ISSN: 0973-7510
E-ISSN: 2581-690X
The present study was on the analysis of phytochemical constituents of Coleus forskohlii rhizome collected from Thiruvannamalai of Tamil Nadu and testing for its antimicrobial potential against human bacterial pathogens. The dried tubers were extracted using different solvents such as ethanol, aqueous, acetone, chloroform and petroleum ether and screened. The total tannin and saponin content in the extract was estimated. The crude ethanolic solvent extract of C. forskohlii was tested for its antimicrobial activity using disc diffusion method. Phytochemical screening of the crude solvent extracts revealed the presence of tannins, phenols, saponins, quinone, flavonoids, cardiac glycoside, terpenoids, steroids, alkaloids, and coumarins. Tannic acid was found in the level of 53.47 ±0.37 mg TAE/g. Saponin content was 2.42±0.63 mg/g dry weight. The crude ethanolic rhizome extract was tested in three different concentrations (10, 20 and 30mg/ml). Maximum antibacterial activity was noted against Bacillus subtilis (15 mm), followed by Bacillus cereus (14 mm), P. aeruginosa (12 mm) and S. aureus (12mm). E. coli was not inhibited even at the highest concentration tested (i.e.) 30mg/ml. The minimum inhibitory concentration (MIC) values of the pathogens tested were 1.4±0.02, 1.590±0.02, 1.820±0.02 and 2.350±0.02 mg/ml respectively to B. subtilis, B. cereus, P. aeruginosa , and S. aureus. Similarly, the minimum bactericidal concentration (MBC) for B. subtilis, B. cereus, P. aeruginosa , and S. aureus were respectively of 2.89±0.02, 3.833±0.05, 3.842±0.01 and 4.856±0.05 mg/ml. In time-kill assay 3 Log reduction was found to be around 11 h for B. subtilis, around 12 h for B. cereus for P. aeruginosa around 13 h and above 14 and less than 15 h for S. aureus. The study showed the antimicrobial potential of ethanol extract of C. forskohlii tuber against human bacterial pathogens and hence it seems to be useful in the preparation of drug formulations.
Coleus forskohlii, phytochemical screening, tannin, saponin, antibacterial activity, MIC, MBC, Time-kill assay.
Coleus spp. is available in different countries and used for different ailments. About 150 Coleus spp. are distributed in different parts of the world in which the C. forskohlii is found in Asia, Africa, Australia and Pacific Islands. This plant is an herb belonged to the family Lamiaceae, in which mint and lavenders are also placed. The fragrance is due to the presence of essential oil which is responsible for the aroma. Roots are fibrous in nature. Coleus plants have 3000 years old history in Ayurvedic medicines which are in practice to cure heart and lung diseases. As it is having the capacity to break the adipose tissue it is also useful in weight reduction programs. The rate of metabolism is induced to burn fat tissue by stimulation of the enzyme adenylate cyclase, resulting in cyclic AMP synthesis. It is also seemed to be useful in eye care products to reduce pressure in the eyes. In the small intestine, it helps in adsorption of pre-digested foods. It is also used against allergens (i.e.) as an antihistamine drug in diseases like asthma, bronchitis etc. As it improves the cyclic AMP content, it also been used against diseases like hypothyroidism and skin diseases like eczema and psoriasis. It acts on lipid hydrolysis as well as to relax the arterial walls and hence used as angina. Due to its antioxidant and anticancer properties, now focus is on using its extract against secondary or metastatic cancers especially gastric cancers.
In India it is for dysentery, digestive disorders and recently to reduce body weight in human beings1. In Brazil also, for a similar purpose, they are used. Ayurvedic medicine recommended this for various disorders like heart disease, abdominal colic, respiratory disorder, burning sensation, insomnia, convulsions, asthma, bronchitis, epilepsy and angina2. Its leaves are used as an expectorant, emmenagogue and as a diuretic in various countries. The present study was on phytochemical screening and testing of antimicrobial activity of crude ethanolic root extract of C. forskohlii plant.
Phytochemical screening of rhizome extract of Coleus forskohlii
Coleus forskohlii rhizomes were collected from Tiruvannamalai, phytochemical screening was done by adopting the described standard methods3-5. Visible color change or precipitate formation was taken into consideration for the presence (+) or absence (-) of a particular active constituent.
Estimation of tannin content in rhizome extracts of Coleus forskohlii
Tannin content was quantified by adopting a standard method6. 1 g of powdered tubers was extracted thrice with 10 ml of absolute ethanol. From this 1 ml was taken in a test tube and 0.5 ml of Folin-Ciocalteau’s reagent was mixed followed by 1 ml of saturated sodium carbonate (Na2CO3) and made up to 10 ml using distilled water. After 30 min. incubation at room temperature it was centrifuged at 6000 rpm for 10 min. and the supernatant was used to read in a UV-Vis spectrophotometer (Systronics) at 725 nm. Tannic acid was used as the standard.
Determination of saponin in Coleus forskohlii
Saponin content was done according to described standard method7. In which ethanol, diethyl ether, and butanol were used and washed with 5% aqueous NaCl. Evaporation was done in a water bath to get constant weight which represented the saponin content.
Antibacterial activity of rhizome extracts of Coleus forskohlii
Bacterial strains and inoculum preparation
The antibacterial potency activity of ethanol extract of tuber was tested against five different pathogens such as Bacillus subtilis, Bacillus cereus, Staphylococcus aureus Pseudomonas aeruginosa and Escherichia coli at three different concentrations (i.e.) 10, 20 and 30 mg/ml. Each strain was subcultured in 5 ml of Mueller Hinton broth medium (Hi-media) and incubated overnight at 37°C for 18-24 h to attain viable cell count of 107 CFU/ml.
Antibacterial activity testing using disc diffusion test
Antibacterial activity was measured using the standard method of disc diffusion on agar plates8. 0.1 ml of overnight grown each bacterial culture was spread on Mueller Hinton agar (Himedia) plate surfaces. Paper discs (6mm in diameter) loaded with 20µl of different concentrations (10, 20 and 30 mg/ml) of ethanolic rhizome extract of Coleus forskohlii were placed on the agar medium. Plates were incubated at 35°C for 24 h. Antibacterial activity was measured and assessed based on the size of the zone of clearance.
Determination of Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) against bacterial pathogens
The Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) were determined by broth/tube dilution method according to the recommendations of the National Committee for Clinical Laboratory Standards Institute9. Different concentration of ethanolic plant extract 100, 50, 25, 12.5, 6.25, 3.125, 1.563, 0.781, 0.390, 0.195 mg/ml were prepared in tubes containing 5 ml Muller Hinton broth. Pathogenic bacterial strains (Bacillus subtilis, Bacillus cereus, P. aeruginosa and S. aureus) were inoculated in 5ml of Muller Hinton broth (MHB) and incubated at 37°C until the turbidity value of 0.5 McFarland (approximately 106 CFU/ml) was attained10. 100 µl of each bacterial strain was inoculated into tubes containing different concentrations of plant extract were incubated at 37°C for 24 h and then observed for turbidity. The Tube containing sterile Muller Hinton broth alone was used as a negative control. The MIC was defined as the lowest concentration that completely prevented visible growth after incubation at 37°C for 18-24 h.
After determination of MIC for each bacterium, 100µl from all tubes in which no visible bacterial growth observed were inoculated into sterile MHB without the plant extract individually. The tubes were then incubated for overnight at 37°C. The MBC is defined as the lowest concentration of antimicrobial agent that kills >99.9% of the initial bacterial population where no visible growth of the bacteria was observed in the Muller Hinton broth tubes.
Time-kill assay
Time-kill assays were performed to further evaluate the antibacterial activity of ethanolic extract against all tested pathogens according to the procedure described previously11,12. The Overnight culture was used to inoculate fresh Muller-Hinton broth containing 10mg/ml of tuber extract with 106 CFU/ml of cells. The culture was incubated at 37°C for 24 h. Surviving bacteria were counted for every 3 h up to 24 h (i.e. 0-24 h). 100µl of samples were subcultured on Muller-Hinton plates and the surviving colonies were counted and expressed as CFU/ml. A bactericidal effect was defined as a ³ 3 log10 CFU/ml decrease compared to the initial inoculum.
Statistical analysis
Each test was performed in triplicates and the results were expressed as mean ± standard deviation.
In the present investigation, rhizome samples were collected from Thiruvannamalai of Tamil Nadu. The dried rhizome sample was extracted with different solvents such as ethanol, aqueous, acetone, chloroform and petroleum ether. Each solvent extract was screened for the presence of phytochemicals. The results revealed the presence of tannins, phenols, saponins, quinone, flavonoids, cardiac glycoside, terpenoids, steroids, alkaloids and coumarins (Table 1). Quantitative estimation of tannic acid content showed 53.47 ±0.37 mg TAE/g and the saponin content in the C. forskohlii was 2.42±0.63 mg/g dry weight (Table 2). When testing the antibacterial activity of crude ethanolic rhizome extract using disc diffusion method against various human pathogens such as Bacillus subtilis, Bacillus cereus, P. aeruginosa, S. aureus and E.coli at the maximum concentration 30mg/ml, the zone of clearance of 15 mm, 14 mm, 12 mm and 12 mm and 0 mm respectively (i.e.) E. coli was not inhibited (Fig. 1 and Table 3).
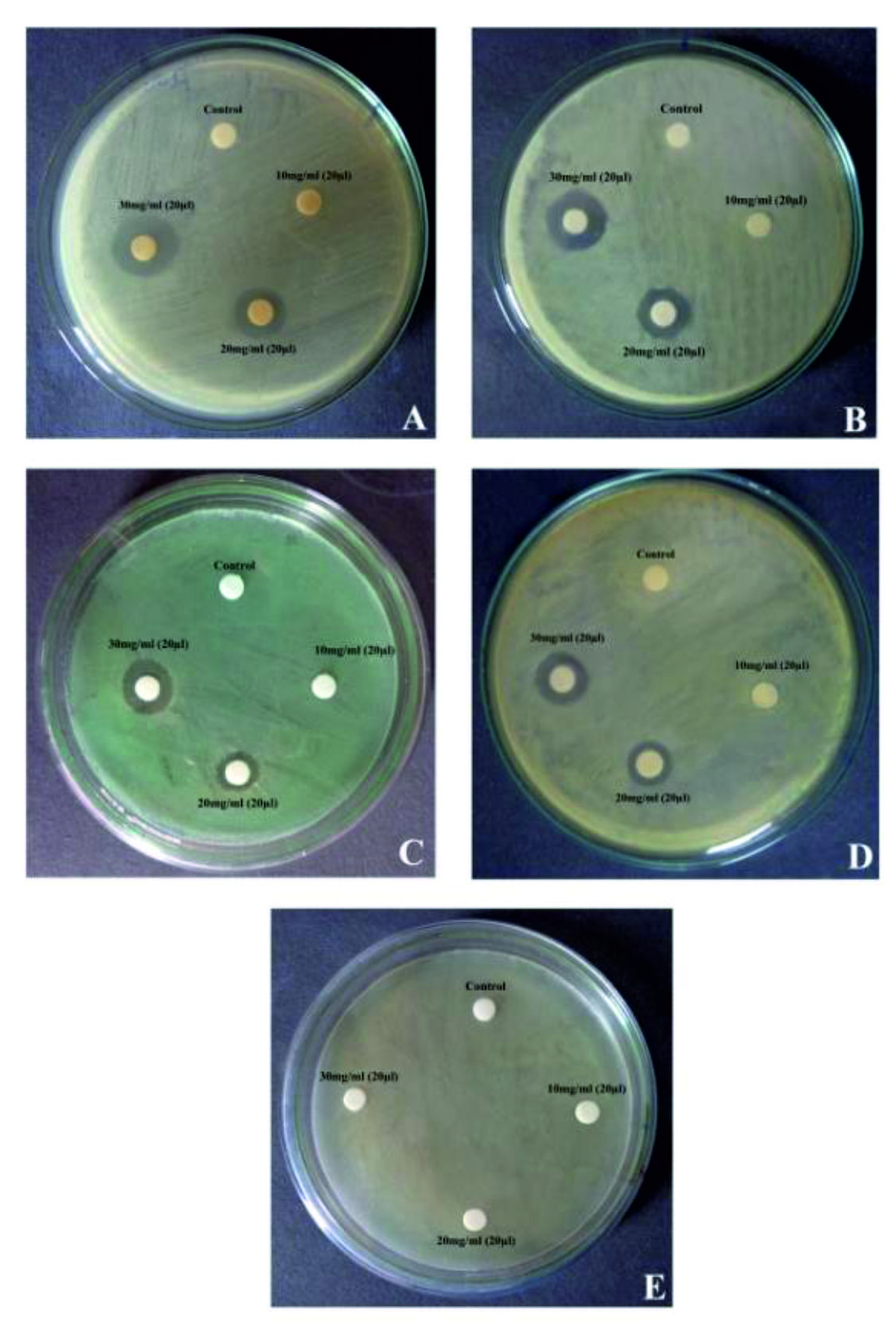 Fig. 1. Antibacterial activity of ethanolic rhizome extract of Coleus forskohlii on bacterial pathogens A: Bacillus subtilis; B: Bacillus cereus; C: Pseudomonas aeruginosa; D: Staphylococcus aureus and E: Escherichia coli
Fig. 1. Antibacterial activity of ethanolic rhizome extract of Coleus forskohlii on bacterial pathogens A: Bacillus subtilis; B: Bacillus cereus; C: Pseudomonas aeruginosa; D: Staphylococcus aureus and E: Escherichia coliTable (1):
Phytochemical screening from rhizome extract of Coleus forskohlii
| Phytochemicals | Rhizome extract of Coleus forskohlii | ||||
|---|---|---|---|---|---|
| Ethanol | Aqueous | Acetone | Chloroform | Petroleum ether | |
| Tannin | ++ | ++ | ++ | + | + |
| Saponin | + | + | + | – | – |
| Quinone | ++ | + | + | – | – |
| Flavanoid | ++ | + | + | – | – |
| Phenol | + | + | + | + | + |
| Glycosides | – | – | – | – | – |
| Cardiac glycoside | ++ | – | – | + | + |
| Terpenoids | ++ | ++ | ++ | – | – |
| Steroids | ++ | + | + | – | – |
| Alkaloid | + | – | ++ | – | + |
| Coumarins | ++ | + | + | + | + |
Key: + Positive: ++ Strong positive: – negative
Table (2):
Quantitative estimation of tannin and saponin content from rhizome extract
of Coleus forskohlii
Sample |
Tannin content (mg TAE/g) |
Saponin content (mg/g dry weight) |
|---|---|---|
Coleus forskohlii rhizome extract |
53.47 ± 0.37 |
2.42±0.63 |
Table (3):
Antibacterial activity from rhizome extract of Coleus forskohlii
| Microorganisms tested | Zone of inhibition (mm) | ||
|---|---|---|---|
| Concentrations of ethanolic rhizome extract used | |||
| 10mg/ml | 20mg/ml | 30mg/ml | |
| Bacillus subtilis (MTCC No. 100224) | – | 12 | 15 |
| Bacillus cereus (MTCC No. 10221) | – | 11 | 14 |
| Pseudomonas aeruginosa (MTCC No. 14676) | – | 12 | 12 |
| Staphylococcus aureus (MTCC No. 9542) | – | 10 | 12 |
| Escherichia coli (MTCC No. 1563) | – | – | – |
| Negative control (Ethanol) | – | – | – |
Inhibition Zone in diameter (mm)*
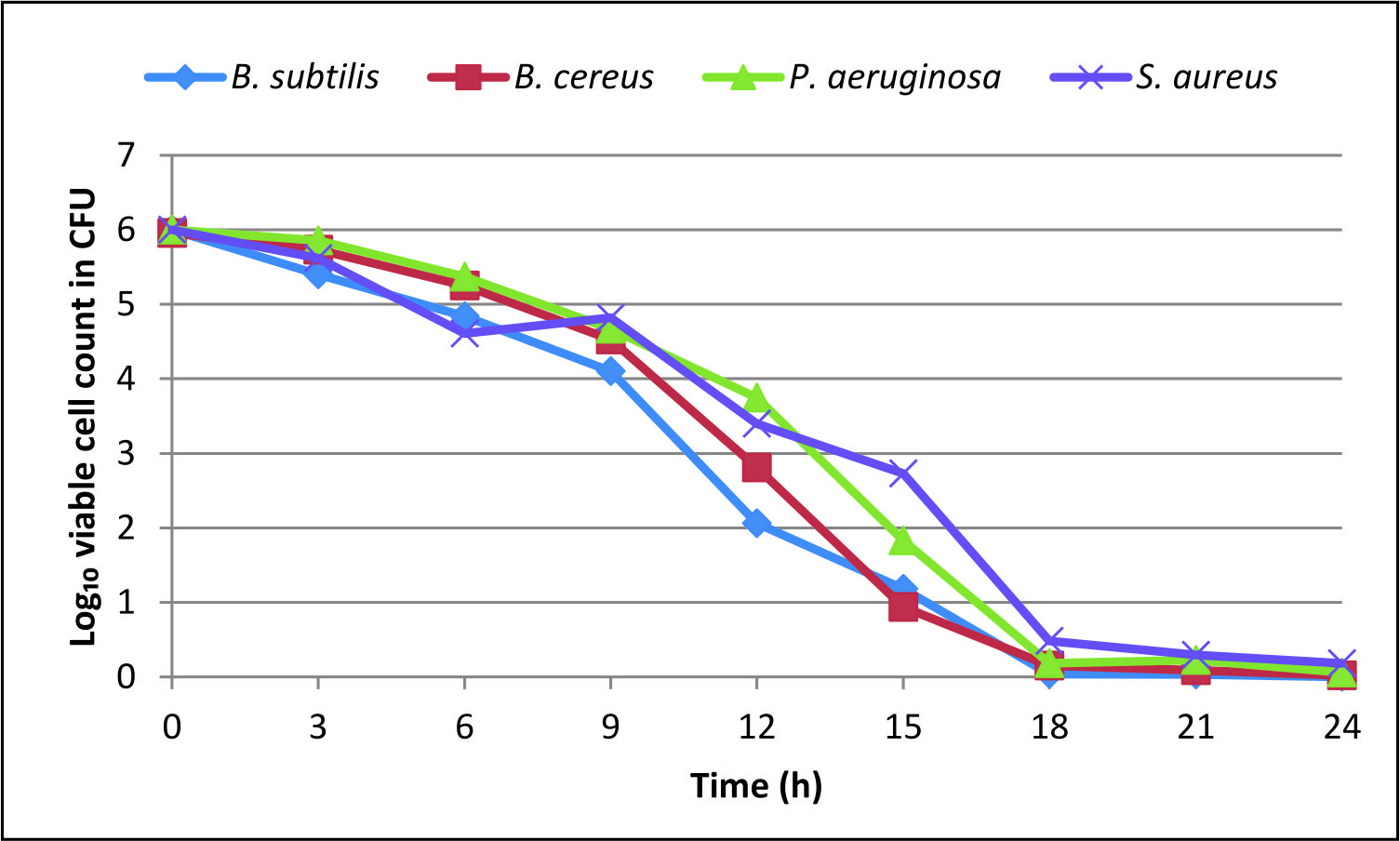
The minimum inhibitory concentration (MIC) values of the tested pathogens viz., B. subtilis, B. cereus, P. aeruginosa and S. aureus were respectively of 1.4±0.02, 1.590±0.02, 1.820±0.02 and 2.350±0.02 mg/ml. Likewise, the minimum bactericidal concentration (MBC) for B. subtilis, B. cereus, P. aeruginosa, and S. aureus were respectively of 2.89±0.02, 3.833±0.05, 3.842±0.01 and 4.856±0.05 mg/ml (Table 4). The time-kill assay for the ethanolic rhizome extract of C. forskohlii against the tested human pathogens Bacillus subtilis, Bacillus cereus, P. aeruginosa, and S. aureus revealed the viable cells in the range of 0 to 0.182 log10CFU/ml after 24 hours of incubation. Whereas at 3 hours incubation, it was in the range of 5.4-5.86 log10CFU/ml and at 12 hours it was ranged from 2.06-3.75 log10CFU/ml. At 18 hours it was at the range of 0.04-2.73 log10CFU/ml (Fig. 2). Time-kill assay showed 3 Log reduction around 11 h for B. subtilis, around 12 h for B. cereus for P. aeruginosa around 13 h and above 14 and less than 15 h for S. aureus.
Table (4):
MIC and MBC
| Pathogens tested | Minimum Inhibitory concentration / Minimum Bactericidal concentration (mg/ml) | |
|---|---|---|
| MIC | MBC | |
| B. subtilis | 1.4±0.02 | 2.89±0.02 |
| B. cereus | 1.590±0.02 | 3.833±0.05 |
| P. aeruginosa | 1.820±0.02 | 3.842±0.01 |
| S. aureus | 2.350±0.02 | 4.856±0.05 |
Qualitative phytochemical analysis of the ethanolic extract of C. forskohlii rhizome showed the presence of tannins, quinone, flavanoids, cardiac glycoside, terpenoids, steroids, and coumarins in relatively higher level, whereas saponins, phenols, and alkaloids in lower level. In chloroform and petroleum ether extracts, only tannin, phenol, cardiac glycoside and coumarins were present. Other solvents also showed varying results. The solvent used in the extraction procedure seemed to decide the phytochemical component that is derived. Hence to know the components present and to delineate their biological or physiological activities useful phytochemical screening is important13.
Earlier studies found C. forskohlii rhizome was rich in phenol and flavonoids14-16. The present investigation revealed the high content of tannin. The total tannin content in the ethanolic rhizome extract of Coleus forskohlii was determined by UV spectrophotometric method and it was found to be 53.47 ± 0.37 mg tannic acid equivalent (TAE) /g (Table 2). The given value is mean ± SD for three different determinations. Presence of tannins was reported in various parts like leaves, stems, and roots of popular medicinal plants collected from Northern India17.
Saponin content observed in the present work in the C. forskohlii was 2.42±0.63 mg/g dry weight. The antibacterial activity of saponin was reported in Sorghum bicolor extract18. Interestingly it had more inhibitory activity towards Gm positive bacteria as observed in the present study. The Antibacterial and fungistatic effect had been reported in various plant extracts in earlier work also19,20.
Among five pathogens tested four were inhibited by the extract of C. forskohlii. Maximum inhibitory activity was found against Bacillus subtilis followed by Bacillus cereus, Pseudomonas aeruginosa and Staphylococcus aureus. However, it was not inhibitory to E. coli. The ethanolic extract of C. froskohlii roots collected from Rewa (Madhya Pradesh) was found to be most inhibitory against B. cereus (32 mm), followed by M. luteus (31 mm), K. pneumoniae (30 mm), S. aureus (29 mm), P. aeruginosa (15 mm) and E. coli (15 mm)16. In their study also, lowest inhibitory activity was shown against E. coli. In another study done on aqueous and methanolic extracts of 12 plants belonging to different families, aqueous extract did not show any activity whereas methanol extract inhibited B. cereus and S. epidermidis21.
When ethanolic extracts of 50 medicinal plants of Nigeria tested, 28 were inhibitory to one or more pathogens tested, which included B. subtilis, E. coli, S. aureus, P. aeruginosa and C. albicans22. Leaves and flowers of C. hirsute, extracted using ethanol and dichloromethanol as well as the similar extract of leaves and stems of C. squalid were found to inhibit Staphylococcus and Streptococcus. However only rhizomes were used in the present investigation.
Coleus canius (Roth) Vatke extract showed inhibitory activity against E. coli when 50mg/ml of hydro-alcoholic extract was used23. Surprisingly they found most inhibitory activity against E. coli whereas in the present investigation it was not so, even with 30mg/ml of the rhizome. The methanolic leaf extract of Acasis nilotica was inhibitory against E. coli, S. aureus and Xanthomonas axonopodis24. Both Gram positive and Gram negative bacteria were found to be inhibited by ethanol extract of the root of this plant. It is true that ethanol, as well as methanol extracts, were more inhibitory compared to other solvents. It is opined that methanol might be more inhibitory compared to ethanol25. This might be due to the aromatic nature of the inhibitory compounds26 which may be easily extracted in organic solvents like ethanol, methanol etc., It was also proved the same through a study on Leucas aspera27. In general Gram-positive bacteria are more susceptible28,29. The cell wall of Gram-positive bacteria is thinner and made of single layer whereas that of Gram-negative is thicker and is a multilayered structure30. However resistance seemed to be strain dependent31 as P. aeruginosa was inhibited and E. coli was not.
The antimicrobial activities of ethanolic extract may be due to the presence of phytochemicals identified. Antimicrobial activity of tannins was already reported by many researchers32-36. Tannic acid works like a siderophore and it quenches iron and the microorganism may die due to lack of iron to do functions like reduction of the ribonucleotide precursor of DNA, a formation of haem and various other important functions37. Inhibition of 15 bacterial pathogens tested by tannic acid was observed37. However, in a study, it was not shown due to lack of iron as they added extra iron to the medium32. The astringent property of tannin form complexes with enzymes and substrates is another view37. They also suggested its action on membrane and complexation of metal ion which are involved in important cellular functions of the pathogens. Tannic acid increased the inhibitory activity of b-lactam antibiotics32.
The MIC values of the pathogens tested were 1.4±0.02, 1.590±0.02, 1.820±0.02 and 2.350±0.02 mg/ml respectively to B. subtilis, B. cereus, P. aeruginosa, and S. aureus. Similarly the MBC for the tested pathogens such as B. subtilis, B. cereus, P. aeruginosa, and S. aureus were respectively of 2.89±0.02, 3.833±0.05, 3.842±0.01 and 4.856±0.05 (Table 4).
MIC values were comparable to other studies in related plants, as well as to various other potent medicinal plants38,32.
MBC values were ranged from 2.89±0.02 to 4.856±0.05 mg/ml. Similar reports were done in ethanolic extracts of Coleus aromaticus (Muniandy et al., 2014). The MBC values were found to be higher than that of MIC. Approximately 2 MIC values in all the pathogens tested. Similar results (i.e.) 2-3 times more MIC for MBC values was reported by many in plant extracts as well as regarding antibiotics also14,32,36.
The study results revealed that within 15 to 18 h the pathogens can be killed totally and hence this plant extract can be used for various drug formulations.
The results obtained in the present study were more potent compared to previous studies in this plant and related species38,39. Time-kill assay in the present study showed 3 Log reduction found to be around 11 h for B. subtilis, around 12 h for B. cereus for P. aeruginosa around 13 h and above 14 and less than 15 h for S. aureus. Thus the results obtained in the present investigation were appreciable.
The present study clearly indicated the antibacterial potential of an ethanolic crude extract of C. forskohlii collected from Thiruvannamalai district of Tamil Nadu.
Acknowledgements
The authors sincerely thank the Management, Karpagam Academy of Higher Education, Coimbatore – 641 021, Tamil Nadu, India, for their encouragement and support.
Conflict of Interest
The authors declares that there is no conflict of interest.
Authors’ Contribution
SS collected plant materials, did laboratory research work, consolidation and interpretation of data, and wrote the manuscript. BVP reviewed and corrected the manuscript.
Funding
None.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
-
- De Souza NJ, Dohadwalla AN, Reden J. Forskolin: A labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med. Res. Rev., 1983; 3: 201-19.
Crossref - Ammon HPT, Muller AB. Forskolin: From an ayurvedic remedy to a modern agent. Planta Med., 1985; 46: 473-77.
Crossref - Raaman N. Qualitative phytochemical screening. Phytochemical techniques, New India Publishing Agency, New Delhi, 2006. p. 19-22.
- Thamaraiselvi LP, Jayanthi P. Preliminary studies on phytochemicals and antimicrobial activity of solvent extract of Eichhornia crassipes (Mart.) Solms . Asian J. Plant. Sci. Res., 2012; 2(2): 115-22.
- Rajesh H, Rao SN, Rani M, Shetty N, Prathima K, Rajesh EP, Chandrashekhar R. Phytochemical analysis of methanolic extract of Curcuma longa Linn. Int. J. Univ. Pharm. Bio. Sci., 2013; 2(2): 39-45.
- Pearson D. Laboratory Techniques in Food Analysis. 1st ed. Butterworth, London. 1976. P. 22-25.
- Obadoni BO, Ochuko PO. Phytochemical studies and Comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta States of Nigeria. Global J. Pure Appl. Sci., 2001; 8: 203-08.
Crossref - Faten M, Hanen F, Fellah R, Chedly A. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci., 2014; 8(3): 216-24.
Crossref - CLSI (Clinical and Laboratory Standards Institure). Performance standards for antimicrobial susceptibility testing. CLSI / NCCLS approved standard. Clinical and Laboratory Institute, Wayne, Pennsylvania 2007:M100-S17.
- Andrews JM. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother., 2001; 48: 5-16.
Crossref - McKay GA, Beaulieu S, Arhin FF, Belley A, Sarmiento I, Parr, T, Moeck G. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother., 2009; 63:1191-99.
Crossref - NCCLS (National Committee of Clinical Laboratory Standards). Methods for determining bactericidal activity of antimicrobial agents: Approved guideline M26-A. NCCLS, Wayne, USA, 1999.
- Suresh SN, Nagarajan N. Preliminary phytochemical and antimicrobial activity analysis of Begonia malabarica Lam. J. Basic Appl. Biol., 2009; 3(1, Suppl2): p. 59-61.
- Anbuselvan S, Muralikrishnan V. Antimicrobial activity of Coleus forskohlii root extract against human pathogens. Int. J. Phytopharmacol., 2013; 4(1): 42-49.
- Kala S. Antimicrobial activity of Coleus forskohlii (Wild) Briq. and Costus igneus N.E.Br. IOSR J. Pharm. Biol. Sci., 2014; 9(5): 01-06.
Crossref - Singh S, Singh N. Phytochemical and antimicrobial activity of various root extracts of Coleus forskohlii medicinal plant. Int. J. Res. Appl. Sci. Eng. Technol., 2016; 4(2): 150-55.
- Minakshi B, Jharna D, Chanbi Devi E, Nayan T, Partha PK, Kundal N and Manash PS . Phytochemical analysis of traditional medicinal plants and their antimicrobial activity: An experience from North East India. J. Pharm. Res., 2016; 1(1): 1-7.
Crossref - Soetan KO, Oyekunle MA, Aiyelaagbe OO, Fafunso MA. Evaluation of the antimicrobial activity of saponins extract of Sorghum bicolor L. Moench. Afr. J. Biotechnol., 2006; 5: 2405-407.
- Farnsworth N. Biological and Physiochemical screening of plants. J Pharmaceutical Sci., 1966; 55: p.225.
Crossref - Tschesche R, Wulff G. Chemistry and Biology of saponins Fortschr. Chem. Org. Naturist., 1973; 30: p.461.
Crossref - Parekh J, Chanda S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr. J. Biomed. Res., 2007; 10: 175-81.
Crossref - Kubmarawa D, Ajoku GA, Enwerem NM, Okorie DA. Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria. Afr. J. Biotechnol., 2007; 6(14): 1690-96.
- Chithrashree, Srinivas C. Antimicrobial, phytochemical and antioxidant study of hydroalcoholic extracts of Coleus caninus (roth) vatke. Int. J. Pharm. Sci. Rev. Res., 27(1); 288-91.
- Mahesh B, Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J. Agric. Sci., 2008; 4: 839-43.
- Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol., 1998; 60: 1-8.
Crossref - Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev., 1999 12(4): 564-82.
Crossref - Preethi R, Devanathan VV, Loganathan M. Antimicrobial and antioxidant efficacy of some medicinal plants against food borne pathogens. Adv. Bio. Res., 2010; 4(2): 122-25.
- Lin J, Opoku AR, Geheeb-Keller M, Hutchings AD, Terblanche SE, Jager AK, et al. Preliminary screening of some traditional Zulu medicinal plants for anti-inflammatory and antimicrobial activities. J Ethnopharmacol 1999; 68: 267-74.
Crossref - Parekh J, Chanda S. In vitro antimicrobial activities of extract of Launaea procumbens Roxb. (Labiateae), Vitis vinifera (Vitaceae) and Cyperus rotundus (Cyperaceae). Afr. J. Biomed. Res., 2006; 9: 89-93.
Crossref - Yao J, Moellering R. Antibacterial agents. In: Manual of clinical microbiology, Murray P, Baron E, Pfaller M, Tenover F, Yolken R. editors, ASM, Washington DC, 1995; p.1281-90.
- Cetin TE, Gurler N. Bakterilerin antibiyotiklere duyaarlilik deneyinin yapilmasi. Kukem Dergisi, 1989; 12: 2-5.
- Akiyama H, Kazuyasu F, Yamasaki O, Oono T and Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother., 2001; 48(48): 487-91.
Crossref - Lim S, Darah HI, Jain K. Antimicrobial activities of tannins extracted from Rhizophora apiculata barks. J. Trop. For Sci., 2006; 18(1): 59-65.
- Banso A, Adeyemo SO. Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr. J. Biotechnol., 2007; 6(15): 1785-87.
Crossref - Dzialo M, Mierziak J, Korzun U, Preisner M, Szopa J, Kulm A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci., 2016; 17: 1-41.
Crossref - Nirwana I, Rianti D, Soekartono RH, Listyorini RD, Basuki DP. Antibacterial activity of fig leaf (Ficus carica Linn.) extract against Enterococcus faecalis and its cytotoxicity effects on fibroblast cells. Vet. World, 2018; 11(3): 342-47.
Crossref - Chung KT, Wong TY, Wei Y, Huang YW, Lin Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr., 1998; 38: 421-64.
Crossref - Muniandy K, Hassan Z, Isa MHM. Effect of heat and filter sterilization on the efficiency of Coleus aromaticus as an antibacterial agent against diabetic wound pathogens. Int. J. Pharm. Pharm. Sci., 2014; 6(10): 438-43.
- Malleswari D, Bagyanarayana B, Hindumathi A. Antibacterial activity of Coleus forskohlii extracts against some pathogenic bacteria. J. Nat. Prod. Plant Resour., 2013; 3(4):75-78.
- De Souza NJ, Dohadwalla AN, Reden J. Forskolin: A labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med. Res. Rev., 1983; 3: 201-19.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.