Pratibha Kumari1, 2, Babloo Sharma3*, Reena Kumari4 and B.R. Murya2
1International Rice Research Institute, Philippines-Cereal Systems Initiative for South Asia (CSISA, India office, Patna, Bihar)
2Department of Soil Science and Agricultural Chemistry, Banaras Hindu University, Varanasi-221005, India.
3Department of Soil and Water Conservation, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, Nadia-741 252, India.
4Agricultural Engineering Department, N. M. College of Agriculture,Navsari Agricultural University, Navsari-396450, India.
ABSTRACT
An incubation study was carried out during 2009-2010 in net house at BHU, Varanasi on alluvial soils, to investigation the consequences of enriching the soil with different organic amendments viz. FYM, sludge, poultry manure, fresh cow dung and Lantana Camera on microbial inhabitant, CO2 evolution, Soil microbial biomass carbon (SMBC) and C: N ratio of soil at 15,30 and 45 days of incubation (DAI).The results indicated a significant rise in microbial inhabitant, CO2 evolution, Soil microbial biomass carbon (SMBC) and C: N ratio. Microbial inhabitant under the sludge application (45 DAI) was significantly higher than that gained under other organic materials, while CO2 evolution, SMBC and C: N ratio was significantly higher under FYM application (45 DAI).Highest soil respiration was reported with FYM followed by sludge and fresh cow dung with increasing incubation periods. Highest value of CO2 evolution was observed in FYM (28 mg CO2) at 45 DAI. A marked increase in SMBC was recorded with application of FYM followed by sludge and fresh cow dung. Maximum SMBC occurred (392 µg g-1 soil) at 45 DAI. Treatment with FYM application found to be efficient in increasing C: N ratio and showed superior over all treatments.
Keywords: Organic amendments; Soil microbial inhabitant; CO2 evolution;
INTRODUCTION
Soil microbial biomass carbon; C: N ratio; Incubation period.Use of organic amendments is an integral part of sustainable agriculture1. Organic amendments supply nutrients and replenish the soil organic matter (OM) pool. According to several studies, organic materials improve soil chemical, physical and biological properties and thereby contribute to the maintenance of overall soil fertility and productivity2, 3. Addition of organic amendments is a suitable strategy to achieve soil recuperation in semi-arid areas, where the organic matter (OM) content and biological quality are low4. Applications of organic amendments can cause changes in the physical, chemical, and biological properties of soils. Applying organic amendments has been shown to increase soil microbial activity5, microbial diversity6, and bacterial densities7. Soil microbial communities are extremely diverse, and the relation between their diversity and function influences soil stability, productivity and resilience; on the other hand, organic matter, water activity, soil fertility, physical and chemical properties influence microbial biomass in soils8.
Organic amendments are materials that have ever been alive either as plant or animal added to a soil to improve its physical properties, such as water & nutrient retention, permeability, water infiltration, drainage, aeration, & structure. The goal is to provide a better environment for roots. Organic amendments are an eco-friendly in nature which can maintain the soil health in terms of soil biological fertility and productivity besides producing quality produce. Microbial biomass and microbial activity are closely related to soil organic matter content; they are positively influenced by organic amendment.
It is well established that microbial life only occupies a minor volume of soil localized in the hot spots such as the rhizosphere soil9 where microflora has continuous access to a flow of low- and high-molecular-weight organic substrates derived from roots. The rhizosphere is the soil volume surrounding the rhizoplane, and the term was first coined by Hiltner in 190410. The soil is the habitat of both fungi and bacteria, which have positive and negative effects on the growth and development of plants11. Microorganisms are largely responsible for the cycles of the elements within a soil and are involved in the decomposition of the organic matter at the ecosystem level via a variety of enzymes. In this sense, the addition of different organic amendments, such as solid organic wastes, sewage sludge, agricultural wastes, and animal manures, is a method of replenishing degraded soil quality through improvement of the biological status of the soil, which usually implies an increase in both microbial and enzyme activity12. This study was undertaken (i) to determine effect of organic amendments on microbial inhabitant, (ii) to investigate the effect of organic amendments on CO2 evolution, (iii) to evaluate effect of organic amendments on Soil microbial biomass carbon (SMBC) and (iv) to examine effect of organic amendments on C: N ratio of soil.
MATERIALS AND METHODS
Description of experiment and site
During 2009-2010 pot culture experiment was conducted in net house at Department of Soil Science and Agricultural Chemistry, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi (25.26º N latitude and 82.99º E longitude and 128.93 m above sea level) located in India. The area receives an average of 1100 mm of annual rainfall. For experiment, Agricultural Research Farm, Banaras Hindu University soil was used. The soil in the study area is an alluvial soil. Alluvial soil is formed by accumulated sediments transferred by the rivers and rapids, thus, it is amongst the most fertile soils. Alluvial soils are rich in potash, phosphoric acid, lime and organic matter but deficient in nitrogen and humus contents. The soils used in this experiment were collected after 15, 30 and 45 days of incubation (DAI) for laboratory analysis. The experiment was set up using complete randomized design with 6 treatments viz. control, FYM, poultry manure, fresh cow dung, sludge, Lantana camara replicated four times. Soil was amended with different organic amendments @10 tha-1.
Isolation and Counting of Microorganisms
The serial dilution plate technique13 was employed to enumerate the rhizosphere soil bacteria, actinomycetes and fungi. Thornton’s Medium14, Kenknight & Munaier’s medium15 and Martins Rose-Bengal-Streptomycin-Agar medium were used for isolation of total bacterial, Actinomycetes and fungal counts, respectively. After the incubation period, the colony-forming units were counted and expressed as cfu×106 g-1 of soil.
CO2 evolution
CO2 evolution was determined using 0.1 N KOH by Zibilske method16. The amount of CO2 evolution was calculated by using the following formula:
CO2 (mg) = (B-V) × (N×E)
Where, B= Volume (ml) of standard acid needed to titrate the trap solution from the empty flask (blank); V= Volume of HCl required to titrate sample reading; N= Normality of the acid in milli equivalents mL-1; E= 22 if the data is to be expressed as CO2 (i.e. mg CO2)
Soil Microbial biomass carbon (SMBC)
SMBC was measured by chloroform fumigation extraction17. Soil MBC was estimated from the relationship between C org extracted from fumigated and subtracted from non-fumigated soil samples by using following formula:
Where, ECF = Extractible carbon in fumigated soil samples; ECNF = Extractible carbon in non-fumigated soil samples; KEC = 0.25 ± 0.05
C: N ratio
The soil C org was analysed by wet-oxidation technique using by potassium dichromate, sulfuric acid and phosphoric acid18. N Total in soil was estimated by modified Kjeldahl method using alkaline potassium permanganate19.
Statistical Analysis
The data thus recorded were subjected to statistical analysis by adopting appropriate method of “Analysis of variance” by C.R.D. (Completely Randomized Design) as detailed by Chandel20. The significance of the treatment effects was judged with the help of “F” (variance ratio) test.
Table 1. Effect of organic amendment on Corg, Ntotaland C: N ratio at different DAI.
| Organic amendment | C org(%) | N total(%) | C: N ratio | ||||||
| 15 DAI | 30 DAI | 45 DAI | 15 DAI | 30 DAI | 45 DAI | 15 DAI | 30 DAI | 45 DAI | |
| Control | 0.30 | 0.33 | 0.34 | 0.17 | 0.19 | 0.20 | 1.60 | 1.71 | 1.77 |
| FYM | 0.35 | 0.39 | 0.41 | 0.20 | 0.22 | 0.23 | 1.72 | 1.84 | 1.87 |
| Sludge | 0.33 | 0.35 | 0.37 | 0.18 | 0.19 | 0.20 | 1.72 | 1.84 | 1.83 |
| Poultry manure | 0.31 | 0.35 | 0.36 | 0.19 | 0.19 | 0.21 | 1.64 | 1.71 | 1.78 |
| Fresh cow dung | 0.34 | 0.38 | 0.40 | 0.20 | 0.21 | 0.22 | 1.72 | 1.84 | 1.82 |
| Lantana camara | 0.31 | 0.34 | 0.36 | 0.20 | 0.21 | 0.21 | 1.54 | 1.64 | 1.70 |
| SEm± | 0.007 | 0.006 | 0.005 | 0.007 | 0.006 | 0.005 | 0.036 | 0.010 | 0.012 |
| CD (P=0.05) | 0.016 | 0.013 | 0.012 | 0.0150 | 0.014 | 0.012 | 0.075 | 0.021 | 0.025 |
| CD (P=0.01) | 0.020 | 0.017 | 0.010 | 0.020 | 0.017 | 0.014 | 0.10 | 0.028 | 0.034 |
RESULTS AND DISCUSSION
Microbial Inhabitant
The microbial counts were significantly influenced by application of organic material. The application of sludge, microbial population was recorded 83, 89 and 97 cfu×106 g-1 soil at 15, 30 and 45 DAI, respectively followed by FYM, Fresh cow dung, Poultry manure and Lantana. While, minimum microbial count was recorded 50, 49 and 52 cfu×106 g-1 soil at 15, 30 and 45 DAI in control, respectively (Fig. 1). Krishnakumar et al. observed that among all the treatments FYM had significantly more influence on the microbial population21. The attributed reason could be the enhanced Corg content of the soil as a result of organic material application.
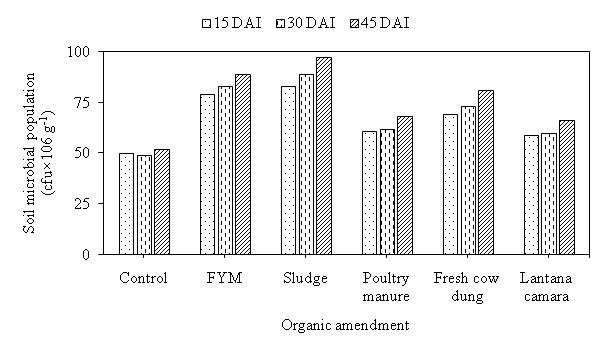
Fig. 1. Effect of organic amendmentsonsoil microbial population (cfu×106 g-1) at different days after incubation.
In microbial inhabitant, the maximum account was obtained by bacteria (> 50%) except sludge followed by actinomycetes and fungi in all treatments. The maximum bacterial count observed under FYM followed by sludge, fresh cow dung, poultry manure, Lantana camara and control during all incubation period (Fig. 2). Whereas, the maximum actinomycetes and fungal population recorded in sludge followed by FYM, fresh cow dung, poultry manure, Lantana camara and control during all incubation period. Cwalina-Ambroziak et al. found that the application of sludge increased fungal colony as compare to other organic and inorganic amendment22.
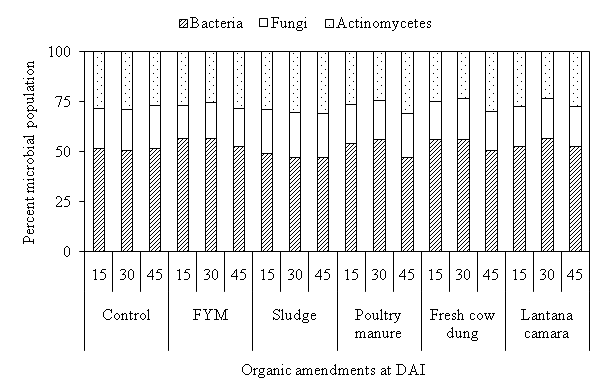
Fig.2. Effect of organic amendmentson percentage of different microbial population in soil at different days after incubation.
Evolution of carbon dioxide
Soil respiration is considered to reflect the availability of carbon for microbial maintenance. The maximum respiratory activity was recorded 16, 21 and 28 mg CO2 at 15, 30 and 45 DAI in FYM amendment, respectively (Fig. 3). The CO2 evolution significantly higher recorded in all treatment at 15 DAI whereas, During 30 and 45 DAI, Lantana camara was not significantly as compare to control and rest other treatment was significantly higher. Jaruhar stated that CO2 evaluation was higher with application of FYM amended due to be improves the physicochemical properties of soil and availability of substrate carbon23.
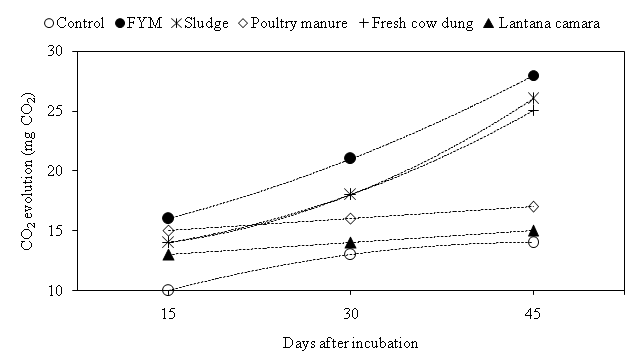
Fig. 3.Evolution of CO2 through microbial community under different organic materials during incubation.
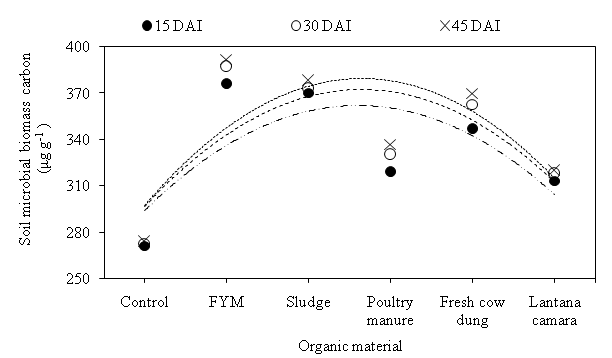
Fig. 4. Effect of organic materials on soil microbial biomass carbon (μg g-1 soil) at different days after incubation.
Soil microbial biomass carbon (SMBC)
The soil microbial biomass is involved in the decomposition of organic materials and thus, the cycling of nutrients in soils. The SMBC was largest in the FYM amended 376, 387 and 392 µg g-1 soil at 15, 30 and 45 DAI, respectively. At 15 DAI, SMBC of FYM amended was 1.60, 7.71, 15.16, 16.76 and 27.93% higher as compare to sludge, fresh cow dung, poultry manure, Lantana camara and control, respectively (Fig. 3). The SMBC recorded 373, 363, 330, 318 and 273 µg g-1 soil in sludge, fresh cow dung, poultry manure, Lantana camara and Control, respectively, at 30 DAI. At 45 DAI, the lowest SMBC found 274 µg g-1soil in control, which was 13.42, 15.05, 21.90 and 26.76 % lower than Lantana camara, poultry manure, fresh cow dung and sludge, respectively. Soil treated with FYM, compost, and other organic manure showed a significant increase in total C org and biomass C in response to the increasing amount of C org added. Application of organic amendments increased the soil MBC24. There is evidence that increasing inputs of crop residues increase soil organic matter and microbial biomass.
C: N ratio
The maximum C org was obtained FYM amended and significantly higher as compare to control at all incubation period. Maximum C org content (0.35%) was recorded in FYM treatment followed by fresh cow dung which appeared significantly higher when compared with control (0.30%), poultry manure and Lantana camara. The similar trend was followed at 30 and 45 DAI. This finding was in accordance to the observation of Goyal et al.25. They concluded that highest amounts of both carbon and nitrogen were recorded in soils receiving farmyard manure.
At 15 DAI, N total of soil significantly influenced by application of organic material. FYM, fresh cow dung and Lantana camara showed similar value (0.20%) of N total which was found significant over control (0.17%). Poultry manure (0.19%) and sludge (0.18%) found to be non-significant over control. The highest N total (0.22%) was observed with FYM followed by fresh cow dung, Lantana camara and they showed significant increase in N total over control, sludge, and poultry manure at 30 DAI. At 45 DAI, FYM amended recorded maximum N total (0.23%), it was higher 4.55, 9.52 and 15.00 % in fresh cow dung, Poultry manure/Lantana camara and Sludge/Control, respectively. Das and Dkhar elucidated the importance of soil C org, N total, and P avail, which were found to be greater in manure amendments, with a significant effect on the composition and quantity of the microbial community in soybean rhizosphere11.
The C: N ratio, which is an important tool of amendment evaluation, cannot explain all differences in nitrogen mineralization, since organic materials with similar C: N ratio may mineralize different amounts of nitrogen. C: N ratio increased significantly with application of organic material. At 15 DAI, FYM, sludge and fresh cow dung (1.72) caused increase in C: N ratio which was found non-significant over poultry manure (1.64) and control (1.60) but significant over Lantana camara (1.54). At 30 DAI, again FYM, sludge and fresh cow dung had similar C: N ratio (1.84) and was found to be significant over poultry manure or control (1.71) and Lantana camara (1.64). Lantana camara reported non-significant over control. FYM was recorded maximum C: N ratio (1.87) which showed its significant superiority to all rest treatment, followed by sludge (1.83), fresh cow dung (1.82), poultry manure (1.78), control (1.77) and Lantana camara (1.70) at 45 DAI. Treatment of soil with poultry manure and Lantana camara was found non-significant over control. Kirchner et al. also concluded that organic amendment increased the C org of the soil, whereas C org and C: N ratio significantly affect microbial community structure26.
CONCLUSION
Our results showed that the enrichment of soil with organic amendments resulted in higher soil microbial activity measured by soil respiration, SMBC, C org and N total. This was caused by the higher inputs of C org an energetic substrate for the present microbial communities that were activated to assure the turnover of indigenous nutrients. Among the amendments, FYM had more beneficial effects on soil respiration, SMBC and C: N ratio but only on when C org was in the state of depletion to provide energy for the other soil microorganisms, which in turn increased soil respiration. Treatment of soil with sludge resulted in significant changes on microbial inhabitant. It was also observed that all parameters were much more dependent on incubation periods.
REFERENCES
- Ajwa, H.A. and Tabatabai, M.A. Decomposition of different organic materials in soils. Bio. Fert. Soils, 1994; 18: 175-182.
- Topoliantz, S., Ponge, J.F. and Ballof, S. Manioc peel and Charcoal: a potential organic amendment for sustainable soil fertility in the tropics. Bio. Fert. Soils, 2005; 41: 15-21.
- Bhogal, A., Nicholson, F.A., Young, I., Sturrock, C., Whitmore, A.P. and Chambers, B.J. Effects of recent and accumulated livestock manure carbon additions on soil fertility and quality. Euro. J. Soil Sci., 2011; 62: 174-181.
- Bastida F., Kandeler, E., Moreno, J.L., Ros, M., García, C. and Hernández, T. Application of fresh and composted organic wastes modifies structure, size and activity of soil microbial community under semiarid climate, App. Soil Eco., 2008; 40: 318-329.
- Liu, B., and J. B. Ristaino. Microbial community structure in soils from organic and conventional agroecosystems. Phytopatho., 2003; 96: S. 53.
- Girvan, M.S., Bullimore, J., Ball, A.S., Pretty, J.N. and Osborn, A.M. Responses of active bacterial and fungal communities in soil under winter wheat to different fertilizer and pesticide regimens. App. Environ. Microbio., 2004; 70: 2692–2701.
- Van Bruggen, A.H.C. and Semenov, A. M. In search of biological indicators for plant health and disease suppression. App. Soil Eco., 2000; 15: 13–24.
- Tomich, T.P., Brodt, S., Ferris, H., Galt, R., Horwath, W.R., Kebreab, E., Leveau, J.H.J., Liptzin, D., Lubell, M., Merel, P., Michelmore, R., Rosenstock, T., Scow, K., Six, J., Williams, N., and Yang, L.: Agroecology: A Review from a Global-Change Perspective. Annual Review Enviro. Reso., 2011; 36: 193–222.
- Nannipieri, P., Ascher, J., Ceccherini, M.T., Landi, L., Pietramellara, G. and Renella, G. Microbial diversity and soil functions. Euro. J. Soil Sci., 2003; 54: 655–670.
- Brimecombe, M.J., De Lelj, F.A. and Lynch, J.M. The rhizosphere: The effect of root exudates on rhizosphere microbial populations. In The rhizosphere: Biochemistry and organic substances at the soil–plant interface, ed. R. Pinton, Z. Varanini, and P. Nannipieri, 95–140. New York: Marcel Dekker, 2001.
- Das, B.B. and Dkhar, M.S. Organic Amendment Effects on Microbial Population and Microbial Biomass Carbon in the Rhizosphere Soil of Soybean. Comm. Soil Sci. Plant Ana., 2012, 43: 1938–1948.
- Albiach, H., Canet, R., Pomares, F., Ingelmo, F. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioreso. Techn., 2000; 75: 43-48.
- Schmidt, E.L. and Caldwell, A.C. A practical manual of Soil Microbiology Laboratory Methods. Food and Agriculture Organization of the United Nations Soils Bull., 1967; pp. 72-75.
- Thornton, H.G. On the development of standardized agar medium for counting soil bacteria with special regard to the repression of spreading colonies. Ann. App. Bio., 1922; 2: 241-274.
- Rao, N.S.S. Soil Microbiol (Fourth Edition of Soil Microorganisms and Plant Growth), Science Publishers, New York, NY, USA, 1999.
- Zibilske, L.M. Carbon mineralization. Methods of Soil Analysis Part 2 Microbiological and Biochemical Properties. S.S.S.A., Madison, W.I., 1994; 835-863.
- Jenkinson, D.S. and Powlson, D.S. The effects of biological treatments on metabolism in soil. V.A method for measuring soil biomass. Soil Bio. Bioche., 1976; 8: 209-213.
- Walkley, A. and Black, C.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci., 1934; 37: 29-38.
- Subbiah, B.V. and Asija, G.L. A rapid procedure for the determination of available nitrogen in soil. Curr. Sci., 1956; 25: 259-260.
- Chandel, S.R.S. A Handbook of Agricultural Statistics, Achal Prakashan Mandir, Pandu Nagar, Kanpur, 1999.
- Krishnakumar S., Saravanan A., Natarajan S.K., Veerabadran V. and Mani S. Microbial Population and Enzymatic Activity as Influenced by Organic Farming. Rese. J. Agri. Biol. Sci., 2005; 1(1): 85-88.
- Cwalina-Ambroziak, B., Bowszys, T. and Wierzbowska, J. Fungi colonizing soil fertilized with composted sewage sludge and municipal waste. J. Elem., 2010; 15(1): 39–51.
- Jaruhar, H.B. Study of the effect of organic manure and earthworm (Pheretima posthuma) inoculation on quality of coal mine soil. Ind. J. Plant Sci., 2013; 2(1): 6-9.
- Mahmood, T., Azam, F., Hussain, F. and Malik, K.A. Carbon availability and microbial biomass in soil under an irrigated wheat–maize cropping system receiving different fertilizer treatments. Bio. Fert. Soils, 1997; 25: 63–68
- Goyal, S., Sakamoto, K., Kazuyuki, I. and Kamewada, K. Long-term effects of inorganic fertilization and organic amendments on soil organic matter and soil microbial properties in Andisols. Arc. Agro. Soil Sci., 2006; 52(6): 617 – 625.
- Kirchner, M.J., Wollum II, A.G., and King, L.D. Soil microbial populations and activities in reduced chemical input agroecosystems. J. Soil Sci. Soc. America, 1993; 57: 1289–1295.

