ISSN: 0973-7510
E-ISSN: 2581-690X
The aim of this study was to determine the prevalence of diphtheria by a cross-sectional in Saudi population. The size of the population consisted of five-hundred healthy subjects from the western regions of Saudi Arabia from six months to 96 years age. ELISA assay kits was used to titers anti-diphtheria IgG. According to the widely used criteria, 31.7% of the total population was susceptible to diphtheria (IgG level <0.15 IU/ml), 15.7% had basic protection (0.15–1.0 IU/ml), and 52.6% had full protection (>1.0 IU/ml). The majority (82%) of the population from 6 months to 96 years had a protective level of IgG against diphtheria. The frequencies of susceptibility were relatively high in middle-aged periods (30.2 – 37.5% of subjects aged 21–50 years). Significantly, more females (20.7%) than males (15%) were unprotected against diphtheria. In conclusion, monitoring immunization status and administering the diphtheria vaccine as required are essential to ensure adequate and long-lasting antibody levels.
Diphtheria, Sero-prevalence, Immunity.
Childhood immunity against diphtheria occurred from infection prior to vaccination, in which available protection using natural boosters had been used against the toxin of diphtheria bacilli. The epidemiology of diphtheria had changed in many countries throughout routine vaccination, so that immunity against the disease is now provided by primary vaccination during the first months of life. Immunization led to a marked decrease in the incidence of the disease and to a subsequent decrease in the reservoir of toxigenic Corynebacterium diphtheria organisms (Galazka and Dittmann, 2000).
In this setting, immunity against diphtheria can only be maintained in adults if routine booster doses of diphtheria toxoid are administered. Moreover, vaccination coverage must be high and uniform in all age groups, which may minimize the pools individuals’ susceptibility when an imported source of infection occurs, the presence of susceptible groups can favor the circulation of C. diphtheria toxigenic strains. Eventually, that can cause a diphtheria epidemic as the one that occurred in the newly independent states of the former Soviet Union (Dittmann et al., 2000). The diphtheria antibodies were measured by an in-house double-antigen ELISA as described by Kristiansen et al. (1997), which shows a good correlation with established toxin neutralizing assays and is functionally specific for IgG antibodies. Saudi Arabia has achieved during the last three decades a tremendous coverage terms of basic vaccination coverage, this has been carried out, mainly by structured vaccination programs delivered, mainly through a wide network of primary health care centers. (Health statistical book, 2014). The main aim of this study was to assess the prevalence of diphtheria antibodies in Jeddah, Saudi Arabia.
Serum samples collection
Blood samples were randomly collected from five hundred patients, from different ages, from Jeddah, Saudi Arabia between April 2015 to November 2015. Sera samples were stored at -20°C until use. Samples were tested for diphtheria IgG antibodies using enzyme-linked immune-sorbent assay (ELISA) test (IBL International, GmbH, Hamburg, Germany). The procedure was followed as indicated by the manufacturer instruction Diphtheria antibody concentrations lower than 0.01 IU/ml were considered without protection; the levels between 0.01 and 0.09 IU/ml and levels higher than 0.1 IU/ml were considered as basic immunity and full protection, respectively. Prior the beginning of the study, the Ethics Committee, King Abdulaziz University approved the protocol, and written informed consent was obtained from each subject before enrollment.
Statistical analysis.
ANOVA, and paired-samples T test were used for statistical analysis (Chicago, IL, USA). Data are presented as mean ± SD or as an absolute number and percentage. A P-value of <0.05 was considered significant.
To the best of our knowledge, this is the first study to assess sera levels of diphtheria antibody in a Saudi population. The national vaccination schedule in which each child should receive the DPT triple vaccine four times at 2, 4, 6 and 18 months of age, and a booster dose at six years which offer levels of immunity against diphtheria appear normal, especially in young children.
A total of five-hundred blood samples with a mean age of (29.1 ± 22.6) were enrolled in this study. About 47.8% of them were male, and 52.2% were female. Cases were classified into seven age groups as follows: Group 1 (£10 years; n = 159), Group 2 (11- 20 years; n =68), Group 3 (21-30 years; n = 63), Group 4 (31-40 years; n = 60), Group 5 (41- 50 years; n = 39), Group 6 (51-60 years; n = 66), and Group 7 (> 60 years; n = 45). Overall, 82% of cases had sufficient immunity levels against diphtheria Table (1).
Table (1):
Population studied according to age and sex.
Age groups (years) |
Males |
Females |
Total |
Mean age (years) |
Standard Deviation of Mean (years) |
|---|---|---|---|---|---|
0-10 |
81 |
78 |
159 |
5.89 |
2.28 |
11-20 |
28 |
40 |
68 |
14.25 |
2.6 |
21-30 |
25 |
38 |
63 |
27.08 |
2.3 |
31-40 |
28 |
31 |
59 |
36.3 |
3.0 |
41-50 |
17 |
23 |
40 |
45.4 |
3.1 |
51-60 |
31 |
31 |
62 |
57.2 |
3.2 |
>60 |
29 |
20 |
49 |
71.3 |
8.1 |
All |
239 |
261 |
500 |
29.1 |
22.6 |
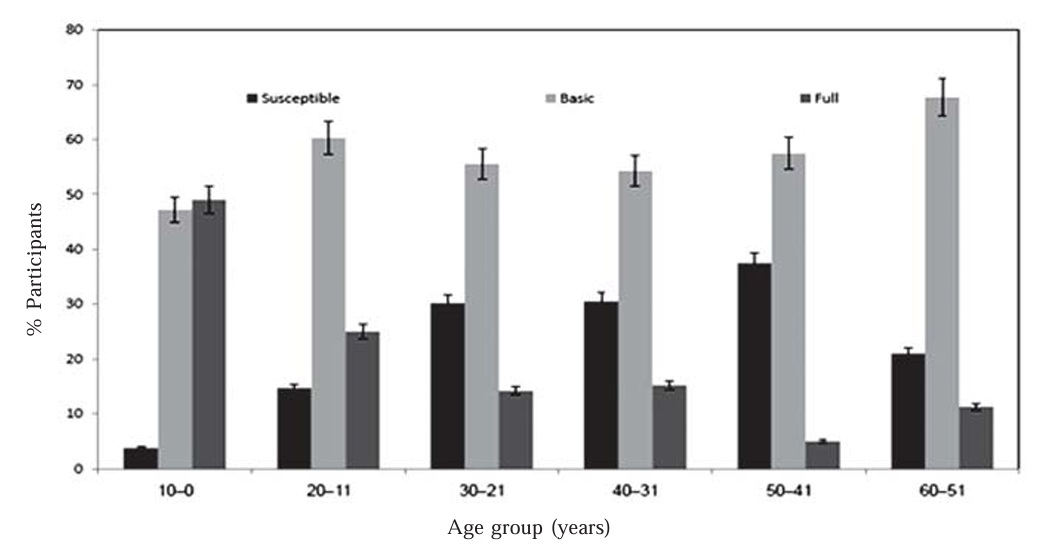
The observed level of IgG in the serum samples (Table 2) indicated that about 18% of the subjects were susceptible to diphtheria, whereas approximately 55.2% had basic protection and about 26.8% appeared to be fully protected. The frequencies of susceptibility were relatively high in middle-aged stage-groups (30.2 – 37.5% of subjects aged 21–50 years were susceptible), which indicate that many adults failed to have a boosted vaccination. This results agree with (Heininger et al., 2006). Low frequencies of adult protection have also been reported from Spain (Galazka and Dittmann 2000) and in Scandinavia and Poland (Walory et al., 2000). However, the proportion of protective antibodies against diphtheria also decreased from infancy to adolescence. In the United States (McQuillan, et al., 2002) and Spain (Pachon, et al., 2002).
Table (2):
Diptheria Immunity by age for both genders.
| Age groups (years) |
No. of subjects |
Susceptible (<0.15 IU/ml) | Basic(0.15-1.0 IU/ml) | Full (> IU/ml) | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| 0-10 | 159 | 6 | 3.8 | 75 | 47.2 | 78 | 49 |
| 11-20 | 68 | 10 | 14.7 | 41 | 60.3 | 17 | 25 |
| 21-30 | 63 | 19 | 30.2 | 35 | 55.6 | 9 | 14.2 |
| 31-40 | 59 | 18 | 30.5 | 32 | 54.3 | 9 | 15.2 |
| 41-50 | 40 | 15 | 37.5 | 23 | 57.5 | 2 | 5 |
| 51-60 | 62 | 13 | 21 | 42 | 67.7 | 7 | 11.3 |
| >60 | 49 | 9 | 18.4 | 28 | 57.1 | 12 | 24.5 |
| All | 500 | 90 | 18 | 276 | 55.2 | 134 | 26.8 |
The youngest age group of children aged (0–10 years) was subdivided into children aged 0–1, 1–2, 2–3 and 3–10 years (data not shown). The children aged 3-10 year were more likely to be susceptible than those aged  three years when children should have the DTP doses. Similar observations have already been made in France, Russia, U.S.A. and Norway, where >50% of children aged 7–10 years were found to have insufficient protection (Galazka and Robertson, 1996 and Skogen et al., 2000), indicating 98% immunization schedule for (DTP) among 1year olds was covered. From the 11- to 21-year-old group on, mean antibody levels decreased progressively until the 40- to 59-year-old group and increased again in individuals 60 years or over. Other studies indicated that oldest subjects (those aged >60 years) were less likely to appear susceptible than the middle-aged, with no real evidence of waning immunity in old age Figure (1). The obtained results in the line with Skogen et al. (2000). In the U.S.A, the lowest level of sero-protection was observed in people aged >50 years (Walory et al., 2001). Researchers in the U.S.A, Turkey, England, and Wales also showed that there was a decrease in the prevalence of diphtheria protective antibodies from young adults to individuals 60 years or over (Maple et al., 2000; McQuillan et al., 2002).
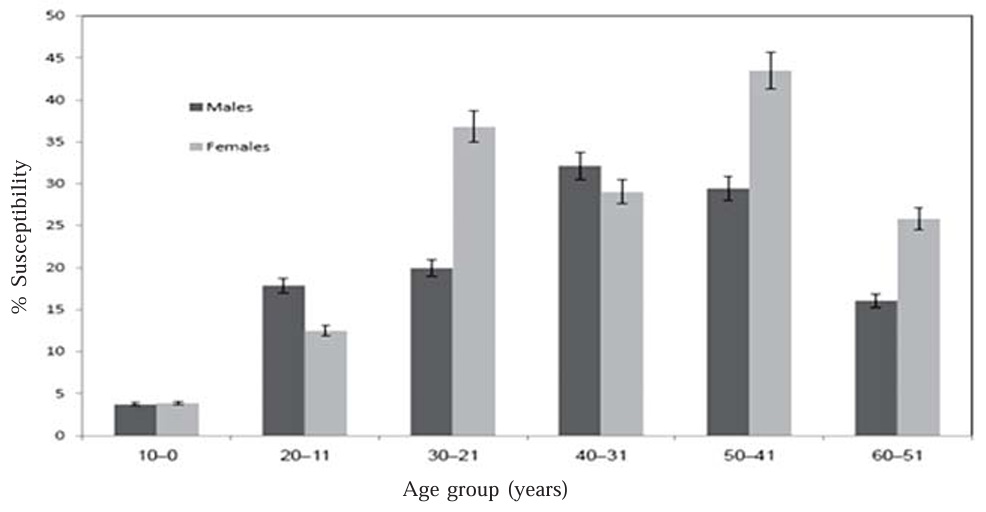
Regarding genders the results indicated that, male subjects were slightly less susceptible (15%) and fully protected (73%) than females (20.7% ) and (61% ), respectively Figure (2). In most countries, in contrast to the present results, women are less likely to be fully protected than men (Gasparini, 2000), this trend probably reflects the opposed frequencies with which the lifestyle of Saudi may affect the resulted observations.
Previous studies in Saudi Arabia on diphtheria and immunity is scattered and fragmented (El-Gezery and Almaie, 1999). According to Al-Mazrou et al. (2007), children at the age of 1, 6, and 17 years are well protected against diphtheria, pertussis, and tetanus. It can be argued that the present study population may not be representative of Saudi population. However, since it involved all age category, it permitted comparison of immunity to diphtheria according to age. Our results showed that children under 3 year and adults 40 to 59 years of age were the two fully protective groups for the disease. Interestingly, during the diphtheria epidemic in the Republic of Georgia in 1993 to 1996, children who did not have the complete primary vaccination series with diphtheria toxoid and adults between 40 and 49 years of age were those with the highest fatality rate among the 659 patients identified with diphtheria (Quick et al., 2000). These findings highlight the need for maintenance of protective antibody levels through basic immunization in children. Efforts must also be undertaken to encourage boosters in adolescents and adults. In the case of the Brazilian population, that seems especially important for the 20 to 40 year age stratum. Several factors have probably contributed to the poor anti-diphtheria immunity in Saudi Arabia. The general effectiveness of health education programs about the importance of vaccination. The Saudi primary immunization schedule is perhaps unable to maintain an adequate protection level for a long time. Levels of natural exposure to C. diphtheriae may also be low (Galazka and Dittmann, 2000). Although the type, number and spacing’s of the diphtheria vaccinations used, in Saudi and elsewhere, appear to confer incomplete protection on an individual basis, reduction in the general prevalence of infection with toxigenic C. diphtheriae, to almost undetectable levels, has virtually eliminated diphtheria from those countries in which the vaccination of the entire population has been achieved (Schneerson et al., 1996). In Saudi, booster vaccinations given later in life (e.g. as children begin and end their school education) and/or improvements in the coverage achieved by the current program of routine immunization would be beneficial. The scheduled immunization of Saudi children who receive their final dose of DPT at 4-6 years supported the observation of zero susceptibility high coverage’s were improved in Saudi Arabia throughout resistant socio–economic immunization programs for the community. In conclusion, the epidemiological status of diphtheria immunity observed in Saudi Arabia appears healthy.
ACKNOWLEDGMENTS
The authors thank all the subjects who participated in the study (for their cooperation), Professor Dr. Khalid Al-Ghamdi for helpful discussions and administration.
- Al-Mazrou, Y.Y., Khalil, M.K., Elgizouli, S.A., Al-Jeffri, M.H., Bakhsh, M.M., Mishkais, A.A., Diphtheria, pertussis, and tetanus serosurvey in Saudi Arabia. Saudi Med. J. 2007; 28(8), 1230-1233.
- Dittmann, S., Wharton, M., Vitek, C., Ciotti, M., Galazka, A., Guichard, S., and Kreysler, J. Successful control of epidemic diphtheria in the states of the Former Union of Soviet Socialist Republics: lessons learned. Journal of Infectious Diseases, 2000; 181(Supplement 1), S10-S22.
- El-Gezery, M., and Almaie, S., Healthy diphtheria carries in two students in the Eastern province, Saudi Med. J., 1999; 20(7), 548-550.
- Galazka, A. M., and Dittmann, S. The changing epidemiology of diphtheria in the vaccine era. Journal of Infectious Diseases, 2000; 181(Supplement 1), S2-S9.
- Galazka, A. M., and Robertson, S. E. Immunization against diphtheria with special emphasis on immunization of adults. Vaccine, 1996; 14(9), 845-857.
- Gasparini, G. Full-time or part-time work: realities and options , European Foundation for the Improvement of Living and Working Conditions, Dublin 2000.
- Health Statistical Year Book, Vaccination coverage and incidence of diseases targeted by the expanded program of immunization, 1996-1999. In: 1420/1421 H (Arabic version). 2000; Ministry of Health, Riyadh, Saudi Arabia:
- Heininger, U., Loos, K., Lorenz, I., and Rascher, W. Compliance with recommended immunizations in adolescents. European journal of pediatrics, 2006; 165(10), 671-676.
- Kristiansen M, Aggerbeck H, Heron I. Improved ELISA for determination of anti-diphtheria and/or anti-tetanus antitoxin antibodies in sera. APMIS 1997; 105: 843-853.
- Maple, P. C., Jones, C. S., Wall, E. C., Vyse, A., Edmunds, W. J., Andrews, N. J., and Miller, E. Immunity to diphtheria and tetanus in England and Wales. Vaccine, 2000; 19(2), 167-173.
- McQuillan, G. M., Kruszon-Moran, D., Deforest, A., Chu, S. Y., and Wharton, M. Serologic immunity to diphtheria and tetanus in the United States. Annals of Internal Medicine, 2002; 136(9), 660-666.
- Pachón, I., Amela, C., and De Ory, F. Age-specific seroprevalence of poliomyelitis, diphtheria and tetanus antibodies in Spain. Epidemiology and infection, 2002; 129(03), 535-541
- Quick, M. L., Sutter, R. W., Kobaidze, K., Malakmadze, N., Strebel, P. M., Nakashidze, R., and Murvanidze, S. Epidemic diphtheria in the Republic of Georgia, 1993–1996: risk factors for fatal outcome among hospitalized patients. Journal of Infectious Diseases, 2000; 181(Supplement 1), S130-S137.
- Schneerson, R., Robbins, J. B., Taranger, J., Lagergard, T., and Trollfors, B. A toxoid vaccine for pertussis as well as diphtheria? Lessons to be relearned. The Lancet, 1996; 348(9037), 1289-1292.
- Skogen, V., Jenum, P. A., Danilova, E., Koroleva, V. N., Halvorsen, D. S., and Sjursen, H. Immunity to diphtheria among children in Northern Norway and NorthWestern Russia. Vaccine, 2000; 19(2), 197-203.
- Walory, J., Grzesiowski, P. and Hryniewicz, W. Comparison of four serological methods for the detection of diphtheria anti-toxin antibody. J Immunol Meth; 2000; 245: 55–65.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.