ISSN: 0973-7510
E-ISSN: 2581-690X
A novel thermophilic lignocellulolytic bacterium, Geobacillus stearothermophilus TP-3, was isolated and characterized from the Tapovan hot spring in India. The cellulase production of TP-3 was optimized using a One-Factor-at-a-Time (OFAT) approach followed by a Plackett-Burman design, leading to a three-fold enhancement in enzyme yield. Phylogenetic analysis based on 16S rRNA sequencing revealed high sequence similarity with Geobacillus sp. H6a. The cellulase enzyme exhibited optimal activity at 50 °C under alkaline conditions (pH 8.0) and retained ~68% of its activity across a broad temperature range (40-70 °C) for up to three hours, demonstrating remarkable thermo-alkali stability. The ANOVA revealed that three factors-glucose, carboxymethyl cellulose (CMC), and yeast extract-significantly affected cellulase production, with yeast extract emerging as the most influential factor. Notably, TP-3 efficiently degraded agronomic residues, including wheat bran and sugarcane molasses, highlighting its potential for sustainable agricultural waste valorization and bioethanol production. The exceptional thermostability and lignocellulolytic potential of G. stearothermophilus TP-3 position it as a promising candidate for industrial bioconversion processes.
Lignocellulosic, Thermophilic Cellulase, Geobacillus, Agro-waste Management, Saccharification
Lignocellulosic materials are highly abundant, biodegradable, renewable, biocompatible polysaccharides with immense industrial potential. Their diverse sugar composition makes them an attractive resource for sustainable development, particularly in bioethanol production-a promising alternative to fossil fuel dependency.1 Agricultural residues-including sugarcane molasses, bagasse, rice hulls, woody crops, corn stover, and forest waste-constitute a substantial portion of lignocellulosic materials and can serve as potential feedstock for bio-based industries.2,3 However, the efficient utilization of lignocellulosic biomass remains a challenge due to its low solubility and recalcitrant structure, thus making them less effective for their actual applications.4 Therefore, prior treatment of the lignocellulosic biomass is the prerequisite for its subsequent employment as an energy source. Enzymatic hydrolysis offers a sustainable and eco-friendly solution by breaking down lignocellulosic biomass into valuable compounds such as acetone and ethanol.5,6
Hot springs are natural reservoirs of novel microbial communities harbouring glycosyl hydrolases for cellulose and hemicellulose degradation.7 A few thermostable cellulases isolated from geothermal springs are CelDZ1,8 Bacillus velezensis strain MRC 5958,9 Geobacillus sp. TP-110 and Bacillus licheniformis PANG L.11 Alkali-thermostable cellulases are valuable for their resilience in harsh industrial conditions, making them ideal for applications where conventional cellulases fail. Key uses include bio-stoning, fabric biopolishing, bio-bleaching, deinking, laundry detergents, industrial cleaning, dark fermentation, bioethanol production, and waste treatment.12-14 Their effectiveness under extreme conditions reduces chemical use and costs and enhances sustainability, making them highly desirable for lignocellulosic biomass valorisation.15
Statistical optimisation of production conditions (pH, temperature, carbon and nitrogen sources, and incubation time) is essential for enhancing microbial enzyme production’s efficiency, yield, and cost-effectiveness. By identifying the ideal combinations of these parameters, statistical optimisation reveals interactions that maximise enzyme yield and activity.16 This approach ensures a more predictable and reproducible process, which is crucial for consistency in industrial-scale production.
Traditional one-variable-at-a-time (OVAT) optimisation is labour-intensive and inefficient. Statistical methods like Response Surface Methodology (RSM) and Design of Experiments (DoE) enable simultaneous evaluation of multiple variables, cutting down time and costs.17 Key techniques include RSM, Plackett-Burman Design (PBD), Box-Behnken Design (BBD), and Central Composite Design (CCD). PBD is particularly useful for screening numerous variables to identify the most significant ones affecting enzyme production. It requires fewer experimental runs, making it cost-effective and ideal for initial screenings. However, PBD only estimates the main effects and doesn’t consider interactions. RSM and CCD are better for detailed optimisation but require more runs as they assume all factors are important.16 Thus, a combined approach-PBD for screening and RSM/CCD for optimisation, is often best.
The present study focuses on the isolation and characterisation of a thermostable cellulase from the thermophilic bacterium Geobacillus stearothermophilus TP-3, obtained from the Tapovan hot spring in India. The study optimizes growth conditions for enhanced cellulase production using a combination of One-Factor-at-a-Time (OFAT) and Plackett-Burman Design (PBD), allowing for the identification of key factors influencing enzyme yield. Additionally, the potential of lignocellulosic waste as a carbon source for cellulase production was investigated, providing insights into its feasibility for enzyme biosynthesis. Given the enzyme’s thermostability and activity under alkaline conditions, G. stearothermophilus TP-3 bacteria, this work contributes to advancing green technologies for efficient lignocellulose degradation, bioethanol production, and industrial waste management.
Chemicals
Luria Bertani broth, Zinc chloride, Ammonium chloride, Sodium acetate, Glycine, Sodium chloride, Peptone, Yeast extract & Magnesium sulphate were purchased from Himedia, India. Carboxymethyl cellulose, Glucose, Beef extract, Ammonium sulphate, Sodium nitrate, Maltose, Lactose, Sucrose, Sodium succinate, Sodium citrate was purchased from SRL (India). DNSA (3,5-dinitrosalicylic acid), Urea, Sodium hydroxide, and HCl were purchased from Sigma Aldrich.
Sample collection and sampling procedure
Soil samples were collected from the Tapovan hot springs (30.490897°N and 79.646662°E) of India (Figure 1).18 They were collected using a sterile spatula from 4-5 cm below the surface soil and stored in sterile polybags. The samples were brought to the laboratory and maintained at 4 °C in a refrigerator until further processing. The soil temperature was recorded during sampling using a thermometer, and it was ~80 °C. During laboratory analysis, the sample exhibited a pH of 8.0.
Figure 1. Sampling site of Tapovan hot spring (30.490897°N and 79.646662°E) in Uttarakhand State, India (Source: Google map)
Isolation of thermophilic cellulase-producing bacteria
To isolate bacteria, 50 mL of enrichment medium and 5 g of soil were mixed in a 250 mL Erlenmeyer flask. Here, the enrichment medium was used where 1% carboxymethyl cellulose (CMC) was added to the medium as an additional carbon source and as an inducer.19 For 24 hours, the flask was shaken at 50 °C and 150 rpm in a shaker incubator. To obtain the thermophilic cellulase-producing bacteria, 100 µL of the overnight culture was spread on a CMC agar plate and cultured for 24 hours at 60 °C. The colonies showing distinct morphology were streaked on a nutrient agar plate, and pure colonies were obtained by using multiple streaking under similar cultivation conditions. The pure isolates of bacteria were maintained on slants for short-term use. The glycerol stocks were prepared in a 1:1 ratio using 50% (v/v) glycerol and kept at -20 °C.
Primary screening of cellulolytic bacterial isolates
The isolates were inoculated in 10 mL of minimal salt media supplemented with CMC to produce cellulase at 50 °C for 24 hours. Bacterial cells were separated from the fermentation broth by centrifugation. The supernatant was then used as a crude enzyme to test the thermophilic isolates for cellulase activity using the well diffusion method. The enzyme was added to the well of CMC-agar plates and then incubated at 50 °C for 48 hours to allow the enzyme to diffuse. The cellulase activity was confirmed by observing a clear zone of hydrolysis. For this, the plates were flooded with 1% (w/v) Congo red solution after complete diffusion and then repeatedly destained with 1 M NaCl solution.20 The potent cellulase-producing isolates were further processed.
Cellulase activity assay
The 3,5-dinitrosalicylic acid (DNSA) technique was used to test the cellulase activity in triplicates.21 Glycine-NaOH buffer-50 mM (pH 8) was used to prepare the substrate by suspending 1% (w/v) CMC. Equal quantities of crude enzyme and substrate were mixed to form the reaction, which was kept at 50 °C for 10 min. The process was stopped by adding an equal volume of DNSA reagent and boiling at 100 °C for 10 minutes. In the final reaction, 400 µL of 33% (w/v) sodium potassium tartrate was added and cooled. The released glucose was quantified by using a spectrophotometric method at the wavelength of 540 nm. A bacterium was selected for further studies based on the highest cellulase activity. The Bradford protein assay was used to estimate the protein content in the fermentation broth.22
One-unit cellulase activity can be defined as the amount of enzyme that releases 1 µmole of glucose per minute per mL under assay conditions.
Morphological and growth characteristics
After being cultured on a nutrient agar plate for 20 hours at 50 °C, the bacterial strain’s phenotypic traits, colour, and margin were examined for their morphological characteristics. The cell morphology was examined using a compound light microscope (Olympus model CX41) and scanning electron microscope (SEM Model: JSM 6490 LV, JEOL, Japan).
Identification of bacterial isolate
The bacterial-specific 16S rRNA gene was amplified using genomic DNA from the chosen bacterial strain. The PCR condition was set as initial denaturation (pre-PCR) for 5 min at 95 °C followed by 30 cycles (denaturation 90 sec at 95 °C, 30 sec at 58 °C, 90 sec at 72 °C) and final extension (post-PCR) for 10 min at 72 °C. The bacterial 16S rRNA gene universal forward (5′ -AGAGTTTGATCCTGGCTCAG-3′) and reverse (5′-GGTTACCTTGTTACGACTT-3′) were used to amplify the 16S rRNA gene.23 The amplified gene was sequenced using Sanger sequencing, and the obtained sequence was compared to that of other closely related bacterial species using BLASTn at NCBI. Phylogenetic analyses were conducted in the T-Coffee multiple sequence alignment program, and the Neighbour-Joining (NJ) method was applied to infer evolutionary relatedness.
Selection of media for cellulase production and OFAT analysis
A few media compositions cited in the literature were used for cellulase production (Supplementary Table). A medium was chosen for further analysis based on the highest cellulase production. One-Factor-at-a-Time (OFAT) analysis was performed to optimize the carbon source, nitrogen source, pH, temperature, and inoculum size to enhance cellulase production.
Plackett-Burman design for statistical optimization of cellulase production
Our study used the Plackett-Burman (PB) factorial design (PBD) to pinpoint the key medium elements affecting cellulase production. The parameters from OFAT analysis were used as a reference for PB designing for cellulase enzyme production. The PB analysis was used to identify the most significant culture variables and medium components that might greatly improve cellulase outputs.24 As shown in Table 1, various independent factors were selected for this study on two different levels, i.e., low (-1) level and high (+1). Seven variables that influenced cellulase production, namely carbon source (Glucose), inducer (CMC), nitrogen source (Yeast extract concentration), pH, temperature, inoculum size, and incubation time, were taken and varied according to PB design. Design Expert Software 6.1.10 was used to examine predicted responses. Each experiment was performed in triplicates, and the conclusion was determined by averaging the outcomes. The experimental outcome was the estimated mean of cellulase production (dependent variable). The experimental design is based on the first-order model shown in Equation 1.25
Y= b0 + Σ bi xi … 1
Y represents the cellulase enzyme activity response, b0 the model intercept, bi the linear variable coefficient, i is the variable number, and xi is the independent variable. The DNSA test was used to determine particular enzyme activity by quantifying the quantity of glucose generated using a standard curve.
Table (1):
Plackett-Burman design with experimental levels of independent variables for cellulase production from Geobacillus stearothermophilus TP-3
Factor |
Production parameter |
Low level (-1) |
High Level (+1) |
|---|---|---|---|
A |
Glucose (%) |
0.4 |
0.6 |
B |
Carboxy methyl cellulose (%) |
1.0 |
1.5 |
C |
Yeast Extract (%) |
0.4 |
0.6 |
D |
pH |
7 |
9 |
E |
Temperature (°C) |
30 |
50 |
F |
Inoculum Size (%) |
4 |
8 |
G |
Incubation Time (hrs.) |
20 |
28 |
Low-cost wastes as the substrate for cellulase production
The bacterial isolate was inoculated in optimized media from the PB experiment for cellulase production, where inexpensive agricultural waste materials replaced the carbon source. A range of low-cost agricultural waste products, including sugar cane molasses, rice straw, wheat bran, and corn cob, were employed as the only carbon source to determine the optimal substrates for cellulase production. These wastes were dried, crushed, and used at 2% w/v (for solids) and 2% v/v (for liquids) concentrations. The enzyme production was measured by using the DNSA method.
Characterisation of cellulase
The cellulase activity of strain Geobacillus stearothermophilus TP-3 was measured at different pH (5-9) and temperatures (40-70 °C). The thermostability of the cellulose enzyme was assessed at various temperatures from 40 °C to 70 °C by varying the incubation time from 10 min up to 3 hours using the DNSA method.
Isolation and selection of thermophilic cellulase-producing bacteria
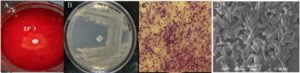
A total of ten bacterial isolates were obtained on CMC-containing enrichment media plates from Tapovan soil samples. Two isolates (TP-1 and TP-3) showed comparatively higher cellulase activity based on the zone of hydrolysis (diameter in mm) on agar plates containing 1% Carboxyl Methyl Cellulose (CMC) medium. Of these two isolates, TP-3 was selected for further analysis due to the maximum zone of clearance during the plate diffusion assay (Figure 2A). Therefore, TP-3 was selected for further optimizing the cellulase production. The isolate exhibited optimum growth at 50 °C; hence, it is considered a thermophilic bacterium.
Morphological and growth characteristics
Bacterial colonies were grown on the nutrient agar plate and subjected to phenotypic characterization. It had a spherical colony, off-white, transparent, and slimy appearance with flat, slightly undulating margins (Figure 2B). Gram staining identified it as a Gram-positive bacterium. A compound light microscope at 100X magnification revealed long rod-shaped bacteria (Figure 2C), and SEM corroborated similar findings (Figure 2D). Gram-positive bacterial cells with terminal and sub-terminal endospores were observed. The TP-3 strain exhibited enzyme activity of 0.692 µmoles/min/mL using the DNSA assay of glucose estimation.
Figure 2. Cellulase enzyme screening and morphological features of isolate. (A) Zone of hydrolysis on CMC-containing agar plates after Congo red staining; (B) TP-3 isolate grown on the nutrient agar plate showing slimy off-white coloured, translucent, circular shaped with flat elevation with slightly undulating margins colonies; (C) Rod-shaped Gram-positive purple cells at 100× magnification using a compound light microscope; (D) Rod-shaped cells visualized using SEM (Scanning Electron Microscope).
Identification of TP-3 cellulose-degrading bacterial strain
A ~1.5 kb fragment of the 16S rRNA gene was successfully amplified and sequenced to identify the cellulolytic bacterial isolate (Figure 3A). Phylogenetic analysis based on the sequence data confirmed that the isolate, designated as TP-3, belongs to the genus Geobacillus. The 16S rRNA gene of TP-3 exhibited the highest sequence similarity with multiple Geobacillus stearothermophilus strains, including G. stearothermophilus YE-6 (98.53%), G. subterraneus E-55 I (98.03%), G. stearothermophilus D6a (98.02%), Geobacillus sp. TC-S8 (97.64%), Geobacillus sp. G1 (97.57%), G. kaustophilus HTA426 (97.44%), and G. thermoleovorans SURF-48B (97.37%). The 16S rRNA gene sequence has been deposited in GenBank under accession number OP962434 (Figure 3B).
Using the Neighbor-Joining (NJ) method with the T-Coffee multiple sequence alignment tool, phylogenetic analysis revealed that TP-3 shares the closest evolutionary relationship with Geobacillus sp. H6a (Figure 3C). These findings suggest that TP-3 is a novel strain within the Geobacillus genus, exhibiting significant cellulolytic potential. Its close phylogenetic relationship with G. stearothermophilus and other thermophilic species underscores its adaptation to high-temperature environments, making it a promising candidate for industrial applications involving lignocellulose degradation.
Figure 3. Identification of the isolate using 16S rRNA sequencing: (A) PCR amplicon of 16S rRNA gene of Geobacillus sp. strain TP-3 using universal primers; (B) NCBI submitted sequencing data of 16S ribosomal RNA gene of Geobacillus sp. strain TP-3; (C) Phylogenetic analysis to infer relatedness amongst Geobacillus strains and other Bacillus species after BLASTn and for determining identity of the bacterium using T-Coffee method
Selection of media for cellulase production and OFAT analysis
The maximum cellulase production was observed in a medium reported by Sakthivel et al.26 of the six different media. The selected medium was optimised using a One-Factor-at-a-Time (OFAT) approach to enhance cellulase production by Geobacillus stearothermophilus. This method systematically assessed the effect of individual parameters, with results presented in Figure 3.
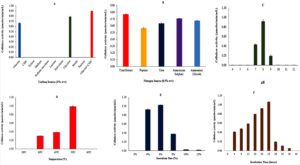
Screening of various carbon sources, including glucose, carboxymethyl cellulose (CMC), xylose, and maltose, identified glucose and CMC as the most effective combination for maximizing cellulase activity (Figure 4A). Similarly, among the nitrogen sources tested, yeast extract yielded the highest cellulase activity (Figure 4B).
The effect of pH on enzyme production was evaluated by adjusting the pH of the production medium (M5) from 4 to 12. Optimal growth was observed within the pH range of 7-9, with peak cellulase production occurring at pH 8 (Figure 4C). Temperature optimization, conducted over a range of 20 °C to 60 °C, revealed that maximum cellulase production occurred at 50 °C (Figure 4D).
The impact of inoculum size was also assessed, with variations tested to determine the ideal concentration. A 6% inoculum size resulted in the highest cellulase activity (Figure 4E). Additionally, cellulase production was monitored at different incubation times (0-60 hours), showing a steady increase up to 24 hours, followed by a gradual decline (Figure 4F). Following OFAT optimization, enzyme activity significantly improved, increasing approximately 1.5-fold from 0.692 µmol/min/mL to 1.06 µmol/min/mL. This enhancement highlights the effectiveness of systematic parameter optimization in boosting cellulase production for potential industrial applications.
Figure 4. Optimization of various factors on cellulase production by using OFAT method (A) Effect of different carbon sources; (B) Effect of different nitrogen sources; (C) Effect of pH; (D) Effect of Temperature; (E) Effect of Inoculum size and (F) Effect of incubation time
Plackett-Burman design for statistical optimization of cellulase production
Seven factors were examined to identify the crucial variables appropriate for cellulase production. The model produced a design for 12 trials (Table 2), where each column corresponds to a variable and each row to an experiment. Geobacillus sp. TP-3 produced cellulase in varying amounts, and these differences are shown in Table 2, where they highlight the significance of factor optimization. The eighth experimental run had the highest cellulase activity (0.925 µmoles/min/mL), whereas the seventh run showed the lowest (0.051 µmoles/min/mL). The data in Table 2 were subjected to multiple linear regression analysis based on the PBD to estimate the F-value and p-value of each component. The first-order linear model determines how independent variables affect cellulase production, and it provides these results in equations 2 (coded factors) and 3. (actual factors).
Table (2):
Plackett-Burman experimental trial table with cellulase activity response
Trial |
A: Glucose (gm/L) |
B: CMC (gm/L) |
C: Yeast Extract (gm/L) |
D: pH |
E: T (° C) |
F: Inoculum Size (%) |
G: Incubation Time (hrs.) |
H: Dummy 1 |
I: Dummy 2 |
J: Dummy 3 |
K: Dummy 4 |
Experimental U/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 |
-1.0 (0.4) |
+1.0 (1.5) |
+1.0 (0.6) |
+1.0 (9.0) |
-1.0 (30) |
+1.0 (8.0) |
+1.0 (28) |
-1.0 |
+1.0 |
-1.0 |
-1.0 |
0.078 |
2 |
+1.0 (0.6) |
-1.0 (1.0) |
+1.0 (0.6) |
-1.0 (7.0) |
-1.0 (30) |
-1.0 (4.0) |
+1.0 (28) |
+1.0 |
+1.0 |
-1.0 |
+1.0 |
0.557 |
3 |
-1.0 (0.4) |
-1.0 (1.0) |
-1.0 (0.4) |
-1.0 (7.0) |
-1.0 (30) |
-1.0 (4.0) |
-1.0 (20) |
-1.0 |
-1.0 |
-1.0 |
-1.0 |
0.563 |
4 |
+1.0 (0.6) |
+1.0 (1.5) |
+1.0 (0.6) |
-1.0 (7.0) |
+1.0 (50) |
+1.0 (8.0) |
-1.0 (20) |
+1.0 |
-1.0 |
-1.0 |
-1.0 |
0.273 |
5 |
+1.0 (0.6) |
+1.0 (1.5) |
-1.0 (0.4) |
+1.0 (9.0) |
+1.0 (50) |
-1.0 (4.0) |
+1.0 (28) |
-1.0 |
-1.0 |
-1.0 |
+1.0 |
0.624 |
6 |
-1.0 (0.4) |
+1.0 (1.5) |
+1.0 (0.6) |
-1.0 (7.0) |
+1.0 (50) |
-1.0 (4.0) |
-1.0 (20) |
-1.0 |
+1.0 |
+1.0 |
+1.0 |
0.072 |
7 |
-1.0 (0.4) |
+1.0 (1.5) |
-1.0 (0.4) |
-1.0 (7.0) |
-1.0 (30) |
+1.0 (8.0) |
+1.0 (28) |
+1.0 |
-1.0 |
+1.0 |
+1.0 |
0.051 |
8 |
+1.0 (0.6) |
-1.0 (1.0) |
-1.0 (0.4) |
-1.0 (7.0) |
+1.0 (50) |
+1.0 (8.0) |
+1.0 (28) |
-1.0 |
+1.0 |
+1.0 |
-1.0 |
0.925 |
9 |
-1.0 (0.4) |
-1.0 (1.0) |
-1.0 (0.4) |
+1.0 (9.0) |
+1.0 (50) |
+1.0 (8.0) |
-1.0 (20) |
+1.0 |
+1.0 |
-1.0 |
+1.0 |
0.541 |
10 |
+1.0 (0.6) |
+1.0 (1.5) |
-1.0 (0.4) |
+1.0 (9.0) |
-1.0 (30) |
-1.0 (4.0) |
-1.0 (20) |
+1.0 |
+1.0 |
+1.0 |
-1.0 |
0.100 |
11 |
-1.0 (0.4) |
-1.0 (1.0) |
+1.0 (0.6) |
+1.0 (9.0) |
+1.0 (50) |
-1.0 (4.0) |
+1.0 (28) |
+1.0 |
-.1.0 |
+1.0 |
-1.0 |
0.090 |
12 |
+1.0 (0.6) |
-1.0 (1.0) |
+1.0 (0.6) |
+1.0 (9.0) |
-1.0 (30) |
+1.0 (8.0) |
-1.0 (20) |
-1.0 |
-1.0 |
+1.0 |
+1.0 |
0.157 |
Independent variables: High level +1 and low level -1. The mean of triplicate culture trials is used to calculate response values
Final Equation in Terms of Coded Factors
Y (cellulase activity) = +102.23*A-119.99*B-147.79*C-87.35*D+94.79*E-65.97*H+67.73*J-128.17*K
…(2)
Final Equation in Terms of Actual Factors
Y (cellulase activity) = 1022.29171* Glucose-479.95132*CMC-1477.88043*Yeast Extract-87.35206*pH+9.47902*Temperature-65.96677*dummy1+67.73334*dummy2-128.16932*dummy3
…(3)
Y = b0 + Σbi + xi;
where Y represents the response of the cellulase enzyme activity, b0 the model intercept, bi the linear variable coefficient, i the variable number, and xi the level independent variable. Design Expert 6.1.10 software.
The factors affecting the generation of cellulase by Geobacillus stearothermophilus TP-3 were examined using an analysis of variance (ANOVA), and the findings were presented in Table 3. The p-value of 0.0371 in the statistical design made it clear that the model is significant. Three factors (glucose, CMC, and yeast extract) were determined by p–value analysis to impact cellulase production significantly. However, the yeast extract was the most important factor, with a p–value of 0.0187. A p–value greater than 0.05 indicates that the studied component was not statistically significant; however, it did not play a specific function in enzyme synthesis. The F-value of 10.98 for the model suggests that it is significant. A “Model F-value” this large might happen due to noise with a mere 3.71% probability. Because the “Pred R-Squared” of 0.4717 is not as close to the “Adj R-Squared” of 0.8789, the coefficient (R2) revealed that the model has a block effect. By the criterion known as “Adeq Precision,” a measure of signal-to-noise ratio that can be used to explore the design space, our model’s ratio value of 9.885 shows that it has a satisfactory signal-to-noise ratio.
Table (3):
Plackett-Burman design ANOVA analysis using the partial sum of squares for Geobacillus sp. TP-3 cellulase production
Source |
Sum of Squares |
Degree of Freedom |
Mean Square |
F-Value |
p-value Prob > F |
Inference |
|---|---|---|---|---|---|---|
Model |
1.064E+006 |
8 |
1.330E+005 |
10.98 |
0.0371 |
Significant |
A: Glucose |
1.0254E+005 |
1 |
1.254E+005 |
10.35 |
0.0487 |
Significant |
B: CMC |
1.728E+005 |
1 |
1.728E+005 |
14.26 |
0.0325 |
Significant |
C: Yeast Extract |
2.621E+005 |
1 |
2.621E+005 |
21.64 |
0.0187 |
Significant |
D: pH |
91564.60 |
1 |
91564.60 |
7.56 |
0.0708 |
Not Significant |
E: T (°C) |
1.078E+005 |
1 |
1.078E+005 |
8.90 |
0.0584 |
Not Significant |
H: Dummy |
52219.38 |
1 |
52219.38 |
4.31 |
0.1294 |
Not significant |
J: Dummy |
55053.66 |
1 |
55053.66 |
4.55 |
0.1228 |
Not significant |
K: Dummy |
1.971E+005 |
1 |
1.971E+005 |
16.28 |
0.0274 |
Significant |
Residual |
36334.24 |
3 |
12111.41 |
|||
Cor Total |
1.100E+006 |
11 |
Std Dev 110.05, mean 311.05, C.V. 35.38, Press 5.813E+005; R squared 0.9670; Adj R-Squared 0.8789; Pred R squared 0.4717; Adeq Precisior 9.885 [*p-value < 0.01 highly significant; 0.01 < P < 0.05 significant]
Low-cost wastes as the substrate for cellulase production
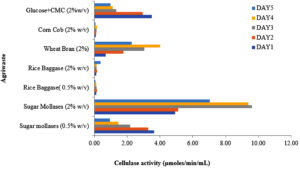
Various agricultural waste products were evaluated as carbon sources to assess their impact on cellulase enzyme induction over five days. Among the tested substrates, cane sugar molasses supported the highest cellulase production (~9.60 µmol/min/mL), reaching its peak on the third day before experiencing a slight decline on the fourth day. In contrast, wheat bran yielded approximately 2.4 times lower cellulase production, with maximum enzyme activity (~4 µmol/min/mL) observed on the fourth day, followed by a significant decline on the fifth.
Untreated rice bagasse, a more complex lignocellulosic substrate, exhibited initial resistance to degradation. However, cellulase activity gradually increased, reaching its peak (~0.37 µmol/min/mL) on the fifth day, suggesting a slower enzymatic response likely due to its recalcitrant structure. Similarly, corn cob demonstrated minimal cellulase production (~0.19 µmol/min/mL) until the fourth day, with enzyme activity dropping to half its initial value by the fifth day (Figure 5).
Figure 5. Effect of different lignocellulosic waste material (as a carbon source) on cellulase production
These findings indicate that readily available and less structurally complex carbon sources, such as cane sugar molasses and wheat bran, facilitate higher cellulase production in shorter timeframes. In contrast, complex lignocellulosic materials like rice bagasse and corn cob require extended incubation periods for enzyme induction, highlighting the necessity of pretreatment strategies for efficient bioconversion. This study underscores the potential of agro-waste valorization for sustainable cellulase production, particularly from substrates with high fermentable sugar content.
Characterization of cellulase enzyme
The cellulase activity of Geobacillus stearothermophilus TP-3 was measured at different pH (5-9) and temperatures (40-70 °C). Optimum cellulase activity was observed at pH 8.0 and 50 °C (Figure 6A, 6B). Moreover, the cellulase enzyme was active at 40-70 °C. Up to 15 minutes, a little increase in cellulase activity was seen at 50 °C and 60 °C. The cellulase enzyme retained ~68% of its original activity at different temperature ranges, 40-70 °C after 3 hours of incubation (Figure 6C). From these results, the cellulase seems to have considerably high thermostability at higher temperatures for a long incubation period.
Figure 6. Characterization of cellulase enzyme (A) Effect of reaction buffer pH on cellulase activity (100% = 9.2 μmol/min/mL); (B) Effect of reaction temperature on cellulase activity (100% = 9.45 μmol/min/mL); (C) Thermal stability profile of cellulase enzyme of Geobacillus sp. TP-3 (100% = 9. 68 μmol/min/mL)
The agricultural and industrial sectors together generate approximately 200 billion tons of lignocellulosic biomass annually, making lignocellulosic waste management a critical challenge in reducing environmental pollution, from air to soil.27 The abundance of cellulose in these materials has drawn attention for their potential conversion into valuable products. However, lignocellulosic biomass is highly recalcitrant, and its depolymerization remains a complex task for extracting fermentable sugars.28 While various physical and chemical methods can break down lignocellulose, these processes can be energy-intensive and environmentally unsustainable. As a result, lignocellulolytic enzymes offer a more eco-friendly alternative for the degradation of lignocellulosic biomass into desired sugars.29
Several microorganisms, including bacteria and fungi, have been identified to produce lignocellulolytic enzymes, which are essential for efficiently breaking down lignocellulosic materials.30 Among these enzymes, cellulases are particularly significant. They specifically target the β 1, 4- glycosidic bonds in cellulose fibers, releasing reducing sugars such as glucose. Thermophilic cellulases are of particular interest, as they can enhance the efficiency of combined bioprocessing methods and accelerate reaction rates during hydrolysis. In this context, the study isolated a novel indigenous thermophilic bacterium, Geobacillus sp. TP-3, which demonstrates potential for cellulase production at elevated temperatures. A comparison of Geobacillus sp. TP-3 cellulase with other cellulases is presented in Table 4.
Table (4):
Comparison of few thermophilic organisms reported in literature for cellulase production
Strain Name |
Substrate used as Carbon source |
pH |
Temp. (°C) |
Production time (h) |
Enzyme Activity |
|---|---|---|---|---|---|
B. licheniformis KBFB356 |
1% CMC |
7 |
50 |
72 |
4.06 IU/mL |
B. licheniformis NIBE2357 |
1% CMC |
7 |
55 |
24 |
20.3 U/mL |
Bacillus sp. PCH9452 |
1% CMC |
7 |
50 |
12 |
3.55 IU/mg |
Bacillus licheniformis TLW-358 |
1% CMC |
7 |
50 |
72 |
452.57 IU/mL/min |
Bacillus smithi NIBE1057 |
1% glucose |
– |
55 |
22 h |
14. 57 U/mL |
Bacillus sp. CX650 |
Wheat straw |
7 |
30 |
18 h |
524.66 U/mL |
Bacillus subtilis MC451 |
CMC (1%) |
7 |
60 |
NA |
10.37 U/mL |
Bacillus subtilis K-1859 |
2% potato peel pH 5 |
– |
50 |
24 |
350 IU/mL/min |
Brevibacillus borstelensis MX1851 |
CMC (1%) |
6 |
50 |
NA |
9.69 U/mL |
Geobacillus sp. KP4360 |
CMC (1%) |
6.5 |
55 |
18 |
0.186 U/mg |
Geobacillus sp. TP-110 |
Sugar Molasses (2%) |
8 |
50 |
20 |
1263 (U/L) |
Geobacillus stearothermophilus TP-3 (current study) |
Sugar Molasses (2%) |
8 |
50 |
24 |
9.60 (U/mL) |
Talaromyces thermophilus61 |
Kans grass (2%) |
6 |
50 |
72 |
~1.8 U/mL |
Geobacillus sp. T162 |
Barley straw (0.5%) |
6.5 |
50 |
24 |
143.5 U/mL |
Geobacillus sp. HTA42663 |
Sugarcane bagasse (1%) |
NA |
60 |
144 |
103.67 U/mL |
Geobacillus species are known for their ability to thrive at temperatures exceeding 45 °C, classifying them as thermophilic bacteria.31 The Geobacillus sp. TP-3 strain secretes cellulase when supplemented with 1% (w/v) carboxymethyl cellulose (CMC) as an inducer, indicating that it produces inducible enzymes. Similar substrate affinities for 1% (w/v) CMC have been reported in other strains, such as Cohnella sp. A0132 and Geobacillus sp. KP43.33 In this study, optimal cellulase production was observed at 50 °C, which aligns with previous reports on Bacillus subtilis K-18.34 This high-temperature tolerance enhances the strain’s applicability in industrial processes, particularly those requiring thermostable enzymes.
The ability of Geobacillus sp. TP-3 to function optimally at higher temperatures is an advantage, as many mesophilic enzymes denature under such conditions. This property makes it a promising candidate for industrial biotechnological applications.35 Furthermore, bacterial cellulases hold great potential for biofuel production from lignocellulosic biomass, offering a more sustainable and cost-effective alternative to traditional chemical methods. The culture medium for this isolate was optimized with a pH of 8.0, leading to maximum cellulase activity, and the enzyme’s performance under alkaline conditions enhances its potential for applications in industries like food, brewing, and wine production.
In terms of enzyme optimization, traditional methods such as the One-Factor-at-a-Time (OFAT) approach have been commonly used to optimize nutrient conditions for enhanced enzyme production.36-41 However, more sophisticated statistical methods like Plackett-Burman (PB) design are increasingly used to identify the most influential factors in enzyme production.42-44 In this study, PB design led to a five-fold increase in cellulase production. Similar approaches have been used for optimizing the production of other thermostable enzymes, such as recombinant xylanases40 and cellulases. Additionally, the use of alternative carbon sources, such as sugar cane molasses and wheat bran, showed significant improvements in cellulase production compared to purified carbon sources, offering a cost-effective solution for large-scale fermentation processes.44,45
Several studies have explored the use of agricultural waste as an alternative carbon source for cellulase productions.45,46 While untreated lignocellulosic waste materials, such as rice straw and corn cob, have demonstrated low cellulase production, treatment methods (acid/alkali/steam) can enhance enzyme production by inducing cellulase enzyme gene expression.47-49 For instance, Ahmad et al. reported a Bacillus sp. CX6 showed the highest ability to saccharify wheat straw.50 Brevibacillus borstelensis and Bacillus subtilis have been shown to achieve significant saccharification efficiencies using corncob as a substrate.51 In this study, Geobacillus sp. TP-3 exhibited optimal cellulase activity at 50 °C and pH 8.0, and its enzyme retained considerable stability, with 68% activity after 3 hours at elevated temperatures. The structural stability of cellulases is a key factor in their industrial application. Studies have shown that thermostable cellulases, such as those from Thermobifida fusca,52 Geobacillus sp. 70PC5353 and Dictyoglomus turgidum,54 maintain their activity even at elevated temperatures. This thermostability is essential for applications in industries that require high-temperature processing, such as textile, detergent, paper, and biofuel production.
The potential of Geobacillus sp. TP-3 was evaluated for saccharification of pretreated sawdust, with promising results. Approximately 50% saccharification was achieved using alkali-pretreated sawdust under optimized conditions of pH 5.5 and 50 °C.55 This indicates that Geobacillus sp. TP-3 is capable of efficiently breaking down the complex lignocellulosic structure of sawdust, particularly when the material undergoes alkali pretreatment, which enhances the accessibility of cellulose for enzymatic hydrolysis. The observed saccharification efficiency suggests that Geobacillus sp. TP-3 could be a viable candidate for industrial applications in biofuel production, particularly in processes involving lignocellulosic biomass as a feedstock. Additionally, the enzyme’s activity under mild acidic conditions (pH 5.5) and at relatively high temperatures (50 °C) highlights its robustness and potential for use in a variety of industrial processes where such conditions are common. These findings further emphasize the importance of optimizing pretreatment methods to enhance enzyme-substrate interactions and improve the overall efficiency of lignocellulosic biomass conversion.
Geobacillus stearothermophilus TP-3, isolated from the Tapovan hot spring, is a novel thermophilic lignocellulolytic bacterium with significant industrial potential due to its strong cellulolytic activity. Optimization strategies achieved a three-fold increase in enzyme yield, with the cellulase demonstrating exceptional thermo-alkali stability and effective degradation of agricultural residues. TP-3 is a promising biocatalyst for sustainable biomass valorization and bioethanol production.
Future research should focus on genetic engineering and optimizing metabolic pathways to enhance cellulase production. Additionally, scaling up fermentation processes and integrating TP-3-derived enzymes into industrial applications could boost bioethanol efficiency. Exploring co-culturing strategies and conducting detailed studies on TP-3 cellulases will facilitate its broader use in biofuel production and other industrial sectors requiring effective biomass degradation.
Additional file: Additional Table
ACKNOWLEDGMENTS
The authors acknowledge the non-UGC NET fellowship given to Meghna Arya and the UGC-SRF fellowship given to Garima Chauhan and Asha Kumari.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
DV and MS conceptualized and designed the study. MA and GC collected the sample, and carried out the experimental work. TF and AK assisted in experimental work. MA wrote the original draft. MS performed supervision. All authors reviewed, edited and approved the final manuscript for publication.
FUNDING
This study was supported by the Uttar Pradesh Council of Science & Technology through grant number CST/8275.
DATA AVAILABILITY
The 16S rRNA gene sequence information is available in the NCBI database repository, with Accession No. OP962434.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Vasic K, Knez Z, Leitgeb M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules. 2021;26(3):753.
Crossref - Ezeorba TPC, Okeke ES, Mayel MH, Nwuche CO, Ezike TC. Recent advances in biotechnological valorization of agro-food wastes (AFW): Optimizing integrated approaches for sustainable biorefinery and circular bioeconomy. Bioresour Technol Rep. 2024;26:101823.
Crossref - Espinoza-Vazquez YM, Hernandez-Camacho NV, Gomez-Castro FI. Agricultural Residues as Raw Materials for a Bio-based Industry. In: Norton A, Scheff D, Gilbertson LM, eds. ACS Symposium Series. American Chemical Society. 2023;1449:77-99.
Crossref - Ben-Othman S, Joudu I, Bhat R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules. 2020;25(3):510.
Crossref - Katsimpouras C, Kalogiannis KG, Kalogianni A, Lappas AA, Topakas E. Production of high concentrated cellulosic ethanol by acetone/water oxidized pretreated beech wood. Biotechnol Biofuels. 2017;10(1):54.
Crossref - Schultze-Jena A, Vroon RC, Macleod AKA, et al. Production of acetone, butanol, and ethanol by fermentation of Saccharina latissima: Cultivation, enzymatic hydrolysis, inhibitor removal, and fermentation. Algal Research. 2022;62:102618.
Crossref - Castaneda-Barreto A, Olivera-Gonzales P, Tamariz-Angeles C. A natural consortium of thermophilic bacteria from Huancarhuaz hot spring (Ancash-Peru) for promising lignocellulose bioconversion. Heliyon. 2024;10(5):e27272.
Crossref - Zarafeta D, Kissas D, Sayer C, et al. Discovery and Characterization of a Thermostable and Highly Halotolerant GH5 Cellulase from an Icelandic Hot Spring Isolate. PLoS ONE. 2016;11(1):e0146454.
Crossref - Sarangthem I, Rajkumari L, Ngashangva N, Nandeibam J, Yendrembam RBS, Mukherjee PK. Isolation and Characterization of Bacteria from Natural Hot Spring and Insights into the Thermophilic Cellulase Production. Curr Microbiol. 2023;80(2):64.
Crossref - Arya M, Chauhan G, Fatima T, Verma D, Sharma M. Statistical Modelling of Thermostable Cellulase Production Conditions of Thermophilic Geobacillus sp. TP-1 Isolated from Tapovan Hot Springs of the Garhwal Himalayan Mountain Ranges, India. Indian J Microbiol. 2024;64(3):1132-1143.
Crossref - Shyaula M, Regmi S, Khadka D, et al. Characterization of Thermostable Cellulase from Bacillus licheniformis PANG L Isolated from the Himalayan Soil. Comi G, ed. Int J Microbiol. 2023;2023(1):1-12.
Crossref - Korsa G, Konwarh R, Masi C, Ayele A, Haile S. Microbial cellulase production and its potential application for textile industries. Ann Microbiol. 2023;73(1):13.
Crossref - Kuhad RC, Gupta R, Singh A. Microbial Cellulases and Their Industrial Applications. Enzyme Res. 2011;2011:280696.
Crossref - Ejaz U, Sohail M, Ghanemi A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics. 2021;6(3):44.
Crossref - Sutaoney P, Rai SN, Sinha S, et al. Current perspective in research and industrial applications of microbial cellulases. Int J Biol Macromol. 2024;264(Pt 1):130639.
Crossref - Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, Tripathi CKM. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front Microbiol. 2017;7:2087.
Crossref - Venkatachalam M, Shum-Cheong-Sing A, Caro Y, Dufosse L, Fouillaud M. OVAT Analysis and Response Surface Methodology Based on Nutrient Sources for Optimization of Pigment Production in the Marine-Derived Fungus Talaromyces albobiverticillius 30548 Submerged Fermentation. Marine Drugs. 2021;19(5):248.
Crossref - Google maps (n.d) Tapovan Hotspring Uttarakhand, India. Available from: https://www.google.com/maps/@30.4665561,79.6594946,13z?entry=ttu&g_ep=EgoyMDI1MDQwNi4wIKXMDSoJLDEw MjExNDU1SAFQAw%3D%3D [Accessed February 25, 2025]
- Mandels M, Reese ET. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol. 1957;73(2):269-278.
Crossref - Zhang YH, Hong J, Ye X. Cellulase assays. Methods Mol Biol. 2009;581:213-31.
Crossref - Miller GL. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem. 1959;31(3):426-428.
Crossref - Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-254.
Crossref - Srinivasan R, Karaoz U, Volegova M, et al. Use of 16S rRNA gene for identification of a broad range of clinically relevant bacterial pathogens. PLoS ONE. 2015;10(2):0117617.
Crossref - Plackett RL, Burman JP. The Design of Optimum Multifactorial Experiments. Biometrika. 1946;33(4):305-325.
Crossref - Yahya S, Jahangir S, Shaukat SS, Sohail M, Khan SA. Production optimization by using plackett-burman design and partial characterization of amylase from Aspergillus tubingensis SY 1. Pak J Bot. 2016;48(6):2557-2561.
- Sakthivel M, Karthikeyan N, Jayaveny R, Palani P. Optimization of Culture Conditions for the Production of Extracellular Cellulase from Corynebacterium lipophiloflavum. J Ecobiotechnol. 2010;2(9).
- Lu H, Yadav V, Bilal M, Iqbal HMN. Bioprospecting microbial hosts to valorize lignocellulose biomass – Environmental perspectives and value-added bioproducts. Chemosphere. 2022;288(part 2):132574.
Crossref - Mujtaba M, Fraceto LF, Fazeli M, et al. Lignocellulosic biomass from agricultural waste to the circular economy: a review with focus on biofuels, biocomposites and bioplastics. J Clean Prod. 2023;402:136815.
Crossref - Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N. Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review. Sustainability. 2020;12(18):7282.
Crossref - Obeng EM, Adam SNN, Budiman C, Ongkudon CM, Maas R, Jose J. Lignocellulases: a review of emerging and developing enzymes, systems, and practices. Bioresour Bioprocess. 2017;4(1):1-22.
Crossref - Najar IN, Thakur N. A systematic review of the genera geobacillus and parageobacillus: Their evolution, current taxonomic status and major applications. Microbiology. 2020;166(9):800-816.
Crossref - Mohammadi S, Tarrahimofrad H, Arjmand S, Zamani J, Haghbeen K, Aminzadeh S. Expression, characterization, and activity optimization of a novel cellulase from the thermophilic bacteria Cohnella sp. A01. Sci Rep. 2022;12(1):10301.
Crossref - Khadka S, Khadka D, Poudel RC, et al. Production Optimization and Biochemical Characterization of Cellulase from Geobacillus sp. KP43 Isolated from Hot Spring Water of Nepal. BioMed Res Int. 2022;2022(1):6840409.
Crossref - Irfan M, Mushtaq Q, Tabssum F, Shakir HA, Qazi JI. Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Express. 2017;7(1):29.
Crossref - Gong G, Zhou S, Luo R, Gesang Z, Suolang S. Metagenomic insights into the diversity of carbohydrate-degrading enzymes in the yak fecal microbial community. BMC Microbiol. 2020;20(1):302.
Crossref - Czitrom V. One-factor-at-a-time versus designed experiments. American Statistician. 1999;53(2):126-131.
Crossref - Rath BP, Das S, Mohapatra PKD, Thatoi H. Optimization of extracellular chromate reductase production by Bacillus amyloliquefaciens (CSB 9) isolated from chromite mine environment. Biocatal Agric Biotechnol. 2014;3(3):35-41.
Crossref - Ahmed SA, Abdella MAA, El-Sherbiny GM, Ibrahim AM, El-Shamy AR, Atalla SMM. Application of one -factor- at-a-time and statistical designs to enhance a-amylase production by a newly isolate Bacillus subtilis strain-MK1. Biocatal Agric Biotechnol. 2019;22.
Crossref - Mishra R, Chandra R. Optimization of culture parameters for a-glucosidase production from suspension culture of moss Hyophilla nymaniana (Fleish.) Menzel. J Genet Eng Biotechnol. 2020;18(1).
Crossref - Verma D, Satyanarayana T. Production of cellulase-free xylanase by the recombinant Bacillus subtilis and its applicability in paper pulp bleaching. Biotechnol Prog. 2013;29(6):1441-1447.
Crossref - Khusro A, Kaliyan BK, Al-Dhabi NA, Arasu MV, Agastian P. Statistical optimization of thermo-alkali stable xylanase production from Bacillus tequilensis strain ARMATI. Electronic Journal of Biotechnology. 2016;22:16-25.
Crossref - Elleuche S, Antranikian G. Starch-Hydrolyzing Enzymes from Thermophiles. In: Satyanarayana, T., Littlechild, J., Kawarabayasi, Y. (eds) Thermophilic Microbes in Environmental and Industrial Biotechnology. Springer, Dordrecht. 2013:509-533.
Crossref - Gajdhane SB, Bhagwat PK, Dandge PB. Response surface methodology-based optimization of production media and purification of a-galactosidase in solid-state fermentation by Fusarium moniliforme NCIM 1099. 3 Biotech. 2016;6(2):260.
Crossref - Liaqat F, Sozer Bahadyr P, Elibol M, Eltem R. Optimization of chitosanase production by Bacillus mojavensis EGE-B-5.2i. J Basic Microbiol. 2018;58(10):836-847.
Crossref - Vanaja K, Rani RHS. Design of experiments: Concept and applications of plackett burman design. Clinical Research and Regulatory Affairs. 2007;24(1):1-23.
Crossref - Norsalwani TLT, Norulaini NAN. Utilization of Lignocellulosic Wastes as a Carbon Source for the Production of Bacterial Cellulases under Solid State Fermentation. Int J Environ Sci Dev. 2012;3(2):136-140.
Crossref - Xin F, Geng A. Horticultural waste as the substrate for cellulase and hemicellulase production by Trichoderma reesei under solid-state fermentation. Appl Biochem Biotechnol. 2010;162(1):295-306.
Crossref - Saratale GD, Kshirsagar SD, Sampange VT, et al. Cellulolytic Enzymes Production by Utilizing Agricultural Wastes Under Solid State Fermentation and its Application for Biohydrogen Production. Appl Biochem Biotechnol. 2014;174(8):2801-2817.
Crossref - Ja’afar JN, Shitu A. Utilization of Lignocellulosic Agro-Waste as an Alternative Carbon Source for Industrial Enzyme Production. In: Yaser, A.Z., Tajarudin, H.A., Embrandiri, A. (eds) Waste Management, Processing and Valorisation. Springer, Singapore. 2022; 2021:221-233.
Crossref - Ahmed AAQ, Babalola OO, McKay T. Cellulase- and Xylanase-Producing Bacterial Isolates with the Ability to Saccharify Wheat Straw and Their Potential Use in the Production of Pharmaceuticals and Chemicals from Lignocellulosic Materials. Waste Biomass Valor. 2018;9(5):765-775.
Crossref - Priyadharshini R, Brindha T, Uthandi S. Thermophilic microbes producing industrially important enzymes from the Manikaran geothermal springs of Himachal Pradesh (India) and their application in biomass saccharification. Biomass Conv Bioref. 2023;13(16):15161-15172.
Crossref - Posta K, Beki E, Wilson DB, Kukolya J, Hornok L. Cloning, characterization and phylogenetic relationships of cel5B, a new endoglucanase encoding gene from Thermobifida fusca. J Basic Microbiol. 2004;44(5):383-399.
Crossref - Chang CJ, Lee CC, Chan Y Te, et al. Exploring the mechanism responsible for cellulase thermostability by structure-guided recombination. PLoS ONE. 2016;11(3):0147485.
Crossref - Fusco FA, Fiorentino G, Pedone E, Contursi P, Bartolucci S, Limauro D. Biochemical characterization of a novel thermostable b-glucosidase from Dictyoglomus turgidum. Int J Biol Macromol. 2018;113:783-791.
Crossref - Arya M, Tiwari A, Sharma M. Pretreatment effects on the biomass composition and saccharification of wood sawdust. Biochem Cell Arch. 2024;24(1):235.
Crossref - Thankappan S, Kandasamy S, Joshi B, Sorokina KN, Taran OP, Uthandi S. Bioprospecting thermophilic glycosyl hydrolases, from hot springs of Himachal Pradesh, for biomass valorization. AMB Expr. 2018;8(1):168.
Crossref - Sharma S, Kumar SK. Exploration of Thermophilic Lignocellulolytic Enzymes Producing Bacterial Strains from Hot Springs of Western Himalayan Range. Indian J Microbiol. 2024.
Crossref - Kiran T, Asad W, Ajaz M, Hanif M, Rasool SA. Industrially relevant cellulase production by indigenous thermophilic Bacillus licheniformis TLW-3 strain: Isolation-molecular identification and enzyme yield optimization. Pak J Pharm Sci. 2018;31(6):2333-2340.
- Irfan M, Gonzalez CF, Raza S, et al. Improvement in thermostability of xylanase from Geobacillus thermodenitrificans C5 by site directed mutagenesis. Enzyme Microb Technol. 2018;111:38-47.
Crossref - Khadka S, Khadka D, Poudel RC, et al. Production Optimization and Biochemical Characterization of Cellulase from Geobacillus sp. KP43 Isolated from Hot Spring Water of Nepal. Li WJ, ed. BioMed Res Int. 2022;2022(1):6840409.
Crossref - Abdullah R, Tahseen M, Nisar K, et al. Statistical optimization of cellulases by Talaromyces thermophilus utilizing Saccharum spontaneum, a novel substrate. Electron J Biotechnol. 2021;51:79-87.
Crossref - Assareh R, Shahbani Zahiri HS, Akbari Noghabi KA, Aminzadeh S, Khaniki GB. Characterization of the newly isolated Geobacillus sp. T1, the efficient cellulase-producer on untreated barley and wheat straws. Bioresour Technol. 2012;120:99-105.
Crossref - Potprommanee L, Wang XQ, Han YJ, et al. Characterization of a thermophilic cellulase from Geobacillus sp. HTA426, an efficient cellulase-producer on alkali pretreated of lignocellulosic biomass. PLoS ONE. 2017;12(4):e0175004.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.