors acknowledge with thanks Deanship of Scientific Research (DSR) for technical and financial support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors designed the experiments. EH and SS performed the experiments. AA and SS analyzed the data. AA, EH and SS wrote the manuscript. All authors read and approved the manuscript.
FUNDING
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (RG-87-130- 38). The authors acknowledge with thanks DSR for technical and financial support.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are Included in the manuscript.
- Dubey R, Dubey K, Sridhar C, Jayaveera KN Human Vaginal Pathogen Inhibition Studies On Aqueous, Methanolic And Saponins Extracts Of Stem Barks Of Ziziphus Mauritiana. Int JPharm Sci Res. 2011;2(3):659-663.
- El-Kamali HH, El-Amir MY. Antibacterial Activity and Phytochemical Screening of Ethanolic Extracts Obtained from Selected Sudanese Medicinal Plants. Curr Res J Bio Sci. 2010;2(2):143-146.
- Lalitha P, Arathi KA, Shubashini K, Sripathi, Hemalatha, S, Jayanthi P. Antimicrobial Activity and Phytochemical Screening of an Ornamental Foliage Plant, Pothos aurea (Linden ex Andre). An Int J Chem. 2010;1(2):63-71.
- Hussain H, Badawy A, Elshazly A, et al. Chemical Constituents and Antimicrobial Activity of Salix subserrata. Rec Nat Prod. 2011;5(2):133-137.
- Preethi R, Devanathan VV, Loganathan M. Antimicrobial and Antioxidant Efficacy of Some Medicinal Plants Against Food Borne Pathogens. Adv Bio Res. 2010;4(2):122-125.
- Sharma A. Antibacterial activity of ethanolic extracts of some arid zone plants. Int J Pharm Tech Res. 2011;3(1):283-286.
- Enne VI, Livermore DM, Stephens P, Hal LMC Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. The Lancet. 2001;28:1325-1328.
Crossref - Westh H, Zinn CS, Rosdahl VT. An international multicenter study of antimicrobial consumption and resistance in Staphylococcus aureus isolates from 15 hospitals in 14 countries. Microb Drug Resist. 2004;10:169-176.
Crossref - Kim H, Park SW, Park JM, Moon KH, Lee CK. Screening and isolation of antibiotic resistance inhibitors from herb material Resistant Inhibition of 21 Korean plants. Nat Prod Sci. 1995;1:50-54.
- Zhu RX, Seto W-K, Lai C-L, Yuen M-F. “Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region.” Gut Liver. 2016;10(3):332-339.
Crossref - Sia D, Villanueva A, Friedman SL, Llovet JM. “Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis,” Gastroenterology. 2017;152(4):745-761.
Crossref - Nordenstedt H, White DL, El-Serag HB. “The changing pattern of epidemiology in hepatocellular carcinoma,” Dig Liver Dis. 2010;42(SUPPL. 3):206-214.
Crossref - Liu J, Wei X, Wu Y, et al. “Giganteaside D induces ROS-mediated apoptosis in human hepatocellular carcinoma cells through the MAPK pathway.,” Cell Oncol. 2016;39(4):333-342.
Crossref - Siegel RL, Miller KD, Jemal A. “Cancer Statistics, 2017”. 2017;67(1):7-30.
Crossref - Lozano R, Naghavi M, Foremanet K, al., “Global and regional mortality from 235 causes of death for 20 age groups i 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010.,” Lancet. 2012;380(9859):2095-2128.
Crossref - Alagesaboopathi C. Antimicrobial Potential And Phytochemical Screening Of Andrographis Affinis Nees An Endemic Medicinal Plant From India. Int J Pharma Pharmaceutical Sci. 2011;3(2):157-159.
- Michael AS, Thompson CG, Abramovitz M. Artemia salina as a Test Organism for Bioassay. Science. 1956;123(3194):464.
Crossref - Pavithra PS, Janani VS, Charumathi KH, Indumathy R, Potala S, Verma RS. Antibacterial activity of the plant used in Indian herbal medicine. Int J Green Pharma. 2010;10:22-28.
- Hasan MN, Choudhry H, Razvi SS, et al. Synthetic strigolactone analogues reveal anti-cancer activities on hepatocellular carcinoma cells. Bioorg Med Chem Lett. 2018;28(6):1077-1083.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.