ISSN: 0973-7510
E-ISSN: 2581-690X
The cotton leaf worm Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) is considered one of the most harmful and destructive pest, not only for cotton crop, but also for corn, peanuts, vegetables, Lucerne, clover, ornamentals, shade trees and other types of crops and plants. The farmers used traditional chemical pesticides such as organophosphates, pyrethroids and organochlorines but the excessive amounts of these pesticides led to a severe environmental pollution. Bacillus thuringiensis bacteria were used as an ecofriendly biopesticide against cotton leaf-worm, but the main disadvantage in using bacteria was its low killing activity of the pest. Nanomaterials especially titanium dioxide nanoparticles are recently used as a nanopesticide toward several pests. In this study sodium titanate in the form of nanotubes and its composites with Bacillus Thuringiensis were examined to be used as a novel nanopesticides to resist cotton leaf-worm. Different biological features were studied for 2nd and 4th instars larvae such as adult longevity, adult sex ratio, pupation, fecundity and percent of eggs hatching (hatchability). All samples were characterized using Field emission scanning electron microscope (FESEM), X-ray diffraction (XRD), FTIR-spectroscopy and Zetasizer for zeta potential measurements.
Sodium titanate, Leaf worm, Nanopesticides, Bacillus Thuringiensis.
One of the most harmful pests which cause a great damage to several crops is the cotton leaf-worm Spodoptera Littoris (Boisd.) (Lepidoptera:Noctuidae) which is the major pest on cotton, corn, peanuts, vegetables, Lucerne, clover, ornamentals, shade trees and other types of crops and plants(Campion et al. 2009; Farag 2008). This worm puts hundreds of eggs in the form of egg masses and passes through six instars in its life cycle from 15-23 days (Miyahara et al. 1971). In Egypt, cotton leaf-worm is one of the most harmful and damaging pest for cotton crop and farmers used to resist it by using some traditional synthetic organophosphorus pesticides like pyrethroids and methyl parathion, but unfortunately the pest generations gained a resistance over the continuous use of these pesticides (Issa et al. 1984).
The extensive use of organochemical insecticides led to a serious types of environmental pollution (Bulmer et al. 2009; Ditta 2012; En et al. 1999; Yadav 2010). So, finding alternatives to replace these traditional pesticides has become a point of interest for a large number of researchers. Different safe alternative natural organisms have been recently used as biopesticides, from these are bacteria, fungi and viruses. Theses biopesticides have a unique mode of action(Ascher 1993; Rao et al. 1990; Thompson et al. 1999). These biopesticides are called entomopathogenic pesticides and the most used biological species from them are bacteria and fungi. They infect the digestive tract of pests(Rai and Ingle 2012).
Bacteria, Bacillus thuringiensis (Bt), is the most used biopesticide but inspite of all of these ecological and insecticidal advantages of (Bt), it has also disadvantages such as a lack of broad spectrum activity, slow rate of killing pests and low mortality percent (Hesketh and Hails 2015). The mortality % caused by (Bt) in a lab experiment was found to be about 10 % only after 48hr (Servin et al. 2015). Bacillus thuringiensis was used as a commercial pesticide in USA by Edward Steinhaus since 1958 (El-ghareeb 2015).
Nanotechnology is one of the new technologies used in the agriculture field which offers the synthesis of materials having a particle size in the nanoscale with new chemical, physical , optical and magnetic properties. In addition to these properties, it has also antimicrobial and antipesticidal activities toward a variety of micro-organisms and pests (Khot et al. 2012; Mohamed Ragaei and Sabry 2014; Servin et al. 2015). Nanosized particles have a wide applications in biological, physical, chemical, environmental, agricultural, industrial and pharmaceutical science(Biswal et al. 2012). Nano is a Greek word which means dwarf or very small and in scientific and mathematical field it is a metrical unit called nanometer which equals one billionth part of a meter (10-9 m) (Biswal et al. 2012). Nanomaterials exist in different formulations (suspension, emulsion, gels, polymer based, capsules and spheres) (Kah and Hofmann 2014) and morphologies (tubes, sheets, rods, fibers and wires). This variation gives these materials a variety of surface to volume ratios which has a great effect on surface activity. So, several nanomaterials especially those of metal oxides like; SiO2 , ZnO, CuO , MnO and Ag nanoparticles have been used as nanopesticides(Servin et al. 2015). Nanopesticides may be found in the form of creams, gels or liquids. It has a long shelf life time and more killing activity toward the targeted pests (Yang et al. 2007).
Bioinsecticides are currently studied more and more because of the possibility of their use in plant protection as an alternative method to the broad use of conventional toxic and polluting pesticides. On the other hand, nanomaterials in different formulations are now being used but over a limited range of use. Nanopesticides may consist of organic ingredients (polymers) and/or inorganic ingredients such as metal oxides in various forms (particles and micelles). The use of metallic nanoparticles like carbon (C), silver (Ag), copper oxide (CuO), zinc oxide (ZnO), iron oxide (FeO) and titanium oxides (TiO3, TiO2) are the most used nanoparticles in plant protection field (M. Ragaei and A. Sabry 2014). Take for example CdSe, Ag and TiO2 nanoparticles when used as nanopesticides against Spodoptera littoralis, it was found that they increase the larval mortality in the following trend CdSe, TiO2 and Ag respectively (M. Ragaei and A. Sabry 2014). Hydrophobic silica nanoparticles come to achieve high mortality percent at 300 and 350ppm concentrations, respectively, when they are used for tomato to resist Spodoptera littoralis (H. M. El-bendary and A. A. El-Helaly 2013). Silica nanoparticles leave no pesticidal residues in food or soil and can be used with other pest management activities (Laing, M. D. and M. C. Gatarayiha 2006).
The aim of the present work is to examine the use of sodium titanate nanotubes (TNTs) as a new nanopesticide and to enhance the activity of the ecofriendly Bacillus thuringiensis (Bt) based biopesticides via making nanocomposite between it and (TNTs) to be used as a nanopesticide against cotton leaf-worm Spodoptera littoris (Boisd.)(Lepidoptera:Noctuidae).
Chemicals
TiO2 powder was purchased from Luba Chemie-India, sodium hydroxide and hydrochloric acid were purchased from EL Nasr Company- Egypt and Bacillus was obtained from Biopesticides unit-Agricultural research center (ARC) – Egyptian ministry of agriculture.
Insect rearing
The cotton leaf worm, Spodoptera littoralis was reared in the laboratory for several generations at room temperature ranged between 25 – 28oC and 60 – 65 % R.H. Larvae were fed on castor bean leaves, Ricinus communis (L.) in wide glass jars until pupation period and adults emergence. The newly emerged adults were mated inside glass jars supplied with a piece of cotton wetted with 10% sugar solution as a feeding source for the emerged moths and branches of Tafla (Nerium oleander L.) or castor bean leaves as an ovipositor site (Mansour, N. A. 1966). Egg masses were kept in plastic jars until hatching. The obtained second and fourth instars larvae were used for bioassay tests. The bioassay evaluations were performed under the same laboratory condition for 12h photophase.
Synthesis of nanocompounds
Synthesis of sodium titanate nanotubes
All the reactants used were of analytical grade and were used without further purification. Five grams of pure bulk anatase TiO2 powder was mixed with 250 ml 10M aqueous NaOH solution under magnetic stirring for about 45 min till a milky white solutions were obtained. Then, the obtained solution were transferred to 500 ml capacity teflon-lined stainless steel autoclave, and after that temperature treatment was carried out at 16°C for 16 hr for the preparation of sodium titanate nanotubes. The autoclave chamber was allowed to cool down till it reached to room temperature. The formed white precipitate was collected and washed several times using distilled water(A A Farghali et al. 2014; Ahmed A. Farghali et al. 2016).
Synthesis of Bt-TNTs anate nanocomposite
The composite of sodium titanate with (Bt) was prepared in the ratio of 1:2gm nanomaterial and bacillus, respectively. The powders were mixed according to this ratio in 200 ml distilled water, sonicated for 10 min, stirred for 1hr using magnetic stirrer and finally dried at 50oC for 24 hr.
Characterization
Sodium titanate, Bacillus and their nanocomposite were characterized by X-ray diffraction (XRD), Field Emission Scanning Electron Microscope (FESEM), Zetasizer for zeta potential measurements and FT-IR spectroscopy.
Bioassay
A weighted amount of 0.25gm powder of sodium titanate (TNTs), Bacillus (Bt) and their nanocomposite (Bt-TNTs) was dissolved in 250 ml distilled water. After that we studied the impacts appeared on the different biological features of 2nd and 4th instars larvae of S. littoralis after feeding on castor bean leaves (Ricinus communis) (Schuster 1973) treated with these materials using dipping method. larval and pupal mortality, pupation and adult emergence %, larval and pupal duration, fecundity, eggs hatching, adult longevity and sex ratios were studied for 48hr. Four replicates (i.e. eight groups divided to four sets) each of 2nd and 4th instars of S. littoralis were prepared to contain 10 larvae for each replicate. The replicates were placed over a sawdust inside a transparent plastic can (10x10x4cm3). The larvae in the first three replicates were fed on the recinus leaves immersed in the prepared solutions of (TNTs), (Bt-TNTs) and Bacillus (Bt), respectively, whereas the larvae in the fourth replicate were fed on untreated leaves (control sample).
Statistical analysis
The total percent of larval mortality of 2nd and 4th instars larvae until pupation was recorded and corrected using Abbott’s formula(Abbott 1925) and different biological parameters were evaluated at the tested concentration. The obtained data of the biology were statistically analysed to determine F-value, P-value and L.S.D (least significant difference) at 0.05 and 0.01 degrees of freedom.
Physical characterization
Figure 1 shows the XRD patterns of all samples, it is clear from patterns that the mean peaks of the as prepared samples Na-titanate nanotubes (TNT); at 9.8o, 24.2o, 28.2o, 48.2o; are the characteristic peaks of the tubular titanate. This figure also shows the XRD pattern of Bacillus bacteria where many peaks are observed at 18o, 22o, 31.9o, 45.3o which may be attributed to the presence of crystalline proteins in this bacteria. While in case of Bt-TNT only the peaks of TNT and TNS can be observed with some change in their intensities and this may be attributed to the coverage of the Bacteria surfaces with TNT and TNS.
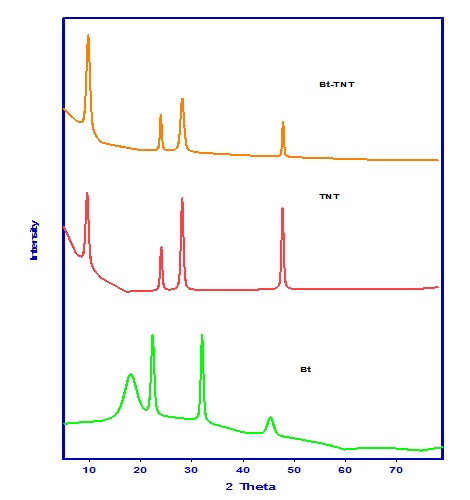
Fig. 1. XRD patterns of Bt, TNTs and Bt-TNTs nanocomposites.
Figure (2-a) shows (FESEM) picture of (TNTs) at the nanoscale appearing as agglomerations and bundles of nanotubes and figure (2-b) shows (FESEM) of Bacillus thuringiensis (Bt) colony. Figure (2-c) shows (FESEM) picture of (Bt-TNTs) nanocomposite and demonstrates the adsorption of nanotubes on the bacterial surfaces revealing the success of interaction between them and explains the difference in the bacterial activity after composite formation.

Fig. 2. FESEM monographs of a-) TNTs, (b) Bt and (c) Bt-TNTs.
Latent effect
Total mortality, pupation and emergence
The data presented in Table (1) show 20% total mortality percent for both 2nd and 4th instars in case of (BT-TNTs) composite while Bacillus Thuringiensis (Bt) resulted in 30% total mortality compared to 0% in case of control. The table also illustrate that all treatments showed 100% pupation except (Bt) which caused 90% pupation for the 4th instar.
Table (1):
Effect of different samples on larval and pupal mortality %, pupation and emergence % of the 2nd and 4th instars of Spodoptera littoralis
| Samples | % of larval mortality | % of Pupal mortality | Total Mortality % | Pupation % | Emergence % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2nd instar | 4th instar | 2nd instar | 4th instar | 2nd instar | 4th instar | 2nd instar | 4th instar | 2nd instar | 4th instar | |
| (TNTs) | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 |
| ( Bt-TNTs) | 0 | 0 | 20 | 20 | 20 | 20 | 100 | 100 | 80 | 80 |
| (Bt) | 0 | 10 | 0 | 20 | 0 | 30 | 100 | 90 | 100 | 70 |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 |
It is obvious also from the data presented in table (1) that (Bt-TNTs) resulted in 80 % emergence for both 2nd and 4th instars. There was also a decrease in emergence percent to be 70 % for the 4th instar in case of (Bt) alone compared to 100 % emergence for both 2nd and 4th instars in case of control sample. There was no effect on the emergence % after using (Bt) for the 4th instar and (TNTs) for both instars.
Larval and Pupal duration %
The data presented in table (2) illustrate the deviation in larval duration period compared to that of control sample. The use of (TNTs) led to 4 and 25 % decrease in larval duration for 2nd and 4th instars, respectively. On the other hand (Bt-TNTs) caused 11% increase in larval duration period for the 2nd instar. Bacillus Thuringiensis (Bt) treatment led to 16 and 18% larval duration increase for 2nd and 4th instars, respectively, compared to control. The table also illustrates that (TNTs) treatment resulted in 7 and 18% pupal duration increase for the 2nd and 4th instars, respectively, while its composite (Bt-TNTs) led to 32% pupal duration increase for the 4th instar. There was no effect on the pupal duration % after using (Bt).
Table (2):
Effect of different samples on larval and pupal duration and malformations of 2nd and 4th instars of S. littoralis
| Samples | Larval duration Mean +S.D. (Days) | Larval malfo. % | Pupal duration Mean +S.D. (Days) | % Pupal malfo. | Adult malfo. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2nd instar | 4th instar | 2nd instar | 4th instar | 2nd instar | 4th instar | 2nd instar | 4th instar | 2nd instar | 4th instar | |
| (TNTs) | 18.5+3.8 n.s. | 14.2+0.6 | 0 | 0 | 18+3.1 | 20.1+1.5 | 0 | 0 | 20 | 30 |
| i ** | n.s. | ª** | ||||||||
| (Bt-TNTs) | 21.3+3.5* | 18.9+2.1 | 0 | 0 | 18.6+1.5 | 22.4+1.6 | 0 | 0 | 0 | 0 |
| n.s | ** | c ** | ||||||||
| (Bt) | 22.2+4.3 | 22.3+5.2 | 0 | 0 | 16.8+2.3 | 17+4.4 | 0 | 0 | 0 | 12.5 |
| ** | ** | n.s | n.s | |||||||
| Control | 19.2+1.6 | 18.9+1.5 | 0 | 0 | 16.9+0.3 | 0.317 | 0 | 0 | 0 | 0 |
| F value | 7.438 | 17.75 | 42.941 | 95.546 | ||||||
| P value | 0.0298 | 0.03 | 0.00922 | 0.01 | ||||||
| L.S.D at 0.05 | 1.513 | 1.48 | 0.6375 | 0.892 | ||||||
| 0.01 | 2.128 | 2.1 | 0.9275 | 1.268 | ||||||
Malformations
Table (2) shows that there was no effect of all samples on the larval and pupal malformation of both 2nd and 4th instars. TNTs resulted in 20 and 30 % adult malformation for 2nd and 4th instars, respectively, while (Bt) caused 12.5 % adult malformation for the 4th instar. Bt-TNTs showed no effect on the adult malformation %.
Adult fecundity and hatchability %
Table 3 show that (TNTs) resulted in 37 and 72 % adult fecundity decrease for 2nd and 4th instars, respectively, while (Bt-TNTs) resulted in 42 % fecundity increase and 82 % fecundity decrease for 2nd and 4th instars, respectively. On the other hand, (Bt) alone resulted in 79 and 86 % adult fecundity decrease for 2nd and 4th instars, respectively, compared to control.
Table (3):
Effect of different samples on adult fecundity and eggs hatchability
| Samples | Adult fecundity (eggs/female) Mean+S.D. | Eggs hatchability % | ||
|---|---|---|---|---|
| 2nd instar | 4th instar | 2nd instar | 4th instar | |
| (TNTs) | 71.7+30* | 37.3+32** | 34.9 | zero |
| (Bt-TNTs) | 160+53n.s | 24+3.6** | zero | 16.7 |
| (Bt) | 25+16** | 19.3+10.1** | 76.7 | zero |
| Control | 113+23 | 133.3+2 | 100 | 100 |
| F value | 127.0408 | 33.0572 | ||
| P value | 0.02 | 0.0324 | ||
| L.S.D at 0.05 | 17.77 | 41.1417 | ||
| 0.01 | 32.622 | 75.518 | ||
** = Highly Significant (p<0.01)
* = Significant (p<0.05)
S.D.=Standard deviation L.S.D. = Least significant difference
L.S.D. = Least significant difference.
s = nonsignificant (p>0.05)
The data presented in table 3 show 34.9 % hatchability for the 2nd instar after using (TNTs) and 16.7 % hatchability for the 4th instar after using (Bt-TNTs) while (Bt) resulted in 76.7 % hatchability for the 2nd instar only compared to 100 % in case of control.
Adult longevity and Sex ratio
The data presented in table 4 illustrate 12 and 4% adult longevity decrease for 2nd and 4th instars, respectively, after using (TNTs) whereas (Bt-TNTs) caused 33% adult longevity increase and 22% decrease for 2nd and 4th instars, respectively. The treatment using (Bt) resulted in 7% adult longevity increase and 7% decrease for 2nd and 4th instars, respectively, compared to that of control.
Also the data shown in table 4 illustrate the effect of the prepared nanomaterials on the adult sex ratio of females (F) and males (M). The adult sex ratio was (30 F : 70 M), (62.5 F : 37.5 M) and (60 F : 40 M) after using (TNTs), (Bt-TNTs) and (Bt), respectively, for the 2nd instar compared to (40 F : 60 M) in case of control. It is obvious also from the table that the sex ratio of the 4th instar was (40 F : 60 M), (62.5 F : 37.5 M) and (50 F : 50 M) after using (TNTs), (Bt-TNTs) and (Bt), respectively, compared to (60 F : 40 M) of control.
Table (4):
Effect of different samples on adult longevity and sex ratios
| Samples | Adult longevity Mean+S.D. | Sex ratios % | ||||
|---|---|---|---|---|---|---|
| 2nd instar | 4th instar | 2nd instar | 4th instar | |||
| Females | Males | Females | Males | |||
| (TNTs) | 7.5+0.7 n.s | 7.8+0.9 n.s | 30 | 70 | 40 | 60 |
| (Bt-TNTs) | 10.9+2ͩ ** | 6.3+1.6** | 62.5 | 37.5 | 62.5 | 37.5 |
| (Bt) | 8.8+1.5n.s | 6.3+2** | 60 | 40 | 50 | 50 |
| Control | 8.2+1.0 | 8.1+1.7 | 40 | 60 | 60 | 40 |
| F value | 20.2557 | 10.8188 | ||||
| P value | 0.0233 | 0.022965 | ||||
| L.S.D at 0.05 | 0.77 | 1.05 | ||||
| 0.01 | 1.102 | 1.502 | ||||
ͣ ** = Highly Significant (p<0.01) increase
ͩ ** = Highly Significant (p<0.01) decrease
* = Significant (p<0.05)
S.D. =Standard deviation
L.S.D. = Least significant difference
n.s. =none Significant (p>0.05)
All the previous results match to a great extend those results obtained by Salama et al. (1981) who studied the effect of Bacillus Thuringiensis Kurustaki (Bt) on some biological parameters of three Lepidopterous cotton pests such as larval and pupal mortality, larval and pupal period which are increased on using (Bt) specially at low toxin concentration (Salama et al. 1981). Also, pupal and larval weight are reduced under the effect of (Bt) treatment (Arshad et al. 2009; Ashfaq et al. 2001; Salama et al. 1981). In addition to that, weak effect of (Bt) on fecundity, hatchability, pupal and adult deformation was observed (Abdel-Rahim 2011; Salama et al. 1981). Moreover, the bacterial nanocomposites show an improvement in the larval activity and a stronger effect on the pest different biological features than bacteria alone.
The present work proved the promising use of sodium titanate nanotubes and its nanocomposite with Bacillus Thuringiensis as a new nanopesticide against cotton leaf-worm Spodoptera littoralis (Boisd.) (Lepidoptera:Noctuidae) and how they affected the different biological features such as larval and pupal duration, adult fecundity, larval and pupal malformation, adult longevity and adult sex ratio.
- Abbott, WS. “A Method of Computing the Effectiveness of an Insecticideþ.” J. econ. Entomolþþ 1925; 18(2): 265–67.
- Ascher, K R Simon. “Nonconventional Insecticidal Effects of Pesticides Available from the Neem Tree, Azadirachta Indica.” Archives of Insect Biochemistry and Physiology, 1993; 22(3–4): 433–49.
- Arshad, Muhammad, Anjum Suhail, M. J. Arif, and M. A. Khan. “Transgenic-Bt and Non-Transgenic Cotton Effects on Survival and Growth of Helicoverpa Armigera.” International Journal of Agriculture and Biology, 2009; 11(4): 473–76.
- Abdel-Rahim, E. F. M. “‘Latent Effect of Microbial Insecticides against the Second Instar Larvae of the Cotton Leafworm, Spodoptera Littoralis Boisd.’” Egyptian Journal of Agricultural Research 2011.
- Bulmer, Mark S et al. “Targeting an Antimicrobial Effector Function in Insect Immunity as a Pest Control Strategy.” Proceedings of the National Academy of Sciences of the United States of America, 2009; 106(31): 12652–57.
- Biswal, SK, AK Nayak, UK Parida, and PL Nayak. “Applications of Nanotechnology in Agriculture and Food Sciencesþ.” IInternational journal of science innovations and discoveries þ, 2012; 2(1): 21–36.
- Campion, D. G., B. W. Bettany, J. B. McGinnigle, and L. R. Taylor. “The Distribution and Migration of Spodoptera Littoralis (Boisduval) (Lepi-Doptera: Noctuidae), in Relation to Meteorology on Cyprus, Interpreted from Maps of Pheromone Trap Samples.” Bulletin of Entomological Research, 2009; 67(3): 501–22.
- Ditta, Allah. “How Helpful Is Nanotechnology in Agriculture?” Advances in Natural Sciences: Nanoscience and Nanotechnology, 2012; 3(3): 33002.
- En, Risø-r-, Edited S G Hanson, P M Johansen, and L Lading. “Annual Progress Report for 1998.” 1100(May): 519–25.
- El-ghareeb, Doaa K. 2015. “Bioinsecticide Bacillus Thuringiensis a Comprehensive Review.” (December).
- Farag, N. A. “Susceptibility of the Cotton Leafworm, Spodoptera Littoralis 3rd Instar Larvae to Some Bio-Insecticides (Lepidoptera: Noctuidae).” Egyptian Journal of Biological Pest Control, 2008; 18(2): 343–46.
- Farghali, A A, A H Zaki, M H Khedr, and Beni Suef. “Hydrothermally Synthesized TiO 2 Nanotubes and Nanosheets for Photocatalytic Degradation of Color Yellow Sunset.”, 2014; 2(7): 285–91.
- Farghali, Ahmed A., Ayman H. Zaki, and Mohamed H. Khedr. “Control of Selectivity in Heterogeneous Photocatalysis by Tuning TiO2 Morphology for Water Treatment Applications.” Nanomaterials and Nanotechnology, 2016; 6: 1.
- H. M. El-bendary and A. A. El-Helaly. “First Record Nanotechnology in Agricultural/ : Silica Nano- Particles a Potential New Insecticide for Pest Control.” 2013; 4(3): 241–46.
- Hesketh, Helen, and Rosemary S Hails. “Bacillus Thuringiensis Impacts on Primary and Secondary Baculovirus Transmission Dynamics in Lepidoptera.” Journal of Invertebrate Pathology, 2015; 132: 171–81.
- Issa, YH et al. “Survey of Resistance to Organophosphorus Insecticides in Field Strains of the Cotton Leafworm during 1980- 1984 Cotton-Growing Seasons.” Bulletin of the Entomological Society of Egypt, 1984; 14: 399–404.
- Khot, L. R. et al. “Applications of Nanomaterials in Agricultural Production and Crop Protection: A Review.” Crop Protection 2012; 35: 64–70.
- Kah, Melanie, and Thilo Hofmann. “Nanopesticide Research/ : Current Trends and Future Priorities.” Environment International, 2014; 63: 224–35.
- Laing, M. D., M. C. Gatarayiha, and A. Adandonon. “Silicon Use for Pest Control in Agriculture: A Reviewþ.” Proceedings of the South African Sugar Technologists’Association, 2006; 80: 278–86.
- Mansour, N. A., et al. “Toxicological Studies on the Egyptian Cotton Leaf Worm, Prodenia Litura. VI. Potentiation and Antagonism of Organophosphorus and Carbamate Insecticides.” Journalof economicentomology, 1966; 59(2): 307–11.
- Miyahara, Y., A. Tanaka, and T. Wakikado. “Seasonal changes in the number and size of the egg masses of Prodenia litura.” Japanese journal of applied entomology and zoology 1971.
- Muhammad Ashfaq, S. Y. Young, And R. W. Mcnew. “Larval Mortality and Development of Pseudoplusia Includens (Lepidoptera: Noctuidae) Reared on a Transgenic Bacillus Thuringiensis-Cotton Cultivar Expressing CryIAc þ.” J. Econ. Entomol. 2001; 94(5): 1053–58.
- Rao, N V, A S Reddy, and P S Reddy. “Relative Efficacy of Some New Insecticides on Insect Pests of Cotton.” Indian Journal of Plant Protection 18: 53–58 ST–Relative efficacy of some new insectic 1990.
- Rai, Mahendra, and Avinash Ingle. “Role of Nanotechnology in Agriculture with Special Reference to Management of Insect Pests.” Applied Microbiology and Biotechnology, 2012; 94(2): 287–93.
- Ragaei, Mohamed, and Al-kazafy Hassan Sabry. “Nanotechnology for Insect Pest Control.” International Journal of Science, Environment and Technology, 2014; 3(2): 528–545.
- Schuster, Silvia. “Sneh, Silvia Schuster •.”, 1973; 26(2): 179–90.
- Salama, H.S. et al. “Development of Some Lepidopterous Cotton Pests as Affected by Exposure to Sublethal Levels of Endotoxins of Bacillus Thuringiensis for Different Periods.” Journal of Invertebrate Pathology, 1981; 38(2): 220–29.
- Servin, Alia et al. “A Review of the Use of Engineered Nanomaterials to Suppress Plant Disease and Enhance Crop Yield.” Journal of Nanoparticle Research, 2015; 17(2): 21.
- Thompson, Gary D, Scott H Hutchins, and Thomas C Sparks. “Development of Spinosad and Attributes of a New Class of Insect Control Products.” Radcliffe’s IPM World Textbook: 1999.
- Yang, Fan et al. “The Improvement of Spinach Growth by Nano-Anatase TiO2 Treatment Is Related to Nitrogen Photoreduction.” Biological Trace Element Research, 2007; 119(1): 77–88.
- Yadav, Surendra Kumar. “Pesticide Applications-Threat to Ecosystems.” J. Hum. Ecol., 2010; 32(1): 37–45.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


