ISSN: 0973-7510
E-ISSN: 2581-690X
This research concerns the influence of contrasting tillage practices (Zero Tillage (ZT), Permanent Bed (PB) and Conventional tillage (CT)) in main plots and crop rotation (rice-wheat (R-W), rice-maize (R-M) and rice-lentil (R-L)) under rice based cropping system under split plot design with three replication, on soil microbial budgeting in terms of size and structure of microbial population, dehydrogenase activity (DHA), phosphatase activity and fluorescein diacetate (FDA) hydrolysis. Rice base cropping system is advocated as the dominant system prevailing in India due to the better suitability to its landforms and climatic conditions. Within tillage system, SOC was reported higher in zero tillage (0.67%), compared to permanent bed planting (0.66%) and CT (0.62%) at 0-15cm. At 15-30cm depth, zero tillage (0.57%) and PB (0.58%) registered significantly (P < 0.05) higher SOC compared to CT (0.52%). In this study, we estimated the microbial community size and structure, enzymatic activities, mychorrizal root infection and soil organic carbon (SOC). Root and rhizospheric soil samples were collected during two consecutive seasons from a 5 year old long term field experiment on conservation agriculture located at Research farm, Bihar Agricultural College, Sabour and still continuing. ZT treatment resulted higher soil organic carbon content (0.57%), viable microbial population (19.6% higher fungi, 10.63% bacteria, and 12.6% actinomycetes), dehydrogenase activity (5.3-9.11%), phosphatase activity (9.3-10.57%) which was at par with PB and differed significantly to that of CT treatment. Thus tillage practices and crop diversifications are the important factors affecting soil microbial community size and structure.
Zero tillage, permanent bed planting, cropping system, soil micro-flora.
Cereal–cereal systems, especially rice (Oryza sativa L.) –wheat (Triticum aestivum L.) (R-W) or rice- maize (Zea mays), contribute to satisfy the bulk of the food demand in South Asia. Rice followed by wheat or maize forms the predominant cropping systems in the subtropical areas of the Indo-Gangetic Plains (IGP), where rice prevails during the monsoon (kharif) season, while wheat, maize, and sometimes winter pulses like chickpea (Cicer arietinum) or lentil (Lens culinaris L.) are grown during the winter (Rabi) season. The rice-wheat system alone occupies 13.5 million ha of productive land in the IGP. Moreover, the area under rice-maize is also expanding rapidly, especially in the eastern IGP. Maize is the third most important cereal crop in India after rice and wheat. At present, maize cultivation during winter is becoming common in eastern India due to its higher productivity and profitability and grown in 1.2 million ha producing 5.5 mt of grain, with an average productivity of 4.0 t ha-1. In recent years, cereal production growth in terms of grain and residue has slowed with annual growth rates falling below 1% and staying well below annual population growth for the past decade (DACNET, 2014).
Today, the most successful resource conserving technology in the rice–wheat systems has been zero-tillage wheat, particularly in the Indian part of IGP. But now with the introduction of different modified planters and multi crop planters, zero tillage (ZT) has started to pick up pace both in terms of area under adoption and seeding of different crops like maize or lentil. ZT allows for timelier wheat establishment as it greatly reduces the turn-around time, allowing wheat establishment in a single pass almost immediately after the rice harvest. Moreover, the reduced turn-around time is reported to have allowed wheat planting to be advanced by 8– 25 days in Bihar. To date, the most widely adopted resource conserving technology in the Indo-Gangetic Plains (IGP) of South Asia has been zero-tillage (ZT) wheat after rice, particularly in India (Erenstein et al., 2008). Thus, conservation tillage, along with some complimentary practices such as soil cover and crop diversity (Corsi et al., 2012) has emerged as a viable option to ensure sustainable food production and maintain environmental integrity. This implies that conservation tillage is a component of conservation agriculture (CA). Corsi et al. (2012) define CA as a method of managing agro-ecosystems for improved and sustained productivity, increased profits and food security while preserving and enhancing the resource base and the environment. Crop management practices (tillage systems or cropping sequences) can affect soil health. Karlen et al. (2013) observed that deep soil ploughing with mouldboard plough had significant negative impact on soil health and quality parameters. Soil with better health and quality will be able to produce higher crop yield under favourable as well as extreme climatic conditions (Congreves et al., 2015), and soil health acts as a critical component for adaptation and mitigation of climate change effects by the crops (Congreves et al., 2015). Sandy loam (Typic Haplustept) soil is the most dominant soil texture of Indo Gangetic Plains of Bihar. The main production constraints of this type of soil are higher bulk density, poor water retention capacity, higher hydraulic conductivity, lower soil organic carbon and lower biological activities (Singh et al., 2016). Optimization of tillage practices lead to improvement in soil quality in various dimensions, including soil structure, soil fertility, and soil biological properties. Similarly, diversification in crop rotations can also affects soil quality by affecting carbon contents and soil biological activity, due to the difference in chemical composition of different crop residues that are added to soil (Srinivasarao et al., 2013).
Keeping in view the above facts in mind, the investigation was carried out to determine the effects of different tillage practices and intensive rice based crop rotations on organic carbon content and various biological traits in sandy loam (Typic Haplustept) soils of Bihar.
Experimental site
The study site was located at 25° 23’ N and 87° 07′ E, altitude of 37.19 m above mean sea level (AMSL) under Agricultural farm of Bihar Agricultural University. The field experiment has been started from 2011 till date and laid out in a split plot design of three replications with tillage being the main factor. It comes under sub – tropical climatic conditions characterized with hot desiccating summer, cold winter and moderate rainfall. May is the hottest month with an average maximum temperature of 35 – 39°C. January is the coldest month of the year with minimum temperature varying from 5 – 10°C. The annual rainfall of this region is around 1300 mm, precipitating mostly between mid June – mid October.
Crop establishment and management
There were three main plots of different contrasting rice establishment techniques viz. Zero tillage (ZT), permanent bed (PB) and conventional tillage (CT) and sub plot treatments comprising of three cropping systems viz. rice-wheat (R-W), rice-maize (R-M) and rice-lentil (R-L). In the ZT flat system and PB systems 30% anchored residues were retained at the end of each season while in CT system all the residues were removed and rice was puddle transplanted followed by seeding of rabi season crops after tillage. In CT, rice was transplanted at a spacing of 20cm × 15cm and in ZT; rice was direct seeded at an average row distance of 20cm. In CT and ZT planting of rabi crops, wheat was drilled within the rows of rice at a row distance of 20cm, maize was hand dibbled at a row to row and plant to plant distance of 60cm and 20cm while lentil was sown within the rice rows with row to row distance of 30cm. In the permanent beds (PB) with 30% residue retention, one row of rice, wheat and lentil was sown on either side of beds, 67.5 cm wide in the respective treatments while one row of maize was hand dibbled on top of each bed. In conventional tillage rice was puddle transplanted as hills (2 plants/hill) with a row to row and plant to plant distance of 20 and 15 cm while for the other crops the spacing was similar to that of ZT treatment. Rice (Oryza sativa L.) cv ‘Susk Samrat’, was grown during rainy (kharif) season while maize (Zea mays) cv ‘DHM-117’, wheat (Triticum aestivum) cv ‘HD-2888’ and lentil (Lens culinaris) cv ‘HUL-57’was sown during the rabi season.
Root infection by Mychorrhizal fungi
Root infection was assessed on a representative root sample taken from each plot at harvest. At harvest roots of 15 cm were taken from plants evenly distributed in each plot. Mycorrhiza infection of each plant was determined by estimating the percent of root segments colonised with AM with the method as described by Bierman and Linderman (1981).
Percent root infection was obtained as follows:
% Root infection = Number of root segment infected with AM / Total numberof segment X 100
Soil sampling and analysis
Soil sampling was performed in every season at the time of harvesting to minimize the effect of plant growth on microbial communities in order to observe the tillage treatment effect. Field-moist samples were transported to the laboratory on ice and then passed through a 2mm sieve within 24 hours. The soil samples were in laboratory using standard procedures. For organic carbon, available nitrogen, phosphorus and potassium were determined by Walkley and Black (1934), Subbiah and Asija (1956), Olsen et al. (1954) and Hanway & Heidel (1952). Total bacteria, fungi and actinomycetes population were estimated by following the serial dilution and plating techniques as described by Schmidt and Caldwell (1967). Dehydrogenase activity was also determined following the standard protocol by Casida et al., 1964 and Phosphomonoesterases (acid and alkaline phosphatases) activity was estimated as described by Tabatabai and Bremner (1969). All analyses were done in triplicate. The initial soil properties are depicted in Table 1.
Table (1):
Initial status (2011) of soil properties 0-15cm (soil depth) at the experimental site.
Soil Properties |
Value |
|---|---|
pH(1 : 2.5) |
7.35 |
EC(dSm-1) |
0.301 |
OC (%) |
0.53 |
Available nitrogen (kgha-1) |
160.2 |
Available phosphorus (kgha-1) |
26.7 |
Available potassium (kgha-1) |
221.6 |
Particle size distribution (%) Sand |
47.4 |
Silt |
32.6 |
Clay |
19.6 |
Statistical analysis
All the data analyzed for analysis of variance (ANOVA) technique as applicable to split-plot design (Gomez and Gomez 1984). The significance of the treatment effect was determined using F -test and to determine the significance of the difference between the means of the two treatments, least significant differences (LSD) were estimated at the 5% probability level. The differences were considered significant only when P<0.05.
Soil organic carbon (SOC) content
The conservation agriculture (CA) practices (PB and ZT) had significant (P < 0.05) effect on SOC content of surface soil layers (Table 2). The SOC content in PB (56%) and ZT (56%) plots were significantly (P < 0.05) higher than the CT (55%) plots for the 0-15 cm layer of soil depth. However, the SOC content of PB, ZT plots were statistically at par. The soil tilling increases organic matter decomposition and decreases carbon content by increasing organic matter oxidation (Six et al., 1999; Balasdent et al., 2001; Balota et al., 2003; Thomas et al., 2007). Aziz et al. (2015) had observed that No Tillage enhanced the total carbon by 30% and active carbon by 10% in corn-soybean-wheat cowpea rotation over CT. The result demonstrated higher carbon content over other crop rotations; this might be due to differences in quantity and chemical composition of crop residue biomass and/or root exudates among the crop rotations (Congreves et al., 2015). The crop rotations had significant (P < 0.05) interaction effect on SOC content of 0–15 cm soil layers (Table 2). R-L cropping system registered 0.58% and 0.59% higher SOC in kharif and rabi season compared to CT for above soil layer, respectively. Thierfelder et al. (2012) found 31% greater soil carbon by inclusion of cowpea and sunhemp in maize based crop rotations. Saha and Ghosh (2013) also reported the positive effects of legume residue application in cereal cropping systems on soil carbon content. The study by Gosai et al., (2009) revealed higher concentration of soil organic matter in the no-till and shallow-tilled plots compared to other conventionally tilled plots that confirms to the findings of Robbins and Voss (1991) and Angers et al. (1995).Increase in soil organic matter under no-tillage may have been a result of reduced contact of crop residues with soil. Surface residues tend to decompose more slowly than soil-incorporated residues, because of greater fluctuations in surface temperature and moisture and reduced availability of nutrients to microbes colonizing the surface residue (Schomberg et al., 1994).
Table (2):
Soil Organic carbon content.
| OC (%) | ||
|---|---|---|
| Kharif 14-15 | Rabi 14 | |
| Initial | 0.53 | 0.53 |
| T1– Zero tillage | 0.56 | 0.57 |
| T2– Permanent bed | 0.56 | 0.57 |
| T3-Conventional Method | 0.55 | 0.56 |
| CD (P=0.05) | 0.008 | 0.008 |
| Initial | 0.53 | 0.53 |
| S1– Rice-wheat | 0.55 | 0.56 |
| S2– Rice- maize | 0.55 | 0.55 |
| S3– Rice- Lentil | 0.58 | 0.59 |
| CD (P=0.05) | 0.009 | 0.009 |
Effect on soil biological properties
Microbial population dynamics
Soil microbial density as envisaged through the population of bacteria, actinomycetes and fungi observed in the experimental plots. It has been revealed by the observation that the microbial count was found higher in the plots of ZT which was statistically at par with PB and varied significantly when compared to CT. Retention of crop residue and minimal soil disturbance resulted in increased soil micro-flora populations both under ZT and PB.
Bacterial population
Bacterial population depends upon the management of crop residues and tillage operation as well. A significant higher population of total bacteria was detected in ZT treatment followed by PB systems which was 10.63 % and 7.6% higher than the CT treatment irrespective of all the cropping systems in rabi season (Table 3). The similar trend was also found in kharif season with a numerical increment. R-L cropping system proved well among all the other three cropping systems with insignificant difference and accounted 46.20 CFU *10-5 in rabi and 47.30 CFU *10-5 in kharif season in the rhizospheric soil. Reduced tillage indirectly defines the species composition of the soil microbial community by improving retention of soil moisture and modifying soil temperature (Krupinsky et al., 2002). The similar findings are also resulted by Sharma et al., 2011 and Helgason et al., 2009.
Table (3):
Microbial population dynamics.
| Treatments | Bacteria(CFU *10-5) | Fungi(CFU *10-4) | Actinomycetes (CFU *10-5) | |||
|---|---|---|---|---|---|---|
| Rice establishment techniques (Main Plot) | Rabi 14-15 | Kharif 15 | Rabi 14-15 | Kharif 15 | Rabi 14-15 | Kharif 15 |
| T1– Zero tillage | 45.47 | 46.70 | 19.53 | 20.37 | 33.17 | 34.80 |
| T2– Permanent bed | 44.20 | 45.23 | 17.43 | 18.27 | 31.50 | 33.17 |
| T3-Conventional Method | 41.10 | 42.10 | 16.33 | 16.67 | 29.47 | 30.27 |
| CD (P=0.05) | 1.19 | 1.42 | 1.68 | 2.15 | 1.19 | 1.03 |
| Cropping systems (Sub-plot) | ||||||
| S1– Rice-wheat | 42.07 | 42.90 | 17.63 | 18.17 | 31.37 | 32.83 |
| S2– Rice- maize | 42.50 | 43.83 | 16.00 | 16.77 | 28.60 | 30.00 |
| S3– Rice- Lentil | 46.20 | 47.30 | 19.67 | 20.37 | 34.17 | 35.40 |
| CD (P=0.05) | 2.36 | 2.73 | 2.52 | 2.71 | 1.59 | 1.64 |
Actinomycetes population
Maintaining cover crop residues on the surface or incorporation provides a stimulating substrate for microbial growth. The highest actinomycetes population was found in surface soils sampled from ZT and PB plots by 12.6% and 6.9% over CT in the rabi season which was relatively similar to kharif season with a slight increment (Table 3). From this table, it was also observed that actinomycetes population was at its higher site for the R-L system (34.17 CFU *10-5 in rabi and 35.40 CFU *10-5 in kharif) followed by R-M and R-W cropping systems. The highest microbial count has been resulted in the plots treated with substantially proved that it could be the resultant of the degradation of crop residues and slow release of nutrients with balanced C/N ratio and ensured a faster microbial proliferation. Mohammadi et al., 2011 and Sharma et al., 2011 also found the similar results to the current study.
Fungi population
In our study we found increasing fungi population by 19.6% and 6.73% in ZT and PB plots over CT in rabi season and was increased following the same trend in kharif season respectively. On the other hand, highest fungi count has been resulted in the plots of R-L cropping system (19.67 CFU *10-4 in rabi and 20.37 CFU *10-4 kharif) as because R-L system is a legume based cropping system receiving greater organic root exudates and organic acids in the rhizosphere caused a higher build-up of fungal density. The results corroborates with the findings of Helgason et al, 2009 and Sharma et al, 2011.
Dehydrogenase activity
Dehydrogenase (DHA) activity reflects the oxidative activity or intensity of metabolism of soil microflora and can be used as an indicator of microbial activity or populations in soils. Tillage and crop rotations and their interactions had significant (P < 0.05) effect on soil dehydrogenase enzyme activity of top soil layer (fig. 1). Higher DHA is the sign of stable soil health and higher microbial activity. The maximum soil DHA was recorded under ZT plots, which was significantly (P < 0.05) higher than CT plots and was statistically at par with PB treatment. The DHA in the rhizospheric soil was 9.11 and 5.30% higher in ZT and PB treatments compared to CT in rabi season, respectively. Similar trend was also resulted in rabi season with an increasing rate. The DHA was found maximum under R-L crop rotation (16.54 and 16.81 µg TPF/hr/g soil in rabi and kharif) when compared to R-M and R-W cropping system (fig. 1).This can be attributed to the organic carbon content of the soil. Tao et al. (2009) have also observed higher DHA in conservation agriculture with legume crop. Gajda et al. (2013) and Perez-Brandan et al. (2012) also reported higher soil microbial enzymatic activities due to conservation agriculture or legumes. Inclusion of legumes in the R-L system compared to other crop rotations might result in higher SOC due to faster and easier decomposition of lower C:N ratio residues and root nodules which resulted higher DHA activity (Srinivasarao et al., 2013).
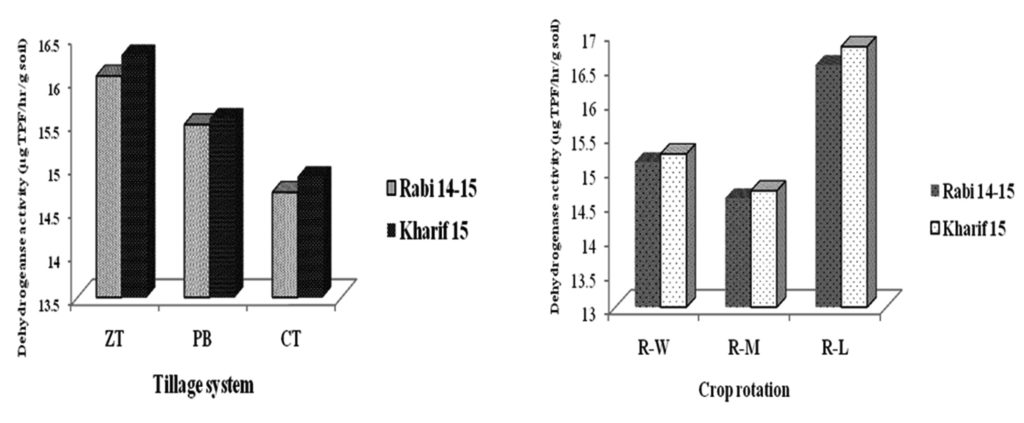 Fig. 1. Influence of tillage practices and crop rotations on dehydrogenase activity
Fig. 1. Influence of tillage practices and crop rotations on dehydrogenase activityPhosphatase activity
Similar to the dehydrogenase activity, phosphatase activity in the crop rhizosphere were assayed during maturity stage (after harvest) in the top soil layer. Results revealed that there was significant difference in enzymatic activities between ZT and PB in comparison with CT (Fig. 2). The maximum activity of acid and alkaline phosphatise activity (ALP) was recorded under ZT and PB which was 10.57 and 9.3% higher over CT, respectively. The minimum ALP activity was recorded under CT possibly due to lower SOC content. Amongst crop rotations; the R-L rotation demonstrated higher ALP activities (158.50 and 160.93 µg PNP released /g soil/hr in rabi and kharif) compared to R-M and R-W cropping system. The acid phosphatase activity has also followed the same trend which is clearly depicted in fig. 2. Dodor and Tabatabai (2003) observed differential activities of ALP under various crop rotations. They also found significant correlation of ALP with SOC. Zero tillage in a volcanic soil in Chile increased dehydrogenase, acid phosphomonoesterase and urease activities mainly in the 0-5 cm layer compared with a soil disk-harrowed to 20 cm (Alvear et al. 2005). Angers et al. (1993) reported 15% larger alkaline phosphatase activity in a barley-red clover rotation than in continuous barley on a clay soil in Quebec.
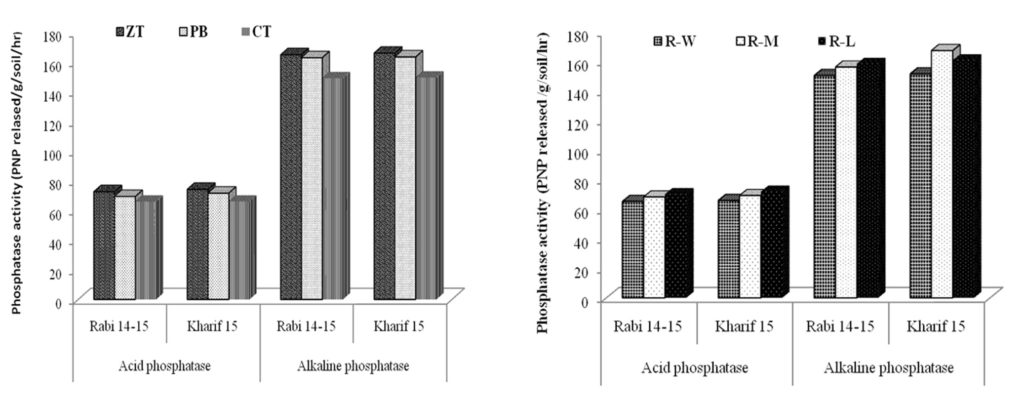 Fig. 2. Influence of tillage practices and crop rotations on phosphatase activity
Fig. 2. Influence of tillage practices and crop rotations on phosphatase activityMychorrhizal infection
Mycorrhiza is a mutualistic symbiosis between certain groups of soil fungi and most plant root systems (Hata et al., 2010). Mychorrhizal root colonization was significantly (P < 0.05) varied with tillage practices and crop rotations. The maximum root colonization was recorded under PB and ZT which was 18.96% and 13.86% higher compared to CT, respectively in rabi season (Fig. 3). The activity of Mychorrizal fungi was highest 61 % in R-M cropping system followed by the 50.67% in R-W and was lowest in R-L system (40.33%) during rabi season. The higher mycorrhizal colonization in maize could be due to the extensive root system of maize crop (Singh et al., 2015). There was any statistical difference for the root colonization in kharif season as because the crop was rice for every tillage systems.
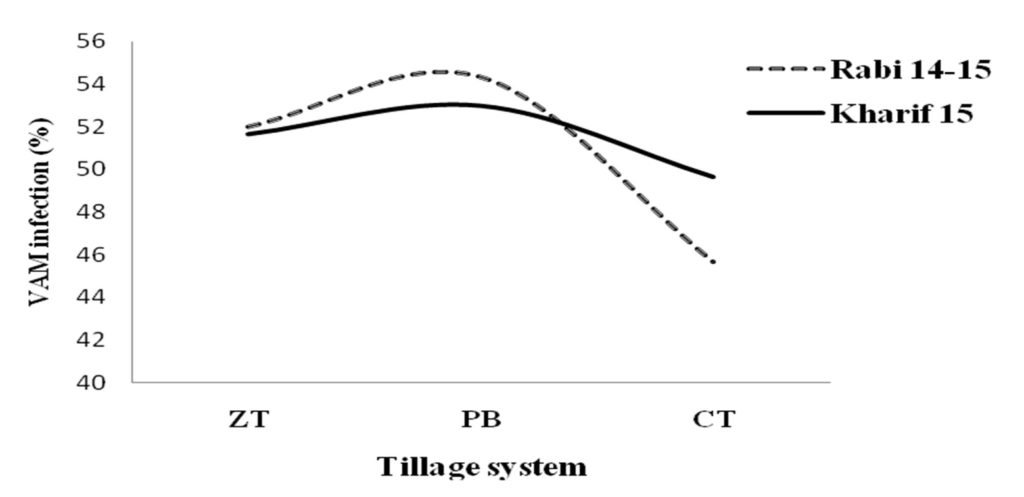 Fig. 3. Influence of tillage practices on root colonization by VAM
Fig. 3. Influence of tillage practices on root colonization by VAMTable (4):
Colonization of roots by VAM.
| Treatments | VAM infection (%) | |
|---|---|---|
| Cropping systems (Sub-plot) | ||
| S1– Rice-wheat | 50.67 | 51.00 |
| S2– Rice- maize | 61.00 | 52.33 |
| S3– Rice- Lentil | 40.33 | 51.00 |
| CD (P=0.05) | 4.22 | NS |
Correlation matrix and regression between soil biological attributes and soil organic carbon
The relationships of soil microbial population and organic carbon illustrated in Table 5. Organic carbon was positively and significantly correlated with bacterial population (r=0.842**), fungal population (r= 0.815**) and actinomycetes population (r = 0.864). From the result presented in table 5, size of the co-efficient of the multiple determinations (R2) indicated that 70.8% of bacterial population was determined by organic carbon. Similarly, 66.4% of fungal population was determined by organic carbon whereas 74.5% of available lead was determined by organic carbon.
Table (5):
Correlation and Linear Regression equation between soil organic content and microbial population.
Mocrobial Population |
Correlation matrix |
Linear Regression equation |
R2X 100 |
|---|---|---|---|
Bacteria population |
0.842** |
y = 133.3x – 30.85 |
70.8 |
Fungi population |
0.815** |
y = 98.94x – 37.55 |
66.4 |
Actinomycetes population |
0.864** |
y = 147.3x – 50.79 |
74.5 |
Where y= microbial population; x is soil organic carbon
** Significant at 1% of level of significance
It was also observed that with ZT followed by PB led to significant improvement in soil biological health. A significant higher population of microorganisms was detected in zero tillage ZT treatment followed by PB systems irrespective of all the cropping systems. Soils managed under zero tillage contained approximately 19.6% higher fungi, 10.63% bacteria, and 12.6% actinomycetes population. Enzymatic activity viz. Dehydrogenase (5.3-9.11%) and alkaline phosphatase (9.3-10.57%) activities also followed the same trend for the tillage systems. Among the rice based crop rotations, Rice –lentil system proved to be the best for the soil microbial activity and organic carbon status of the soil.
- Alvear, M.A., Rosas, J.L. and Rouanet, F.B. Effects of three soil tillage systems on some biological activities in an Ultisol from southern Chile. Soil Tillage Res., 2005; 82: 195–202.
- Angers, D.A. and Mehuys, G.R. 1993. Aggregate stability to water. In M.R. Carter (Ed.). Manual on soil sampling and methods of analysis. CRC Press, Boca Raton, Florida, 1993; pp 651-657.
- Angers, D.A., Carter, M.R., Gregorich, E.G., Bolinder, M.A., Donald, R.G., Voroney, R.P., Drury, C.F., Liang, B.C., Simard, R.R. and Beyaert, R.P. Agricultural management effects on soil carbon sequestration in eastern Canada. In: M. Beran, ed. Prospects for carbon sequestration in the biosphere. NATO ASI, Springer-Verlag, Berlin, Germany. 1995; pp 253–263.
- Aziz, I., Bangash, N., Mahmood, T. and Islam, K.R. Impact of no-till and conventional tillage practices on soil chemical properties. Pak. J. Bot., 2015; 47 (1), 297–303.
- Balasdent, J., Keyes, O., Krik, R. and Lessard, R. Standard procedure in the hydrometer method for particle size analysis. Commun. Soil Sci. Plant Anal., 2001; 32: 633–642.
- Balota E.L., Colozzi A., Andrade D.S. and Dick R.P. Microbial biomass in soils under different tillage and crop rotation systems. Biol. Fert. Soils, 2003: 38: 15–20.
- Biermann B. and Linderman R.G. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol., 1981; 87: 63-67.
- Casida, L.E.J., Klein, D.A. and Santaro, T. Soil dehydrogenase activity. Soil Sci., 1964; 98: 371–376.
- Congreves, K.A., Hayes, A., Verhallen, L.L. and Van Eerd, L.L. Long term impact of tillage and crop rotation on soil health at four temperate agroecosystems. Soil Tillage Res. 2015; 152: 17–28.
- Corsi, S., Friedrich,T., Kassam, A., Pisante,M., and deMoraesSà, J.C. Soil organic carbon accumulation and green house gas emission reductions from conservation agriculture: A literature view, integrated crop management. Rome:AGP/FAO, 2012; 16: 101.
- DACNET, The Directorate of Economics and Statistics (DES), an attached office of the Department of Agriculture and Cooperation, Ministry of Agriculture, Govt. Of India. 2014.
- Dodor, D. E and Tabatabai, M. A. Effect of cropping systems on phosphatases in soils. J. Plant Nut. Soil Sci., 2003; 166: 7–13.
- Doran, J.W. Microbial biomass and mineralizable nitrogen distributions in no-tillage and plowed soils. Biol. Fertil. Soils, 1987; 5: 68-75.
- Erenstein, O., Farooq, U., Malik, R.K. and Sharif, M. On-farm impacts of zero tillage wheat in South Asia’s rice-wheat systems. Field Crops Research, 2008; 105: 240-252.
- Gajda, A.M., Przewoka, B. and Gawryjoek, K. Changes in soil quality associated with tillage systemapplied. Int. Agrophys. 2013; 27: 133–141.
- Gomez, K.A. and Gomez, A.A. Statistical Procedure for agricultural Research. 2nd edition. John Wiley and So ns inc., New York (USA), 1984.
- Gosai, K., Arunachalam, A. and Dutta, B.K. Influence of conservation tillage on soil physicochemical properties in a tropical rainfed agricultural system of north- east India. Soil & Tillage Research 2009; 105 (1): 63–71.
- Hanway, J.J. and Heidel, H. Soil analyses methods as used in Iowa State College Soil Testing Laboratory. Iowa Agric. 1952; 57:1-31.
- Hata, S., Kobae, Y. and Banba, M. Interactions between plants and arbuscularmycorrhizal fungi. Int. Rev. Cell Mol. Biol. 2010; 281: 1–48.
- Helgason, B.L., Walley F.L. and Germida J. Fungal and bacterial abundance in long-term no-till and intensivetill soils of the northern great plains. Soil Sci. Soc. Am. J., 2009; 73 (1): 120.
- Karlen, D.L., Cambardella, C.A., Kovar, J.L. and Colvin, T.S. Soil quality response to long-term tillage and crop rotation practices. Soil Tillage Res. 2013; 133: 54–64.
- Krupinsky, J.M., Bailey, K.L., McMullen, M.P., Gossen, B.D. and Turkington, T.K. Managing Plant Disease Risk in Diversified Cropping Systems. Agronomy Journal 2002; 94: 198–209.
- Mohammadi, K., Eskandari, M., Heidari, G. and Nezhad, M. Canola traits and some soil biological parametersin response to fertilization and tillage management. Afr. J. Biotechnol., 2011; 10 (64): 140-167.
- Olsen, S., Cole, C., Watanabe, F. and Dean, L. Estimation of available phosphorus by extraction with sodium bicarbonate (Circular 39). Washington DC: USDA. 1954.
- Parihar, C.M., Yadav, M.R., Jat, S.L., Singh, A.K., Kumar, B., Pradhan, S., Chakraborty, D., Jat, M.L., Jat, R.K., Saharawat, Y.S. and Yadav, O.P. Long term effect of conservation agriculture in maize rotations on total organic carbon, physical and biological properties of a sandy loam soil in north-western Indo- Gangetic Plainshttp://dx.doi.org/10.1016/j.still. 2016.04.001 Soil and Tillage Research, 2016; 161: 116–128.
- Perez-Brandan, C., Arzeno, J.L., Huidobro, J., Grumberg, B., Conforto, C., Hilton, S., Bending, G.D., Meriles, J.M. and Vargas-Gil, S. Long-term effect of tillage systems on soilmicrobiological, chemical and physical parameters and the incidence of charcoal rot by Macrophomina phaseolina (Tassi) Goid in soybean. Crop Prot., 2012; 40: 73–82.
- Robbins, S. G. and Voss, R. D. Phosphorus and potassium stratification in conservation tillage systems. J. Soil Water Conserv., 1991; 46: 298-300.
- Saha, R. and Ghosh, P.K. Soil organic carbon stock, moisture availability and crop yield as influenced by residue management and tillage practices in maize-mustard cropping system under hill agro-ecosystem. Natl. Acad. Sci. Lett. 2013; 36(5): 461–468.
- Schmidt E.L. and Caldwell, A.C. A practical manual of Soil Microbiology Laboratory Methods. Food and Agriculture Organization of the United Nations. Soils Bulletin, 1967; 72-75.
- Schomberg, H.H., Steiner, J.L. and Unger, P.W. Decomposition and nitrogen dynamics of crop residues: residue quality and water effects. Soil Science Society of America Journal, 1994; 58: 372-381.
- Sharma P., Singh G. and Singh R. Conservation tillage, optimal water and organic nutrient supply enhance soil microbial activities during wheat (TriticumaestivumL.) cultivation. Braz. J. Microbiol., 2011; 42: 531.
- Singh, M., Beura, K., Pradhan, A.K., Rakshit, R. and Lal M. Ability of arbuscular mycorrhiza to promote growth of maize plant and enzymatic activity of an alluvial soil. J. App. and Nat. Sci. 2015; 7(2): 1029 – 1035.
- Singh, V.K., Singh, Y., Dwivedi, B.S., Singh, K.S., Majumdar, K., Jat, M.L. Mishra, R.P. and Rani, M. Soil physical properties, yield trends and economics after five years of conservation agriculture based rice-maize system in north-western India. Soil Tillage Res., 2016; 155: 133–148.
- Six, J., Elliott, E.T. and Paustain, K. Aggregate and soil organic dynamics under conventional and no-till systems. Soil Sci. Soc. Am. J., 1999; 68: 1350–1358.
- Srinivasarao, C., Venkateswarlu, B., Lal, R., Singh, A.K. and Kundu, S. Sustainable management of soils of dryland ecosystems of India for enhancing agronomic productivity and sequestering carbon. Adv. Agron., 2013; 121: 253–325.
- Subbiah, B. and Asija, G. A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci., 1956; 25: 259-260.
- Tabatabai, M.A. and Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem., 1969; 1: 301–307.
- Tao, J.J., Gui, J.F. and Zhang, Q.Y. Isolation and characterization of a rhabdovirus from coinfection of two viruses in mandarin fish. Aquaculture, 2009; 262: 1–9.
- Thierfelder, C., Cheesman, S. and Rusinamhodzi, L. A comparative analysis of conservation agriculture systems: Benefits and challenges of rotations and intercropping in Zimbabwe. Field Crop Research, 2012; 137: 237-250.
- Thomas, G.A., Dalal, R.C. and Standley, J. No-till effects on organic matter, pH, cation exchange capacity and nutrient distribution in a Luvisol in the semi-arid subtropics. Soil Tillage Res., 2007; 94: 295–304.
- Verhulst, N., Kienle, F., Sayre, K.D., Deckers, J., Raes, D., Limon-Ortega, A., Tijerina- Chavez, L. and Govaerts, B. Soil quality as affected by tillage-residue management in a wheat–maize irrigated bed planting system. Plant Soil, 2011; 340: 453–466.
- Walkley, A. and Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil science, 1934; 37: 29-38.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


