ISSN: 0973-7510
E-ISSN: 2581-690X
The present investigation was conducted to find out the fungal diversity on leaf litter samples collected from Ratangarh forest, Datia. The samples were collected from Jan 2017-June 2017 at monthly intervals. Serial dilution method and PDA media was used for the isolation of fungi. During present investigation, 14 species belonging to 6 different fungal genera have been isolated and identified. Out of these, 2 species belongs to class Zygomycota and 12 species belongs to class Ascomycota and their anamorphs. Species namely Mucor hiemalis, Rhizopus stolonifer, Aspergillus niger, Aspergillus japonicus and Torula sp. were early colonizers while Aspergillus nidulans, Aspergillus flavus, Aspergillus fumigatus, Emericella nidulans, Ascochyta sp., Penicillium chrysogenum, Penicillium aurantiogriseum, Trichoderma reesei, and Trichoderma viride were found as late colonizers over leaf litter of Acacia catechu. In all stages of decomposition, fungi belonging to the Ascomycota were predominant. The present investigation provides valuable information about diversity of leaf litter fungi of tropical forest.
Forest ecosystems, Fungal diversity, Colonization, Decomposition.
Forest produces a large amount of organic matter in the form of litter which provides unique habitats for fungi. Leaf litter fungi are the major degraders of plant litter which is composed of basically cellulose and lignin and play a major role in carbon and nitrogen cycling in the forest ecosystem1. Decomposition of plant litter is a physical and chemical process which is involved in reducing litter to its elemental chemical constituents and as such a major determinant of nutrient cycles2. Fungi are the major component of soil microbiota in the forest ecosystems which utilize the organic substrates present in leaf litter as energy and nutrient source by the process of decomposition. In their role as decomposers, leaf litter fungi also sequester carbon and move it to the recalcitrant carbon stores of the soil3.
In addition to their vital role in ecosystem services, fungi are of great interest for biotechnological application in food, wine and textiles industries⁴, ⁵, for energy generation⁶, for plastics degradation⁷, and to produce highly-added value compounds such as biosensors, cosmetic products and organic acids⁸,⁹.
The replacement of fungal species on a particular substratum over time has been termed as ‘succession’ and has been the focus of numerous studies10-15. Studies on litter fungi of tropical ecosystems are limited as compared to temperate ecosystems16-18. In recent years numerous studies on litter fungi have been carried out specially on plant species e. g. Manglietia garrettii19, Ficus pleurocarpa20, Magnolia liliifera21, Pandanus sp.22, Ficus sp.23, Hevea brasiliensis24, Anacardium occidentale and Pavetta indica25,26.
For the present study, a tropical deciduous forest ecosystem was chosen in Datia, Madhya Pradesh, India (Fig. 2a). The forest of study area was dominated by ‘kardhai’ and ‘khair’ trees. Some of the common tree species are Anogeissus pendula Edgew (Combretaceae), Albizzia lebbek Benth (Mimosaceae), Holoptelia integrifolia Planch (Ulmaceae), Cassia fistula Linn. (Caesalpiniaceae), Delonix regia (Boj) Ref. (Caesalpiniaceae), Acacia catechu (Mimosaceae), Hardwickia binata Roxb. (Leguminosae), Terminalia arjuna Bedd. (Combretaceae), Pongamia glabra Venl. (Leguminosae), Prosopis spicigera Linn. (Leguminosae), Diospyros melanoxylon Roxb. (Ebenaceae), Anogeissus latifolia Wall. (Combretaceae), Tectona grandis Linn. (Verbenaceae), Terminalia tementosa Roxb. (Combretaceae), Lagerstroemia parviflora Roxb. (Lythraceae), Salmalia malabarcum DC. (Salmaliaceae), Mallotus philippinensis (Euphorbiaceae), Acacia leucophloea Willd. (Leguminosae) and Ficus glomerata Roxb. (Moraceae).
The genus Acacia belongs to family Mimosaceae comprising about 2800 species, 37 in Madhya Pradesh, mostly found in scrub and dry deciduous forests of Balaghat, Bhopal, Chindwada, East Nimar, Gwalior, Hoshangabad, Indore, Jabalpur, Mandala, Panna, Raisen, Sagar, Satna, Shivpuri, Sidhi and Datia. Acacia catechu (L.f.) Willd., locally known as ‘khair’, (Fig. 2b) is a medium sized deciduous tree up to 15 m high with hooked spine. Branchlets armed with pseudo-stipular spines in pairs below the petioles. It occurs in edaphic climax types of dry deciduous as in Anogeissus forest. ‘Khair’ is chiefly used as a source of kattha and kutch. Kattha is extracted from its heart wood and used in pan. It is also used as a timber, fuel, fodder, gum, medicine, furniture material and household articles by the local people. There have been only a few recorded studies on the fungal succession on leaf litter in India. The present study has been aimed to isolate and identify litter decomposing fungi and their ecology in the forest of Datia district at different time intervals during leaf litter decomposition of Acacia catechu.
Study site and sample collection
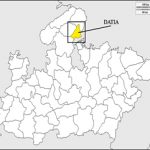
For sampling purpose, the forest area of Ratangarh in Seonda block is selected. This place is 65 Km away from Datia city and situated in the north eastern part of MP, India (25° 28’ to 26° 20’ N latitude and 78° 10’ to 78° 45’ E longitude) (Fig. 1a & 1b). Datia experiences widespread Indian monsoon climate with an average annual rainfall of 825.93 mm. with average maximum and minimum temperatures of 32.64°C and 18.45°C.
Litters of Acacia catechu family have been collected randomly from research field from January 2017 to June 2017 at monthly intervals. Collected leaves were brought to laboratory in sealed polythene bags within 4 hrs of collection for further study.
Approximately 20 g leaves were enclosed in nylon mesh bag27, (with mesh size 2 mm) and placed randomly into 0.61 meters pits (fig. 3a & 3b) (one bag in each pit) for decomposition by soil fungi. One bag from each pit was randomly removed at a regular interval of time (15, 30, 45, 60, 75, 90, 120, 150, 180 days) after their incubation in pit. Each bag was placed in a separate paper bag and transported to the laboratory for isolation work.
Isolation of fungi
The isolation of litter fungi was carried out by using serial dilution method28. 10 g of decaying leaves were transferred into 250 ml Erlenmeyer flask containing 100 ml distilled water and shaken thoroughly for 15 min on a horizontal mechanical shaker for spore suspension. The suspension was further diluted 103 and 104 times. One ml of this suspension was inoculated separately into each of five Petri plates containing 15 ml potato dextrose agar nutrient medium i.e. PDA (Potato 200g, Dextrose 20g, agar 15g, pH 5.5, Distilled water 1000 ml) supplemented with streptomycin to avoid bacterial contamination in culture plates. The plates were subsequently incubated at 28 ± 2°C.
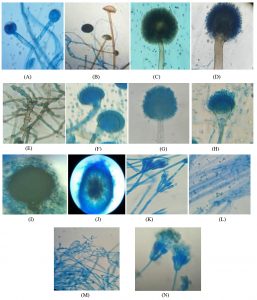
Fig. 5. Schematic diagrams of identified fungal species in order of appearance during the study. A. Mucor hiemalis, B. Rhizopus stolonifer, C. Aspergillus niger, D. Aspergillus japonicus, E. Torula sp. F. Aspergillus nidulans, G. Aspergillus flavus, H. Aspergillus fumigatus, I. Emericella nidulans, J. Ascochyta sp. K. Penicillium chrysogenum, L. Trichoderma reesei, M. Trichoderma viride, N. Penicillium aurantiogriseum.
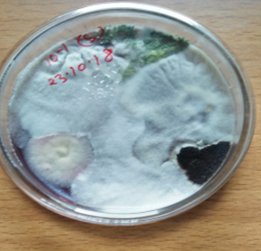
Development of fungal colonies was noticed from third day of incubation. The heterogeneous or mixed fungal colonies appeared after 5 days of incubation. Desired colonies were transferred to freshly poured Petri plates containing PDA media in order to obtain pure and mono culture. The pure cultures were then preserved on PDA slants and maintained at 4ºC for further studies.
Identification of fungi
The majority of sporulating isolates were identified on the basis of their cultural, morphological and spores characteristics with the help of standard texts and keys29-31. Preparation of microscopic slide of the isolated fungi, to aid identification, was done using the pure culture. For the preparation of semi permanent slides of fungal fruiting bodies a portion of mycelium of the representative colony was picked up with the help of a pair of needles and mounted on a clean slide with solution of cotton blue in lactophenol. The slide was examined under Trinocular microscope (Olympus) with 10x & 45x objectives and 10x & 15x eye pieces and the microphotographs of the individual fungal species were taken.
Six fungal genera and fourteen species have been isolated and identified from leaf litter of Acacia catechu over a period of six month of decomposition (Table-1) following serial dilution method. Out of these, two species belongs to the class Zygomycota and twelve species belongs to class Ascomycota and their anamophs. On the basis of their occurrence on leaf litters, fungal species were divided into early and late settlers. Fungal species namely Mucor hiemalis, Rhizopus stolonifer, Torula sp. and Aspergillus nidulans were initially highly frequent on available substrate and regarded as early colonizers. The appearance of Aspergillus flavus, Aspergillus fumigatus, Emericella nidulans, Ascochyta sp. Penicillium chrysogenum, Trichoderma reesei, Trichoderma viride and Penicillium aurantiogriseum increased in 2-6 months and decreased thereafter as the decomposition progressed and thus were termed as late colonizers. The fungi Aspergillus niger and Aspergillus japonicus dominated the overall litter assemblages throughout the 180 days of decomposition.
Table (1):
Fungi identified associated with the litter of Acacia catechu in the forests of Datia district in order to appearance.
| Fungal taxon | Period of decomposition (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 | 30 | 45 | 60 | 75 | 90 | 120 | 150 | 180 | |
| Mucor hiemalis Wehmer | + | + | + | − | − | − | − | − | − |
| Rhizopus stolonifer Ehrenberg | + | + | + | − | − | − | − | − | − |
| Aspergillus niger Tiegh | + | + | + | + | + | + | + | + | + |
| Aspergillus japonicus Saito | + | + | + | + | + | + | + | + | + |
| Torula sp. Peersoon ex Fries | − | + | + | + | + | + | − | − | − |
| Aspergillus nidulans Fennell and Raper | − | − | + | + | + | + | − | − | − |
| Aspergillus flavus Link | − | − | − | + | + | + | + | − | − |
| Aspergillus fumigatus Fresen | − | − | − | + | + | + | + | − | − |
| Emericella nidulans Eidam Vuill | − | − | − | + | + | + | + | − | − |
| Ascochyta sp. Saccardo | − | − | − | + | + | + | − | − | − |
| Penicillium chrysogenum Thom. Bull | − | − | − | − | + | + | + | + | − |
| Trichoderma reesei E. G. Simmons | − | − | − | − | + | + | + | + | − |
| Trichoderma viride Pers | − | − | − | − | + | + | + | + | + |
| Penicillium aurantiogriseum Dierekx | − | − | − | − | − | + | + | + | − |
(+) = Presence, (−) = Absence
These results presented indicate that the total number of species recovered from the decomposed leaf litter of Acacia catechu increased initially and started to fall later. M. hiemalis, R. stolonifer, Torula sp. and A. nidulans were dominated during the early stage of decomposition and Ascochyta, Trichoderma and Penicillium sp. were dominant during the later period of decomposition. Emericella nidulans which is teleomorphs of A. nidulans have been isolated from the decaying litter. The fungi have been isolated in its sexual cycle only. In all stages of decomposition, fungi belonging to the Ascomycota were predominant. During the entire decomposition process, species richness ranged from 4 to 12 species with the highest value found at 90 days of decomposition. After 120 days of decomposition, the numbers of fungal species decreased and were replaced by new colonizers. Findings in present work are similar to that of32-34,19,35,36,20,37,25,24,38. Occurrence of fungi during succession study on senescent leaves of Mangletia garretti for 56 days recorded 22 fungal taxa with the fungal community composition differing at each stage of succession. Greater fungal communities were recorded from the mature stage of decomposition19. A similar trend was observed in the studies of succession of the woody litter of Magnolia liliifera for 35 months at bimonthly samplings, where the number of fungal species was higher during the mature stage of decomposition21. On the other hand, species diversity tends to be richest and the number of fungi usually highest during the early and middle stages of colonization24. Most of the fungi isolated from degrading biomass are the members of Ascomycetes whereas very few fungi belongs to other groups like Zygomycetes39-40, reported similar observations when they isolated mycoflora of Chenopodium leaf litter.
The results of present investigation shows that the tropical leaf litter is colonized by numerous fungi and the type of fungal species changes as decomposition progress. M. hiemalis, R. stolonifer, A. niger, A. japonicus, Torula sp. and A. nidulans appear first on available substrates and they give way to other colonizers with greater ability to degrade the leaf litter. The appearance of A. flavus, A. fumigatus, E. nidulans, Ascochyta sp., P. chrysogenum, T. reesei, T. viride and P. aurantiogriseum were increased with the progress of degradation. The dominance of A. niger and A. japonicus in all stages of decomposition indicates the higher survivability of these species in tropical deciduous forests of district Datia.
In the decomposition process, the initial colonizers are main utilizers of simple organic compounds (sugars) whereas the later once are capable of exploiting complex organic molecules such as cellulose and lignin41. Initial colonizers or pioneers gradually disappear and another inhabitant replaced earlier ones. This is possible because of the availability of different kinds of nutrients in different levels of decomposition. The species of Aspergillus, Penicillium and Trichoderma are capable of using cellulose as a source of carbon for their growth and multiplication. The physiochemical environment, litter quality and the composition of the decomposer community are the three main factors controlling litter decomposition42-44.
Morphological characterization of leaf litter fungi of Datia forest
Mucor hiemalis Wehmer
(J) Colonies white, later grey; reverse pale olivaceous grey both in light and dark; sporangiophore with large sympodial branches originating from a short distance below the previous sporangia, sporangia globose, blackish brown, wall diffluent, leaving a basal collarette; columellae globose or oval, sporangiospores variable in shape, cylindrical-oblong31.
Rhizopus stolonifer Ehrenberg
(K) Initially colonies were white then become blackish with spreading stolons, internodes brown, rhizoids branched at internode, unbranched. Sporangiophores were in group of 3-10 which become dark brown at maturity. Sporangia appears black, Spores were irregular round and oval, angular in shape and grey in color31.
Aspergillus niger Tiegh
(D) Colonies with abundant mycelium, conidial heads are deep black or brownish black; conidiophores arising directly from the substratum, conidia may be globose, spinulose with black pigment, globose to subglobose, sclerotia produced in some strains, at first cream to buff, later vinaceous buff in age31.
Aspergillus japonicus Saito
(B) Colonies spreading rapidly producing purple brown to black conidial heads; conidiophores arising from the substratum, conidia mostly globose, sometimes subglobose, strongly echinulate, bright colored at first, becoming purplish brown31.
Torula sp. Peersoon ex Fries
(C) Colonies small, discrete, olive brown or dark brown or sometimes black in color. Mycelium superficial unbranched or sometime branched but irregularly. Conidia blastic dry, in simple or branched chains which arise from surface of the conidiogenous cells. Cells are cylindrical or spherical may break into with phragmoconidia.
Aspergillus nidulans Fenn and Raper
(F) Colonies are grown at room temperature. In some strains colonies are green and conidiophores light brown, sinuous, smooth, phialides biseriate, conidia globose to subglobose, green in mass31.
Aspergillus flavus Link
(A) Colonies growing rapidly, at young stage conidial heads are yellow then become yellowish green and then greyish green at older, conidiophores develop from the substratum, conidia globose to subglobose, echinulate, yellowish green, sometimes elliptical when young31.
Aspergillus fumigatus Fresen
(C) Initially colonies are velvety to floccose then become bluish green, conidiophores short, smooth, light green and enlarged into a flask shaped vesicle; conidia globose to subglobose, green in mass, echinulate, sclerotia and cleistothecia absent31.
Emericella nidulans (Eidam ) Vuill
(H) Colonies growing plane, somewhat velvety, margins thin, cress green from abundant conidial heads, changing from deep dull to reddish or purple with the formation of abundant cleistothecia; conidial heads loosely radiate when young, later short columnar; conidiophores light brown, sinuous, smooth, occasionally septate; vesicles hemispherical, brown, fertile over one portion only; conidia are globose to subglobose, rugulose, greenish in colour; cleistothecia abundant, globose to subglobose; hiille cells thick walled, globose to subglobose; asci 8-spored, occasionally 16-spored, numerous in each cleistothecia, globose to subglobose, breaking quickly with the liberation of ascospores; ascospores purple-red, lenticular, smooth walled, with two equatorial crests pleated with sinuous and entire margin31.
Ascochyta spp. Saccardo
(I) Colonies spreading rapidly, dark brown; hyphae brown; pycnidia gregarious, globose, subglobose, dark brown, ostiolate, wall pseudoparenchymatous; 2 celled conidia are smooth hyaline, ovoid and oblong31.
Penicillium chrysogenum Thom. Bull
(M) Colonies sometimes smaller, often centrally umbonate, commonly moderately deep and floccose; margins usually deep, conidia blue-green; conidia ellipsoidal to subellipsoidal, less commonly spheroidal, smooth, borne in long irregular columns31.
Trichoderma reesei E.G. Simmons
(P) Colonies growing moderately, aerial mycelium appressed to agar surface, cottony aerial mycelium not forming, white with radial lines and scant conidial production after one week; conidiophores forming on aerial mycelium, tufts minute, only primary branches infrequently branched, phialides arising singly before first branch, side branches producing more than one phialide; conidia obovoid to ellipsoid, smooth, and light green31.
Trichoderma viride Pers.
(O) Colonies appears hairy due to formation of aerial mycelium, glaucous to dark bluish green; conidiophores may be formed on the aerial or creeping hyphae, main conidiophores producing smaller side branches, ultimately a conifer-like branching system is formed, conidia globose or short obovoid, or broadly ellipsoidal, sometimes with distinct apiculus- like base because of distinct minute roughening on their walls, bluish green to dark green31.
Penicillium aurantiogriseum Dierekx
(N) Colonies growing moderately, radially sulcate, moderately deep, surface texture smooth to granular, fascicles seen in young growth at low magnifications; margins usually entire, deep, and white; mycelium white, usually inconspicuous, occasionally dominating the colony appearance in floccose isolates; conidiophores borne singly or in fascicles, mostly from substrate hyphae, with stipes, bearing terminal terverticillate or less commonly biverticillate penicilli; conidia subspheroidal to ellipsoidal, smooth, usually borne in long well defined columns31.
The present investigation provides valuable information about the leaf litter fungal diversity of tropical forest. The fungal communities of the tropical forest were entirely dominated with Ascomycota and co-dominated with Zygomycota. The data presented here indicates that Aspergillus niger, Aspergillus japonicus, Aspergillus flavus, Emericella nidulans, Ascochyta sp., Trichoderma spp. and Penicillium sp. may contribute or be useful in future work in the area of nutrient recycling and may also act as fungal bio-indicators of decomposition in the particular forest area of Datia district.
ACKNOWLEDGMENTS
The authors are thankful to Principal and Head of the Department of Botany and Industrial Microbiology Jhansi (U.P.) and the authorities of Government PG College, Datia (MP) for providing necessary facilities throughout the course of this investigation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript
- Johanna B. Litter decomposing fungi in Boreal forests. Their function in Carbon and Nitrogen Circulation. Ph.D. thesis. 2009; Swedish University of Agricultural Science. Uppsala.
- Aerts R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystem: atriangular relationship. Oikos, 1997, 79: 439-449.
Crossref - Jordan CF. Nutrient Cycling in Tropical Forest Ecosystems, John Wiley & Sons, Chiechester, UK, 1985.
- Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnology advances, 2000; 18(5): 355-383.
Crossref - Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G. Fungal laccases and their applications in bioremediation. Enzyme Research. 2014,
Crossref - Balat M. Production of bioethanol from ligno-cellulosic materials via the biochemical pathway: A review. Energy Conversion Management, 2011; 52: 858-875.
Crossref - Kathiresan K. Polythene and plastic-degrading microbes from the mangrove soil. Revista de Biologia Tropical, 2011; 51(3): 629-634.
- Hasan F, Shah A, Hameed A. Industrial application of microbial lipases. Enzyme and Microbial Technology, 2006; 39: 235-251.
Crossref - Oliveria JMPF, Graaff LH. Proteomics of industrial fungi: trends and insights for biotechnology. Applied Microbial Biotechnology, 2011; 89: 225-237.
Crossref - Kendrick B, Burges A. Biological aspects of the decay of Pinis sylvestris leaf litter. Nova Hedwigia, 1962; 4: 313-342.
- Hering TF. Succession of fungi in the litter of Lake District oakwood. Transactions of British Mycological Society, 1965; 48: 391-408.
Crossref - Frankland JC. Succession of fungi on decaying petioles of Pteridium aquilinum. Journal of Ecology, 1966; 54: 41-63.
Crossref - Hogg BM, and Hudson HJ. Micro-fungi on leaves of Fagus sylvatica. L. 1. The micro-fungal succession. Transaction of British Mycological Society, 1966; 49: 185-192.
Crossref - Kuter G A. Microfungal populations associated with the decomposition of sugar maple leaf litter. Mycologia, 1986; 78: 114-126.
Crossref - Kjoller A, Struwe S. The fungal community; its organization and role in the ecosystem, 2nd Edition.1992; pp. 619-628. In: Carroll, G.C. and Wickow, D.T. (Eds). Marcel Dekker, Inc., New York-10016.
- Sayer EJ. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biological Reviews of the Cambridge Philosophica Society, 2006, 81:1-31.
Crossref - McGuire KL, Fierer N, Bateman C, Treseder KK, Turner BL. Fungal community composition in neotropical rain forests: the influence of tree diversity and precipitation. Microbial Ecology, 2012; 63: 804-812.
Crossref - Xu W, Shi L, Chan O, Li j, Casper P, Zou X. Assessing the effect of litter species on the dynamic of bacterial and fungal communities during leaf decomposition in microcosm by molecular techniques. PLoS ONE., 2013; 8: 12, e84613.
Crossref - Promputtha I, Lumyong S, Lumyong P, McKenzie EHC, Hyde KD. Fungal succession on senescent leaves of Manglietia garrettii in DoiSuthep-Pui National Park, northern Thailand. Fungal Diversity, 2002; 10: 89-100.
- Paulus B, Gadek P, Hyde KD. Successional patterns of microfungi in fallen leaves of Ficus pleurocarpa (Moraceae) in an Australian tropical rain forest. Biotropica, 2006; 38: 42-51.
- Kodsueb R, McKenzie EHC, Lamyong S, Hyde KD. Fungal succession on woody litter of Magnolia lillifera (Magnoliaceae). Fungal Divers, 2008; 30: 55-72.
- Thongkantha S, Lumyong S, McKenzie EHC, Hyde KD. Fungal saprobes and pathogens occurrence on tissues of Dracaena loureiri and Pandanus spp. Fungal Diversity, 2008; 30: 149-169.
- Wang HK, Hyde KD, Soytong K, Lin FC. Fungal diversity on follen leaves of Ficus in northern Thailand. Journal of Zhejiang University SCIENCE B, 2008; 9(10): 835-841.
Crossref - Seephueak P, Petcharat V, Phongpaichit S. Fungi associated with leaf litter of Para rubber (Hevea brasiliensis). Mycology, 2010; 1(4): 213-227.
Crossref - Shanthi S, Vittal BPR. Fungi associated with decomposing leaf litter of cashew (Anacardium occidentale). Mycology, 2010; 1(2):121-129.
Crossref - Shanthi S, Vittal BPR. Biodiversity of microfungi associated with litter of Pavetta indica. Mycosphere, 2010; 1: 23- 37.
- Crossley DA, Hoglund A. Litter-bag method for the study of microarthropods inhabiting leaf litter. Ecology, 1962; 43: 571-573.
Crossref - Waksman SA. Do fungi live and produce mycelium in the soil? Sci. N. S., 1916; 44: 320-322.
Crossref - Gilman JC. A manual of soil fungi. 2nd ed. Iowa, The Iowa State College Press, 1957; pp.450.
Crossref - Barnett HL, Hunter BB. Illustrated Genera of Imperfect Fungi, (3rd edition). Burgess Publishing Company, Minneapolis, 1972; p.331.
- Nagmani A, Kunwar IK, Manoharachary C. Handbook of soil fungi I. K International Pvt. Ltd. 2006; New Delhi. India.
- Senthilkumar K, Udaiyan K, Manian S. Successional pattern of mycoflora associated with litter degradation in a Cymbopogon caesius-dominated tropical grassland,1993; 27: 121-127.
- Bills GF, Polishook JD. Abundance and diversity of microfungi in leaf litter of a lowland rain forest in Costa Rica. Mycologia, 1994; 86: 187-198.
Crossref - Parungao MM, Fryar SC, Hyde KD. Diversity of fungi on rainforest litter in north Queensland, Australia. Biodivers. Conserv. 2002; 11: 1185-1194.
Crossref - Tokumasu S, Aoiki TA. New approach to studying microfungal succession on decaying pine needles in an oceanic, subtropical region in Japan. In: Fungal Succession (eds. K.D. Hyde and E.G.B. Jones). Fungal Diversity, 2002; 10: 167-183.
- Tang AMC, Jeewon R, Hyde KD. Succession of microfungal communities on decaying leaves of Castanopsis fissa. Canadian journal of Microbiology, 2005; 51: 967-974.
Crossref - Duong LM, McKenzie EHC, Lumyong S, Hyde KD. Fungal succession on senescent leaves of Castanopsis diversifolia in DoiSuthep-Pui National Park, Thailand. Fungal Diversity, 2008; 30: 23-36.
- Osono T. Diversity and functioning of fungi associated with leaf litter decomposition in Asian forests of different climatic regions. Fungal Ecol.
- Borkar KM, Thakre RP. Effect of temperature, pH and substrates on CMCase enzyme activity of thermophilic fungus Humicolainsolens, Intl J Life Sci., 2014; A2: 91-94.
- Mehrotra RS, Aneja KR. Microbial decomposition of Chenopodium album litter. Succession of decomposers. J Indian Bot. Soc, 1979; 58: 189- 195.
- Garrett SD. Soil Fungi and Soil Fertility, Peragamon Press, Oxford, 1963.
- Berg B, Berg MP, Bottner P, Box E, Breymere A. Litter mass loss rates in pine forest of Europe and Eastern United States: Some relationship with climate and litter quality. Biogeochemistry, 1993; 20: 127-159.
Crossref - Couteaux MM, Bottner P, Berg B. Litter decomposition, Climate and Litter quality. Trands Ecol. Evol., 1995; 10: 63-66.
Crossref - Cadish G, Giller KE. Driven by nature: Plant Litter Quality and Decomposition. Wallingford: CAB Int.,1997; 432 pp.
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.