ISSN: 0973-7510
E-ISSN: 2581-690X
Skin and soft tissue infections are brought on by invasion of microbes on the skin and underlying soft tissues (SSTIs). They appear in a series of shapes, causes the high level of severeness. Differentiating between SSTI situations that require prompt attention and surgical or medical intervention from those that don’t is difficult. SSTIs are most prevalent in emergency rooms and affect 7% to 10% of hospitalised patients. SSTIs are characterised by inflammatory components as well as other symptoms including fever, quickly growing lesions, and bullae. The creation of a severity categorization approach to specify suitable empirical treatment would improve the management of SSTIs. Based on the patient’s status knowledge of potential infections, an antibiotic medicine is chosen. Oral antibiotics are sufficient for simple mild-to-moderate infections; however, intravenous antibiotics are required for complicated severe infections.
Infection, Skin, Soft-tissue, Pathogenesis, Treatment, Epidemiology
Skin serves as an important barrier against skin infections and is an essential component of the human immune system.1 Skin and soft tissue infections (SSTIs) are a type of disease that is common in humans.2,3 SSTIs are classified as difficult or uncomplicated.4 They can affect subcutaneous tissue, fascia, or muscle.5 They include a wide spectrum of illnesses, from those who are otherwise healthy but have a serious infection to those who have a relatively mild infection but underlying co-morbidities.6,7 They demand extensive treatment that combines cautious antibacterial drug selection with quick surgical intervention. SSTIs may cause metastatic abscesses and bacteremia, which are both limb- and life-threatening conditions.8,9
A variety of categorization approaches and algorithms have been provided to assist the clinician in clinically determining the severity of an illness.9,10 In severe infections, which frequently call for laboratory testing, diagnostic imaging, and surgical investigation, early diagnosis is very crucial.11 The severity, epidemiology, etiology, and extent of the infection should all be taken into consideration when managing SSTIs. Along with the treatment of a serious underlying disease, drainage or debridement, topical, oral, or systemic antibiotic medication may be required.12
This review examines the epidemiology, pathogenesis, site of infection, diagnosis, prevention, treatment, and current advancement of some common bacterial SSTIs, as well as the structures of common antibiotics that go along with each.
Anthrax
Bacillus anthracis is the cause of the deadly infectious disease known as anthrax. 13 Animals and humans may both contract it. It can also spread by inhaling spores or coming into contact with an infected animal.14 Anthrax, a chemical found naturally in soil, regularly causes injury to both domestic and wild animals.15 Contact with sick animals can spread anthrax, though this is uncommon in the United States.16
Epidemiology
Between 1984 and 1983, the Centers for Disease Control and Prevention received three reports of anthrax illnesses.17 In 1976, Pakistan also reported a case of anthrax.18 Most anthrax cases are cutaneous, while the remaining 5% are gastrointestinal or respiratory illnesses.19 Anthrax is classified into three clinical types: gastrointestinal anthrax, inhalational anthrax, and cutaneous anthrax.20
Pathogenesis
Poly-D-glutamic acid is most likely responsible for B. anthracis capsule phagocytosis resistance. The other three proteins that make up the anthrax toxin are the deadly factor, the edema factor, and the protective antigen (PA). The vast size of the plasmids raises the possibility that other pathogenicity genes are still unknown.21 PA, the main anthrax toxin component, is named after its propensity to provide experimentally-based protective immunization against B. anthracis. An 83-kD protein called PA interacts with target cells’ receptors. Only 20 kD makes up the N-terminal segment of a proteolytically 3-kD protein, which interacts with target cell receptors.22
By cleaving the smaller 20-kD N-terminal fragment, proteolytic enzymes enable the bigger cell-bound segment to function as a membrane channel. Edema toxins and potentially lethal toxins are produced. These enzymatic proteins are released into the cell’s cytoplasm after crossing cell membranes, where they carry out their intended duties.23
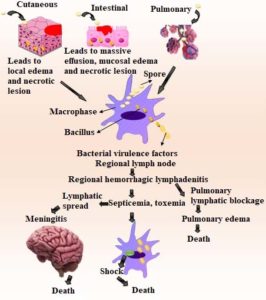
Adenosine triphosphate (ATP) is converted to cyclic adenosine monophosphate by EF, a calmodulin-dependent adenyl cyclase (cAMP). Consequently, intracellular cAMP concentrations rise, resulting in the edema observed in anthrax.24 Polymorphonuclear leukocytes’ phagocytic and oxidative burst activities are both inhibited by edema toxin. Examining LF’s behaviour is being done. Less LF may contribute to high levels of interleukin-1 and tumour necrosis factor (TNF). It has been demonstrated that high dosages of LF encourage macrophage lysis (IL-1). This observation supports the intriguing hypothesis that, early in the anthrax infection, macrophages sustain IL-1 and/or other pro-inflammatory mediators while toxin levels are below the severe concentration required for lysis.25 This inflammatory mediator’s quick release may be the cause of the anthrax victims’ quick demise. TNF and IL-1 antibodies’ ability to protect mice from a fatal dosage of anthrax toxin is evidence of their distinct functions.26 Pathophysiology of anthrax is shown in Figure 1.
Site of infection
The skin, lungs, digestive system, and other body parts are generally affected.27
Diagnosis
There is varied diagnosis methods are available among these chest radiograph, patients sputum, blood test, chest x-ray, CT- scan, stool testing, spinal tap test are important.28
Prevention and Treatment
To prevent anthrax, there is a vaccine (Anthrax Vaccine Adsorbed (AVA) or BioThraxTM); for treatment: A: Benzylpenicillin. Adults need to get 0.6 MU IV every 6 hours until the local edema disappears. After that, give A: 250 mg of phenoxymethylpenicillin every six hours for seven days. Option B: 500 mg of oral erythromycin (O) every eight hours for 10 days. 29–31
Advancement in Management
Recent technological developments have led to the discovery of a biomarker for the determination of anthrax using Dipicolinic acid (DPA), which has an increased level of sensitivity and selectivity, a quick time of response, and actual on-site applicability. That was put to the test by comparing it to a nonporous silica-based nanoparticle system in order to determine the basic properties of pSiNPs-Tb and its capacity for detecting DPA. Using stimulated Bacillus subtilis spores, cellulose-based paper, and successful tests, we were able to show how to employ pSiNP-Tb for DPA sensing from actual water samples.32
Recent research has resulted in the development of a nano aptasensor for anthrax toxin that detects polypeptide entities, protective antigens (PA toxin), using a single-walled carbon nanotube (SWNT) device functionalized with PA toxin ssDNA aptamers. This makes this instrument a blessing because it can quickly identify anthrax and does not interact with either human or bovine serum albumin.33
LF and PA have a strong relationship for increasing LF distribution into cell lines. Raji B cells will get the anti-CD19 immunoliposome nanoparticle-loaded LF. Western blot confirmed that LF has an inhibitory effect on MAPK signalling. To achieve the greatest LF encapsulation and targeted delivery, the liposome nano-formulation was improved. Caspase gene expression was increased as a result of the treatment, demonstrating how LF kills cells via pyroptosis and caspase-dependent apoptosis.34
Antitoxin treatments and the prophylactic use of antibodies are some of the most recent methods for treating anthrax. One of these is raxibacumab, a monoclonal IgG1 antibody that binds to anthrax and inhibits the toxin’s effects. Plasma from BioThrax vaccine recipients is used to make intravenous anthrax immune globulin (AIGIV, also known as Anthrasil). Under the Animal Rule, the FDA approved raxibacumab in 2012 for the treatment of people with inhalational anthrax in combination with antibiotics.35
The Federal Drug Administration has already given its approval to two antibodies that neutralize the poisons of B. anthracis, making them promising therapeutic agents. Using white New Zealand rabbits that had been intravenously infected with anthrax, we created the humanized antibody. This antibody neutralizes anthrax toxins in vivo by binding to the protective antigen. These encouraging findings prompted the establishment of a programme and the availability of funding for the preclinical and clinical phase I research. Sadly, the preclinical development was halted after 5 years owing to technical and scientific problems.36
Boils and carbuncles
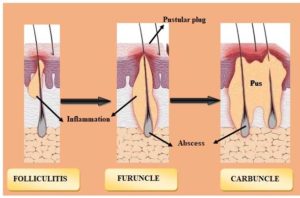
Staphylococcus aureus is commonly found on the skin and within the nose.37 When bacteria invade the skin, a boil occurs that is red, painful, and covered with pus.38 Upon invasion of the skin, a boil occurs that is red, painful, and covered with pus. A bunch of boils are called carbuncles.39 Usually, boils start out as painful, red lumps. Before they burst and discharge, the lumps quickly fill with pus, enlarging and becoming more painful.12 The classification of bacterial infections diseases are described in Figure 2.
Epidemiology
SSTIs occur at a rate of 24.6 per 1,000 years. SSTIs are varied, which is why it takes 10 days to convert.11 Patients report that conversion rates range from 7% to 10%. After chest discomfort, it is the third most typical diagnosis for patients in emergency rooms. There is a chance to raise this between the ages of 45 and 64. Overall, the complication rate is small.40
Pathogenesis
When SSTI develops in an otherwise healthy person, a limited number of bacteria first colonise the site of the symptomatic infection. Staphylococci may easily do this because over 80% of people have intermittent S. aureus colonisation at some point, and 10-15% of those have chronic colonisation. Then, S. aureus must enter the deeper epidermis and dermis layers through minute skin abrasions, traumatic damage, or skin.41 S. aureus has several virulence factors that are in charge of starting and maintaining an infection. Small vesicles with a sieve-like shape emerge after infection, followed by red-hued skin that is pussy. Many of them converge to form a central necrotic ulcer (ash grey slough) surrounded by rosette-shaped fresh vesicles. The cutaneous vesicles get obstructed, which causes the skin to become blocked. Following invasion, the host’s inflammatory response causes microvascular leakage, the release of inflammatory cytokines and chemokines, and leukocyte attraction.42
Site of infection
Afflicted body parts are the face, back side of the neck, armpits, thighs, and buttocks.43
Diagnosis
Itching before the lump emerges, body aches, weariness, fever and chills, skin crustiness or leaking are all possible signs. Pus normally develops one day after the carbuncle forms.
Prevention and Treatment
Boils are treated using Staphylococcus toxoid and autogenous vaccination. Flucloxacillin, oral, adults: For seven days, adults should take 250–500 mg every six hours, while kids should take 250 mg every six hours. For seven days, take 125 mg six hours a day; for one to five years, take 62.5 mg six hours a day. 44
Advancement in Management
A colorectal surgeon is the ideal person to treat big boils, which are often polymicrobial in origin and may not respond to antibiotic therapy alone. If a large abscess needs to be drained, a lengthy, single incision should be avoided since it might hinder wound healing and increase the likelihood of deformity.45
It was previously thought that the mechanism of x-ray-induced healing reduction was a combination of immunological changes that improved phagocytosis and aided in elimination.46
Treatment options include topical and systemic antibiotics, including phototherapy, topical benzoyl peroxide, and surgical procedures (e.g., incision and drainage). Ofloxacin, Norfloxacin, Sisomicin, Gentamicin, and DiedaXiaoyan Gao ointment are among the contemporary topical treatments. Oral treatments include Cefaclor, Flucloxacillin, Cefadroxil, Cefdinir, Cefalexin, Amoxicillin/clavulanate, Erythromycin, and Azithromycin, among others.47,48
Diphtheriae
Corynebacterium diphtheria is the bacterium that causes diphtheria. The most typical means of disease transmission are direct touch between people or interaction with objects contaminated with bacteria, such as a cup or used tissue. Breathing problems, heart failure, paralysis, or even death might ensue from it. To prevent diphtheria, the CDC recommends vaccinations for newborns, children, teenagers, and adults.49
Epidemiology
By 1980, the frequency of diphtheria had been successfully brought down to about 70% thanks to a massive vaccination effort.50 Even though there have been a few disease outbreaks around the world, the most notable post-vaccination outbreaks occurred in many former Soviet Union states, with over 157,000 illnesses and over 5000 deaths recorded. Between 2000 and 2009, outbreaks of C. diphtheria had the highest yearly incidence rate in Latvia.51 93% of the infants that died were not vaccinated, making up a total of 74% of the case-patients. This emphasizes the importance of a thorough immunization regimen. Diphtheria is extremely rare in children because they have been protected from the disease for so long.52, 53
Pathogenesis
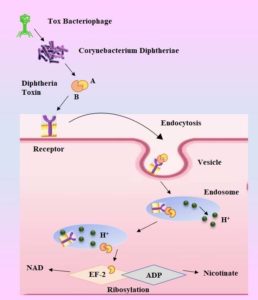
C. diphtheriae pathogenesis entails a number of stages that culminate in host cell invasion, protein synthesis inhibition, and cell death. Diphtheria toxin will be produced if the bacteria successfully invade and populate the host, resulting in illnesses. Adherence And although the particular response of pili attachment is unclear, various research has been conducted in order to create a realistic notion. The two minor pili, SpaB and SpaC, have been identified as being located not only in the pilus but also in monomeric and heterodimeric forms on the bacterial cell wall. In order for SpA to bind to pharyngeal epithelial cells, these two pili are necessary.54 Diphtheria’s toxin mechanism for pathophysiology is presented in Figure 3.
These adhesions can facilitate both distant and intimate interactions during the pathogen’s initial adhesion and subsequent colonisation since they can be found on the cell surface as both complex proteins and fibres. If either minor pilli is absent, there is a considerable reduction in adhesion to the host cells. The SpaD- and SpaH- pili do process of adhesion to the lung and laryngeal epithelial cells, despite the fact that little is really known about the precise activities they perform.55
C. diphtheriae will begin to produce DT as soon as it enters the body and comes into touch with a surface. But a variety of factors affect how the poison manifests. For instance, the time and frequency of DT release are influenced by the extracellular iron concentrations in the tissues of the respiratory tract. This is because when iron is present in the extracellular environment, it functions as a corepressor and represses the toxin gene. Furthermore, DT remains dormant until it is lysogenized by a certain beta phage. The tox genes are located on the phage chromosome rather than the bacterial chromosome, and the phage contains a regulatory gene for the shape of the toxin molecule 56. For the release of DT, a beta phage and low extracellular iron levels are necessary 57.
DT is first produced as a proenzyme, which bacterial proteases subsequently split into two halves, A and B. While fragment B is unstable and devoid of any enzymatic activity, fragment A is the main toxin and is catalytically active. To create a channel through the host cell membrane, the extracellular epidermal growth factor and DT work together. This enables fragment A to pass through and reach the cytoplasm via a hydrophobic region of fragment B. Translation requires elongation factor-2 (EF-2), and Fragment-A works as a catalyst to inactivate it. Since the toxin can no longer interact with RNA during translation as a result of its covalent bond with EF-2, all protein synthesis in that ribosome is inhibited.58
Commonly effected body parts
The most commonly infected areas are the nose and throat. Once infected, the bacteria release toxins, which are harmful compounds. Toxins travel through your bloodstream, causing a thick, grey coating to build in these parts of your body: the airways, nose, throat, and tongue. These poisons can occasionally injure other organs like the kidneys, heart, and brain. Complications including myocarditis (inflammation of the heart muscle), paralysis, and renal failure can have potentially deadly consequences as a result. 49
Diagnosis
The antigen-antibody precipitation theory underlies the Elek test. It is possible to find a specific species that has grown on blood agar plates indirectly in as little as 30 minutes with an accuracy of 97 to 100%. Elisa and PCR testing, among others, have also been helpful. The ejection fraction and other related structural problems can be identified via echocardiography.59
Prevention and treatment
Antitoxin—Diphtheria antitoxin, or DAT, is made from a horse’s serum that has received an antitoxin vaccination. The medication that is preferred is A (Penicillin V, 250 mg four times a day) for a treatment of 14 days, Erythromycin, 125-250 mg every 6 hours for 14 days; Azithromycin, 125-500 mg daily for 3 days; and Penicillin G (Benzyl Penicillin), 25,000 to 50,000 units/kg to a maximum of 1.2, MU IV every 12 hours until the patient is able to take oral medication).60–62
Advancement in Management
It is necessary to modify the vaccine schedule in order to stop diphtheria outbreaks because adult antibody levels do not offer adequate protection against the disease. A dose for high-risk people should be considered.60 Its announcement must be taken into account as part of the national monitoring standards in order to reduce the impact of toxigenic Corynebacterium ulcerans from animal reservoirs on future public health. Toxic Corynebacterium ulcerans, which causes diphtheria, has frequently been discovered in domesticated animals where zoonotic transmission has been demonstrated.63 Consider cutaneous diphtheria if painful skin ulcers with a greyish membrane appear suddenly. The vaccine does not actually prevent bacterial infection, only toxigenic signs. Prompt diagnosis, treatment, and immunisation are necessary to halt the transmission of pharyngeal and cutaneous diphtheria in the future.64
The effective management of a C. ulcerans case requires collaboration between human and animal health organizations. Numerous ethical and practical issues exist, such as the varied licensure of antibiotic kinds for human and veterinary use and the lack of laws requiring owners to treat pets confirmed to be harbouring a hazardous strain of C. ulcerans, particularly when the animals are asymptomatic.65,66 Concerns regarding the safety and reactogenicity of diphtheria-tetanus-whole-cell pertussis (DTwP) vaccinations led to the development of the diphtheria-tetanus-acellular pertussis (DTaP) vaccine. Since they are both safe and effective, the majority of contemporary nations recommend employing the DTaP vaccinations for the infant main series in national immunisation programs.67 Because of concerns about the safety and reactogenicity of diphtheria-tetanus-whole-cell pertussis (DTwP) vaccines, a less reactogenic diphtheria-tetanus-acellular pertussis (DTaP) vaccine was developed.68,69
The phylogeny’s spatio-temporal structure shows that carriage may have had a considerable impact on the C. diphtheriae species’ overall persistence and evolution. As a result, future studies must pay close attention to asymptomatic carriage in both vaccinated and unvaccinated people, particularly in at-risk populations and well-known epidemiological hotspots.68 It is thought that antibiotic resistance is an issue when treating C. diphtheriae. It is believed that the main causes of the emergence of antibiotic resistance in genomes are the widespread use of antibiotics and the regular co-occurrence of numerous resistance elements on the same mobility elements. The resistant genotypes are widespread throughout Asia and Europe and are not confined to any one nation or area. In spite of the fact that erythromycin and penicillin are frequently suggested as the first line of therapy for confirmed cases of early-stage diphtheria, only one macrolide resistance gene and no resistance to lactamases were discovered.70 Antibiotics, isolation, and the diphtheria toxoid vaccine are essential for stopping the spread of the disease, and diphtheria antitoxin is essential for reducing mortality. As a result, there is an urgent need to address the global diphtheria antitoxin shortage.71
Folliculitis
Hair follicles become inflamed as a result of the common skin condition called folliculitis. In most cases, a bacterial or fungal infection is at blame. The initial signs of hair follicles, the tiny pockets from which individual hairs develop, may be small red bumps or white-headed pimples. Crusty, non-healing sores could develop as a result of the infection spreading. Even though the condition is not life-threatening, it can nonetheless be uncomfortable, unpleasant, and embarrassing. If an infection is serious enough, it may leave scars and cause permanent hair loss.72
Epidemiology
Folliculitis of the legs, a persistent condition, affected 50 male patients in a clinic, according to epidemiological research. It mostly affected young individuals between the ages of 16 and 25. Severe pruritus was found in 86 percent of patients, and this symptom emerged prior to the onset of new lesions. Follicular papules were more frequent than pustules in 58% of individuals. Staphylococcus aureus was discovered in 72.5% of the cases. In 50% of the study participants, the lesions were confined to both legs; in 28%, they reached the thigh, and in 10%, they affected the legs, thighs, and forearms.73
Pathogenesis
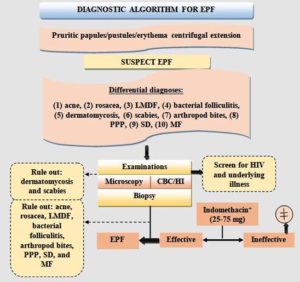
Even though the exact cause of EPF is still unknown, research so far suggests that immunologic pathways play a big role in how the disease develops. Patients with EPF have been found to have anti-basal keratinocyte antibodies, pemphigus-like antibodies, chemotactic factors, and nitrous oxide generation by eosinophils, all of which are regarded as pathogenically relevant.74,75 According to an immunohistochemical study, adhesion molecules are expressed within and around hair follicles, including intercellular adhesion molecule 1 (ICAM-1), endothelial and vascular cell adhesion molecules (ELAM-1 and VCAM-1, respectively), and others.76 The flow chart of diagnostic algorithm for eosinophilic pustular folliculitis is shown in Figure 4.
It may be possible to understand the perifollicular eosinophilic infiltration by thinking about these molecules, which are important for inflammatory cells like eosinophils to stick to. Immune dysfunction may have a role in the pathophysiology of the condition because HIV infection and EPF have been associated in some instances. Type II T-helper cell (TH2) function is encouraged in advanced HIV infection as part of immunological dysregulation. The idea that hypersensitive reactivity to several putative antigens, including infectious agents, medications, and autoantigens, is essential in the pathogenesis of some cases of EPF is compatible with the possibility that cells are simultaneously activated by an antigen. As a result, the levels of the cytokines eotaxin-1 and interleukins (ILs) 4, 5, and 13 rise, which, coupled with other cytokines, promote the growth and recruitment of eosinophils that cause skin inflammation.76
Site of infection
The inflammation of the hair follicles is known as foliculitis. Every hair on your body grows from a follicle, which is a small sac. Any area of the body with hair is susceptible to folliculitis. However, the beard, arms, back, buttocks, and legs are the parts where it occurs most frequently.77
Diagnosis
Pruritus-related scratching that has gotten worse, a history of excessive sweating, recent topical corticosteroid usage, recent and/or ongoing oral antibiotic use, any hot tub or pool use, HIV exposure with a CD4 level under 250, or immunosuppression (ex. a patient on immunosuppressive medicines after a transplant), The scalp, chest, back, and other hair-bearing areas, as well as the bilateral upper and lower extremities, should all be carefully inspected during the physical examination.78
Treatment and prevention
Flucloxacillin, oral; adults: 250–500 mg every six hours for seven days; children: 250 mg every six hours for seven days; 125 mg every six hours for seven days; and 62.5 mg every six hours for seven days.79,80
Advancement in Management
Since their pathophysiology and treatments differ, it is challenging and stressful to identify them, and a false positive or missed diagnosis could result in ineffective treatment.81 The severe type of the disease was linked to an early age of onset. In clinical dermatologic treatment, the proposed unique therapy procedure may be a very helpful tool. S. aureus infections in hair follicles are persistent, although immunodeficiency disorders are not linked to them. Combining rifampicin, clindamycin, doxycycline, and isotretinoin results in a better therapeutic response.82,83 Photodynamic therapy has been used to treat FD, and it has been shown to be effective in certain individuals. In order to comprehend how the ailment affects patients’ quality of life, researchers also took into account FD’s socioeconomic repercussions. 84 Treatment with a topical, systemic antibacterial agent is advised. Compared to molecular biology and other more expensive, advanced investigations, cytology is the most effective and affordable diagnostic approach. 85, 86
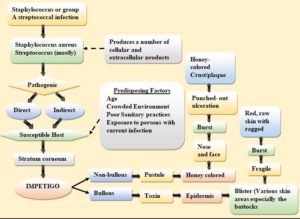
Impetigo
Children ages two to five are most affected by impetigo, a superficial skin condition that is extremely contagious.87 There are two kinds of impetigo: bullous impetigo and non-bullous impetigo. Both of these types are sometimes called impetigo contagiosa.88 The diagnosis is typically made clinically, but in rare circumstances, a culture may be required. While therapy for impetigo can assist with pain management, physical attractiveness enhancement, and stopping the spread of an organism that can cause other illnesses, impetigo frequently recovers in two weeks without leaving any scars (e.g., glomerulonephritis). There are several impetigo treatment options available, and there is no one therapy that works for everyone. Effective topical antibiotics like mupirocin and fusidic acid can occasionally replace oral antibiotics. For individuals with serious illnesses, oral antibiotics should be considered. 89
Epidemiology
This disease is most common in children aged 2 to 5, but it can affect anyone at any age. Summer and fall are the most common times for this to happen. Infants are more likely to develop bullous impetigo. It affects an estimated 162 million children worldwide at any given moment, according to Trusted Source. Impetigo is more prevalent in developing countries and poorer sections of developed countries. Oceania, which includes Australia, New Zealand, and several other countries, has the highest number of instances. 90
Pathogenesis
Impetigo is an infection of the epidermis brought on by wounds, bites, scratches, or other events that produce a break in the skin’s continuity.88 Viruses can enter the system through gaps in the continuity. M-protein strains D and E operate as virulence factors for group-A streptococci, producing streptococcal impetigo.91 Streptococci belonging to Group A have a high potential for invasiveness. They can be isolated from the oropharynx two to three weeks after the skin’s development and from the skin ten days before an infection. Staphylococcus aureus causes impetigo.92 Staphylococci cause toxin-mediated impetigo in the following ways: Toxins produced by staphylococci function as super-antigens and can stimulate T cells. Desmoglen-1 may be hydrolyzed by the exfoliative toxins, decreasing the desmosomes. They are also capable of producing TNF-alpha, interleukin-1, and interleukin-6. These lymphokines can cause bullous impetigo by acting on the skin.93
The aetiology of bullous impetigo is influenced by the following factors: Staphylococcus aureus releases exfoliative toxins, which produce bullous impetigo. Desmoglein 1 can be hydrolyzed by exfoliative toxins, which weakens desmosomes as a result. The two types of toxins, A and B, cause the superficial layer of the epidermis to form bullae that are flaccid and quickly rupture. In almost 80% of instances of impetigo that isn’t bullous, Staphylococcus aureus is implicated as a cause of non-bulbous impetigo. Group-A streptococci, alone or in conjunction with Staphylococcus aureus, have a role in pathogenesis. Superantigens produce toxins, which are produced by bacteria. Toxins encourage T-cell activation. These result in exfoliative skin changes, which induce impetigo.94 Figure 5 shows the pathophysiology of impetigo disease.
Site of infection
Sores around the nose and mouth, as well as other exposed regions of the body that break open, ooze, and eventually crust in adulthood. Around the nose and mouth in children, as well as on the trunk, hands, feet, and diaper area.87
Diagnosis
Impetigo is commonly diagnosed by examining the characteristic lesions, and lab tests are not always essential. 95 Impetigo is often diagnosed based on the clinical presentation. Impetigo typically does not come with a fever. Laboratory tests may reveal leucocytosis with increased neutrophils, increased ESR, hypophosphatemia, hypocalcaemia, low vitamin D levels, hypoalbuminemia, and iron insufficiency.96
Prevention and treatment
For 7–10 days, phenoxymethylpenicillin (O) is the preferred medication. Children should take 25 mg/kg/24 hours every six hours, whereas adults should take 250–500 mg every six hours.97,98
Advancement in Management
Topical ozenoxacin or retapamulin are used to treat impetigo in nonendemic situations, but systemic antibiotics and the mass drug administration (MDA) approach have evidence to support their use in endemic circumstances. The growing global spread of antibiotic-resistant bacteria puts the therapeutic efficacy of antibiotics in peril, emphasising the necessity for careful use of currently available antimicrobials as well as the development of innovative medicines. In an emergency, ozenoxacin can be used to treat nonendemic diseases. In nonendemic settings, topical ozenoxacin or retapamulin are used to treat impetigo, however, evidence exists to support the use of systemic antibiotics and the mass drug administration (MDA) technique in endemic scenarios. The threat posed by the growth of antibiotic-resistant bacteria on a worldwide scale highlights the need for cautious application of currently available antimicrobials as well as the creation of novel pharmaceuticals. Ozenoxacin can be used to treat nonendemic illnesses in an emergency.99 The usual therapies for impetigo have been oral erythromycin and penicillin.100,101 Systemic corticosteroids are the main treatment option for IH. The corticosteroid that is advised is prednisolone, since it is nonteratogenic. Manuka honey, tea effusions, tea tree, olive, garlic, and coconut oils are some examples of natural remedies.102
MRSA (Methicillin resistant staphylococcus aureus)
Infections caused by MRSA (Methicillin-resistant Staphylococcus aureus) can harm a number of organs. It is more challenging to treat than the majority of Staphylococcus aureus, or staph, strains due to its medication resistance. Depending on where you contracted the infection, your MRSA symptoms may fluctuate. The result is typically minor skin problems like boils, abscesses, or rashes. In addition to more severe skin infections, it can infect surgical wounds, the bloodstream, the lungs, and the urinary system. Most MRSA infections do not cause mortality, but occasionally they do. The rise of powerful MRSA strains is causing a lot of public health professionals to worry. MRSA is sometimes referred to as a “super bug” because of how challenging it is to treat.103
Epidemiology
Every year, approximately 132 000 MRSA infections are recorded in German hospitals. Around 18% to 20% of all positive S. aureus culture specimens collected from inpatients include MRSA. CA-MRSA is not yet endemic in Germany. MRSA is to blame for 74% of all S. aureus infections observed worldwide. It is difficult to determine how often S. aureus infections occur in South and East Asia, as well as the Western Pacific. MRSA infections made up more than 60% of S. aureus infections connected to hospital settings in Cyprus, Italy, Portugal, and Romania.104
Pathogenesis
S.aureus is a regular part of the flora in many people’s noses and mouths, especially those who work in hospitals. Transmission of these germs can be difficult to prevent. It is a nonmotile bacterium that is transmitted mostly through human-to-human contact or contaminated surfaces/foods. MRSA often spreads from person to person through contact. In some cases, MRSA can be spread through the cough of someone who has MRSA pneumonia. The most prevalent mode of transmission for these bacteria is horizontal rather than vertical.105
Naturally, S. aureus colonises the upper respiratory tract, nose, mouth, mammary glands, hair, and other parts of the body. Most of the time, S. aureus colonises different parts of the body without doing any harm. These bacteria can thrive at NaCl concentrations of up to 15% and can endure temperatures between 15 and 45 degrees Celsius. With just a 30- to 8-hour incubation period, S. aureus has an infectious dose of more than 100,000 organisms when swallowed.106
Because the germs may be carried for long periods of time before becoming contagious, frequently for months, it can be challenging to determine the infectious dosage and incubation period for other S. aureus infections. Additionally, S. aureus might be carried continuously or irregularly.107
Site of infection
It frequently appears in the armpits, back of the throat, nose, and groyne skin folds.108
Diagnosis
By checking for signs of drug-resistant bacteria in a tissue sample or nasal secretions, doctors can identify MRSA. In a laboratory, the material is put on a plate containing chemicals that encourage bacterial growth. Due to the bacterium’s growth cycle of about 48 hours, newer tests that can find staph DNA in just a few hours are becoming easier to use. 109
Prevention and treatment
Vancomycin is the preferred medication; 30 mg/kg/24 hours is administered intravenously twice, with a 2 g/24-hour maximum unless serum levels are monitored. 110–113
Advancement in Management
Clinical diagnostics are best performed using phenotypic approaches. The most common method for detecting PBP2a is the antigen-antibody latex agglutination test. Some automated Staphylococci antimicrobial susceptibility testing devices provide great sensitivity and specificity for the tested MRSA strains. Chemotactic factors, nitrous oxide generation by eosinophils, anti-basal keratinocyte antibodies, and pemphigus-like antibodies have all been found in individuals with EPF and are regarded as pathogenically relevant. 114 The most reliable non-genotypic technology for the direct identification of pathogens from positive blood cultures is matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS). Multiplex real-time PCR methods are now used to find S. In DNA-based methods, aureus is used to directly identify MRSA from clinical samples. These tests have a high level of validation and produce results within an hour.115
Methicillin-resistant S. aureus (MRSA) infection is a top pathogen, according to the WHO. To battle the biofilm-resistance system and comprehend how the growth of MRSA biofilms links to antibiotic resistance, front-line measures must be developed. As crucial to treating staph infections as the creation of new drugs is the invention of methods to avoid, eradicate, or spread biofilms. The creation of anti-biofilm strategies that may be used in addition to traditional antibiotics to target planktonic bacteria is a cutting-edge strategy in the fight against S. aureus. Non-conjugated human milk oligosaccharides (HMOs), which are common in human milk, regulate the growth and development of biofilms in several bacterial diseases, including MRSA. Creating anti-biofilm techniques that can be used in conjunction with traditional antibiotics to treat planktonic bacteria is a cutting-edge strategy in the fight against S. aureus. Non-conjugated human milk oligosaccharides (HMOs), which are common in human milk, regulate the growth and development of biofilms in a number of bacterial diseases, including MRSA. after being converted from the HMO 2′- fucosyllactose (2′-FL) into an anomeric, amino-variant. 116 There are many antimicrobials that are effective against MRSA and other Gram-positive bacteria that are now treated with medications like vancomycin. Antimicrobial resistance, such as that to drugs like Rifampicin, Ceftaroline, Daptomycin, Linezolid, and Telavancin, is a constraint on the discovery of new antibiotics.117 Additional clinical trials are required to study the benefits of combination therapy and evaluate the effectiveness of these new antibiotics.118
Pyoderma
The skin disorder pyoderma gangrenosum can result in ulcers.119 A common co-occurring condition is rheumatoid arthritis, paraproteinemia, inflammatory bowel disease, or a haematological malignancy. The process of ruling out other causes of cutaneous ulcers confirms the diagnosis, which is mostly based on the clinical features and prior encounters with the ulcers. Pyoderma is the term used to describe any pyogenic (pus-filled) skin condition. Impetigo, ecthyma, folliculitis, impetigo contagiosa Bockhart’s impetigo, carbuncles, tropical ulcers, and other conditions are examples of superficial bacterial infections. Pyoderma gangrenosum is an autoimmune disease.119,120
Epidemiology
From October 1976 to December 1978, a group of 89 children living in an orphanage about 12 kilometres from Vellore, Tamil Nadu, India, were assessed monthly for streptococcal pyoderma. The prevalence rate of pyoderma was 10.1 percent, with monthly fluctuations ranging from 2.1 percent to 17.1 percent, as determined by the isolation of group A streptococci (GAS). Temperature, humidity, and rainfall were found to have no significant relationship with pyoderma seasonal trends. However, Vellore’s hot and muggy climate, which prevails for the bulk of the year, may be responsible for this disease’s endemicity. Studies on a small number of GAS strains showed that the lesions contained several varieties of M types. In this study, PSGN (post-streptococcal glomerulonephritis) was not identified. One in 100,000 people suffers from pyoderma gangrenosum. Although it can afflict people of any age, the disorder is most prevalent in those in their 40s and 50s.121
Pathogenesis
It is an uncommon cutaneous symptom that is clinically identified by excluding inflammation, infection, neoplasia, and thrombophilia. Studies on pathogenetics and treatments are few. Abnormalities in the immune system, neutrophil activity, and inflammatory cytokine activity, together with certain genetic changes, predispose patients to this complicated illness process. 122
Site of infection
Despite the fact that it may affect any part of the body, the legs are most frequently afflicted.123
Diagnosis/ Assessment
A diagnosis of pyoderma gangrenosum cannot be confirmed by a test.124
Prevention and treatment
It can be difficult to treat because it can occur in a range of clinical settings, and there isn’t a clear course of action. The greatest obstacle to effective treatment has been a lack of knowledge about the pathophysiology. The pathophysiology of this disorder has, however, been better understood recently, and PG is now thought to be an autoinflammatory disease process.125
Advancement in Management
The pathophysiology of Pyoderma gangrenosum (PG) is mostly a mix of neutrophil malfunction, inflammatory mediators, and hereditary susceptibility, however, it is still not entirely understood. Since neutrophils are the immune system’s effector cells, their function depends on this fact. The inflammasome’s involvement in T cells and cytokines like IL-8 and IL-1b is the subject of current studies. Genetics plays a crucial role in determining a person’s propensity for PG-associated illnesses and autoinflammatory diseases. A better understanding of the complex pathophysiology of PG is required for identifying those with severe conditions, improving diagnosis, and increasing the chances of developing targeted and specialised treatment options.126 It is important when analysing skin lesions since pyoderma gangrenosum is an uncommon systemic illness that is commonly mistaken for a number of skin illnesses. Early diagnosis can reduce the need for needless procedures and delays in receiving proper care. To reduce morbidity, early identification of the condition and adequate treatment are advised for the purpose of creating consensus recommendations and treatment procedures for skin allergies.127
Even though alternative immunosuppressants and cytostatic medications can be used as steroid-sparing treatments, they are traditionally regarded as the first line of therapy. Multidrug regimens are a useful therapy option for patients with refractory disease, even though they have not been completely characterised in the literature. Pyoderma gangrenosum (PG) and the underlying ailment can both benefit from anti-inflammatory and immunosuppressive medications. As an IL1 antagonist and an anti-TNF agent, respectively, anakinra and infliximab are two examples. Recently, both timolol gel and intralesional activated protein C have demonstrated positive outcomes. Surgery is partially contraindicated because it may aggravate PG due to an allergy. Immunosuppression should also be applied in addition to these therapies. Less common therapies are used when more traditional ones are ineffective. These medications are frequently based on immunosuppressive drug combinations that have been shown to be safe and effective in the treatment of other immunological diseases.128, 129
For the diagnosis of pyoderma gangrenosum, there are several enhanced tools that are separated into major and minor categories. A biopsy of the ulcer’s edge that shows neutrophil infiltration is part of the major Minor conditions are those that do not include infection, allergy, inflammatory arthritis, vesicle ulceration, pustules, papules, or discomfort at the ulceration site. This category of diagnosis also includes multiple ulcerations and “wrinkled paper” scars at the sites of ulcer healing.130,131.
Urticaria
Itchy, red welts known as hives (urticaria) are brought on by a skin reaction. 132 The welts increase in size, frequency of occurrence, and appearance as the response worsens. 133 The illness is referred to as “chronic hives” if the welts persist for longer than six weeks and repeat often throughout months or years.134
Epidemiology
Inducible Urticaria (IU) was discovered in 35.1% of the 1091 cases of Chronic Urticaria (CU), whereas CSU accounted for 61.1% of CU cases. The patients that were left had both IU and CSU. In comparison to patients from IU, patients from CSU were more than twice as likely (12.1 % vs. 6.0 %, p = 0.001) to have a family history of urticaria. However, wheals with large eruptions predominated in CSU (57.9% vs. 42.1%), whereas wheals with localised eruptions were more prevalent in IU. (Localized wheals 54.8% vs. generalised wheals 45.2%, p 0.001). It was shown that the CU was the root cause of absenteeism in almost every fifth patient with this condition. All kinds of urticaria had a lifetime prevalence rate of 8.8% (95 % confidence a: 7.9-9.7%). The lifetime prevalence of CU was 1.8 % (95 % CI 1.4-2.3 %) and the 12-month prevalence was 0.8 % (95 % CI 0.6-1.1 %).135
Pathogenesis
The mast cell is the primary effector cell. Mast cell mediators are created and have the ability to mobilise and activate other cells, including neutrophils, basophils, and maybe eosinophils, as well as cause inflammation. According to a recent study, one-third of CIU patients have functional mast cell-specific histamine-releasing autoantibodies that bind to the high-affinity IgE receptor or, less commonly, to IgE. Additionally, there is no discernible circulating histamine-releasing activity in these individuals. Antihistamines are the most popular type of treatment for CIU; however, immunotherapy utilizing plasmapheresis, intravenous immunoglobulin, and cyclosporine may be beneficial for critically ill patients with incurable diseases. 136,137
Site of infection
Hives can appear anywhere on the body and can quickly change appearance, move around, vanish, and reappear. The lumps, which are red or skin-colored “wheals” with obvious edges, normally emerge and disappear fast.132
Diagnosis
Acute urticaria can identify by looking at the skin rash. The patient may be able to prevent recurrences by pinpointing the trigger. In chronic urticaria, a stool sample to look for parasites, a blood test to look for anaemia, and an erythrocyte sedimentation rate (ESR) test to look for immune system issues; a thyroid function test to determine whether the thyroid is overactive (hyperthyroidism) or underactive (hypothyroidism); a liver function test to monitor whether the liver is overactive (hypothyroidism); and a liver function test to determine whether the liver.138
Prevention and treatment
If acute, rule out medication responses (such penicillin) or infections if they have been present for less than three months. administering oral antihistamines A: Promethazine (O) if insomnia is a characteristic OR A: Chlorpheniramine (O) 4–16 mg once at night Adults should take 25 to 50 mg at night, 10 mg of Cetrizine and 10 mg of Loratadine each day, while children should take 0.1 to 0.2 mg/kg 2-4 times a day or 0.5 mg/kg at bedtime.139
Advancement
More research has been done on how urticaria develops, and the findings point to HIV as a potential cause. A higher CD4 count and a lower HIV viral load were indicators of the considerable improvement in symptoms with HIV medication. It is uncertain why there is a connection between HIV and urticaria. It could be connected to the fact that HIV targets dendritic cells since they have HIV receptors on them. It decreased to a few times a week after taking HIV medication for one month, and after two months, it vanished entirely. He persisted in displaying no symptoms of a respiratory condition.140
Based on biomarkers that may aid in forecasting disease duration, severity, and treatment response, novel therapeutics for urticaria are being developed. Mast cells and basophils, together with the autoimmune pathway, play a major role in urticaria. Omalizumab, an anti-IgE monoclonal antibody that lowers levels of free IgE and has demonstrated safety and efficacy in the therapy of chronic spontaneous urticaria (CSU), is one example of advancement in the treatment of urticaria. Another illustration is the prescription of cyclosporine (CsA) for CSU therapy when omalizumab fails or is not appropriate.141-142
Papular urticaria, a separate type of urticaria, is more common in the Calabar region of Nigeria and affects children attending wealthy schools more frequently than those attending public schools. These are also reported to have a significant prevalence among women, as seen in surveys conducted at hospitals.143
The microorganism’s presence in the skin, its toxins, or the complement activation brought on by circulating immune complexes can all cause urticaria. Urticaria disappears when the infection has been treated.144 One of the most prevalent intestinal protozoan parasites, Blastocystis, has been related to skin lesions and urticaria. A wide range of skin symptoms, primarily urticaria in many cases, were seen with this illness. For patients with this infection, the most often given medications were metronidazole, paromomycin, and tinidazole. The findings demonstrated that urticaria and other skin complaints can have Blastocystis infection as an etiopathogenic factor. Therefore, it is advised that a Blastocystis infection be treated and diagnosed early to stop both gastrointestinal and cutaneous symptoms.145 Currently, omalizumab, cyclosporine, and second-generation H1 antihistamines are used in a stepwise manner to treat urticaria with the goal of achieving a complete response. Targeting mediators, signalling pathways, and receptors on mast cells and other immune cells is the main goal of cutting-edge therapeutic strategies. Future studies should concentrate on locating illness endotypes and associated biomarkers, finding fresh therapeutic targets, and creating more effective drugs.146 Some common antibiotics that are used for these infections are represented in Figure 6.
SSTIs are a group of illnesses that are extremely common but also intricate and varied. The variety of their presentations makes clinical care difficult. The lack of data from well-documented research further complicates their management, and decisions about the site of treatment and the best antimicrobial therapy may be uneven and ineffective.
One approach to addressing site of care decisions is to use a combination of clinical findings to assess illness severity. When figuring out how bad a disease is, it’s important to look at the location, size, systemic symptoms, other health problems, and the main characteristics of the infection. Based on these factors, SSTIs can be classified as mild, moderate, or severe. Following this classification, it is possible to decide where the patient will get care. Small lesions can be treated in the outpatient environment with oral medications, but moderate to severe lesions may require inpatient or outpatient intravenous therapy. SSTIs include diseases including HIV, the use of injectable drugs, diabetes, bite wounds, exposure to salt or freshwater, extended hospitalisation and antibiotic medication, and risk factors for MRSA in the community. In the settings, the frequently detected microorganisms must be included in the empirical therapy for SSTIs. The best methods for determining the duration of therapy and the use of oral therapy are extensive monitoring and perceptive clinical judgment.147 The creation of an innovative drug delivery system is required to increase the bioavailability of pharmaceuticals and their actions, as well as their safety and side effects.148,149 This is due to advancements in all biomedical disciplines and the growing complexity of diagnostic and therapeutic procedures.150,151 Though numerous drugs are available, using polymers and other natural molecules, such infections can be targeted with fewer side effects.152
ACKNOWLEDGMENTS
The authors would like to thank MM College of Pharmacy, Maharishi Markandeshwar (Deemed to be University) Haryana, India, for providing support and assistance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Chopra H, Gandhi S, Gautam RK, Kamal MA. Bacterial Nanocellulose based Wound Dressings: Current and Future Prospects. Curr Pharm Des. 2021;28(7):570-580.
Crossref - Barie PS, Wilson SE. Impact of Evolving Epidemiology on Treatments for Complicated Skin and Skin Structure Infections : The Surgical Perspective. J Am Coll Surg. 2015;220(1):105-116.e6.
Crossref - Chopra H, Bibi S, Kumar S, Khan MS, Kumar P, Singh I. Preparation and Evaluation of Chitosan/PVA Based Hydrogel Films Loaded with Honey for Wound Healing Application. Gels. 2022;8(2):111.
Crossref - Rajan S. Skin and soft-tissue infections : Classifying and treating a spectrum. Cleve Clin J Med. 2012;79(1):57-66.
Crossref - Chopra H, Kumar S, Singh I. Strategies and Therapies for Wound Healing: A Review. Curr Drug Targets. 2021;23(1).
Crossref - May AK. Skin and Soft Tissue Infections. Surg Infect (Larchmt). 2011;12(3):179-184.
Crossref - Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother. 2010;65(Suppl 3):35-44.
Crossref - Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(17):731-732.
Crossref - Stevens DL, Bisno AL, Chambers HF, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft-Tissue Infections. Clin Infect Dis. 2005;41(10):1373-1406.
Crossref - El-menyar A, Asim M, Mudali IN, Mekkodathil A, Latifi R, Al-Thani H. The laboratory risk indicator for necrotizing fasciitis ( LRINEC ) scoring : the diagnostic and potential prognostic role. Scand J Trauma Resusc Emerg Med. 2017;25(1):1-9.
Crossref - Tognetti L, Martinelli C, Berti S, et al. Bacterial skin and soft tissue infections : review of the epidemiology , microbiology , aetiopathogenesis and treatment A collaboration between dermatologists and infectivologists. J Eur Acad Dermatol Venereol. 2012;26(8):931-41 2012.
Crossref - Hospital TG, Medicine I, Training R, Centre HS. Bacterial skin and soft tissue infections in adults : A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008;19(2):173-184.
Crossref - Oncul O, Ozsoy MF, Gul HC, Kocak N, Cavuslu S, Pahsa A. Cutaneous Anthrax in Turkey : A Review of 32 Cases. Scand J Infect Dis. 2002;34(6):413-416.
Crossref - Logan NA. Bacillus species of medical and veterinary importance. J Med Microbiol. 2015;25(1988):157-165.
Crossref - Misgie F, Atnaf A, Surafel K. A Review on Anthrax and its Public Health and Economic Importance. Acad J Anim Dis. 2015;4(3):196-204.
Crossref - Goel AK. Anthrax: A disease of biowarfare and public health importance. World J Clin Cases.2015;3(1):20-33
Crossref - Li S, Ma Q, Chen H, et al. Epidemiological Investigation and Etiological Analysis of a Cutaneous Anthrax Epidemic Caused by Butchering Sick Cattle in Guizhou. Front Public Health. 2020;8:65.
Crossref - Abdrakhmanov SK, Mukhanbetkaliyev YY, Korennoy FI, Karatayev BS, Mukhanbetkaliyeva AA, Abdrakhmanova AS. Spatio-temporal analysis and visualisation of the anthrax epidemic situation in livestock in Kazakhstan over the period 1933-2016. Geospat Health. 2017;12(2):589.
Crossref - Doganay M, Demiraslan H. Human Anthrax as a Re-Emerging Disease. Recent Pat Antiinfect Drug Discov. 2015;10(1):10-29.
Crossref - Doganay M, Metan G, Alp E. A review of cutaneous anthrax and its outcome. J Infect Public Health. 2010;3(3):98-105.
Crossref - Makino S ichi, Sekizaki T. Cross-talk to the genes for Bacillus anfhracis capsule synthesis by atxA , the gene encoding the trans-activator of anthrax toxin synthesis. Mol Microbiol. 1997;23(6):1229-1240.
Crossref - Maynard JA, Maassen CBM, Leppla SH, et al. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat Biotechnol. 2002;20(6):597-601.
Crossref - Cunningham K, Lacy DB, Mogridge J, Collier RJ. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc Natl Acad Sci U S A. 2002;99(10):7049-7053.
Crossref - Toxin AE, Alpha NF. Anthrax Edema Toxin Differentially Regulates Lipopolysaccharide-Induced Monocyte Production of Tumor Necrosis Factor Alpha and Interleukin-6 by Increasing Intracellular Cyclic AMP. Infect Immun. 1994;62(10):4432-4439.
Crossref - Palazon-Riquelme P, Lopez-Castejon G. The inflammasomes, immune guardians at defence barriers. Immunology. 2018;155(3):320-330.
Crossref - Palladino MA, Bahjat FR, Theodorakis EA. ANTI-TNF- a Therapies : The Next Generation. Nat Rev Drug Discovery. 2003;2:736-746.
Crossref - Akbulut A, Akbulut H, Ozguler M, Nuran I, Yalc S. Gastrointestinal Anthrax: A Case and Review of Literature. Adv Infect Dis. 2012;2(3):67-71.
Crossref - Friedlander AM. Clinical aspects, diagnosis and treatment of anthrax. J Apply Microbiol. 1999;87(2).
Crossref - Sidwa T, Salzer JS, Traxler R, et al. Control and Prevention of Anthrax, Texas, USA, 2019. Emerg Infect Dis. 2020;26(12):2815-2824.
Crossref - Huang E, Pillai SK, Bower WA, et al. Antitoxin Treatment of Inhalation Anthrax: A Systematic Review. Health Secur. 2015;13(6):365-377.
Crossref - Parker CM, Karchmer AW, Fisher MC, Muhammad KM, Yu PA. Safety of Antimicrobials for Postexposure Prophylaxis and Treatment of Anthrax: A Review.
Clin Infect Dis. 2022;75(Suppl 3):417-431.
Crossref - Jung Y, Kang S, An J, Jung J, Kim D. Porous silicon-based fluorescent nanoprobe for the detection of anthrax biomarker and its practical sensing applications. Dyes Pigments. 2020;182.
Crossref - Cella LN, Sanchez P, Zhong W, Myung N v., Chen W, Mulchandani A. Nano aptasensor for Protective Antigen Toxin of Anthrax. Anal Chem. 2010;82(5):2042-2047.
Crossref - Banihashemi SR, Rahbarizadeh F, Zavaran Hosseini A, Ahmadvand D, KhoshtinatNikkhoi S. Liposome-based nanocarriers loaded with anthrax lethal factor and armed with anti-CD19 VHH for effectively inhibiting MAPK pathway in B cells. Int Immunopharmacol. 2021;100.
Crossref - Savransky V, Ionin B, Reece J. Current status and trends in prophylaxis and management of anthrax disease. Pathogens. 2020;9(5).
Crossref - Avril A, Tournier JN, Paucod JC, Fournes B, Thullier P, Pelat T. Antibodies against Anthrax Toxins: A Long Way from Benchlab to the Bedside. Toxins (Basel). 2022;14(3).
Crossref - Dutta R, Morgan D, Baker N, Gardner JW, Hines EL. Identification of Staphylococcus aureus infections in hospital environment: electronic nose based approach. Sens. Actuators B Chem. 2005;109(2):355-362.
Crossref - Williamson DA, Carter GP, Howden BP. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin Microbiol Rev. 2017;30(3):827-860.
Crossref - Liu Z, Wei J, Cao Z, Zhu X, Zhang C. Fire Needle Combined Therapy or Surgery Therapy for Carbuncle of Neck? A Case Series. Infect Drug Resist. 2022;15:7293-7299.
Crossref - Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations and Management. Clin Microbiol Rev. 2015;28(3):603-661.
Crossref - Creech CB, Al-zubeidi DN, Fritz SA. Prevention of Recurrent Staphylococcal Skin Infections. Infect Dis Clin North Am. 2015;29(3):429-464.
Crossref - Ginsburg I, Korem M, Koren E, Varani J. Pro-inflammatory agents released by pathogens, dying host cells, and neutrophils act synergistically to destroy host tissues: A working hypothesis. J Inflamm Res. 2019; 12:35-47.
Crossref - Winthrop KL, Albridge K, South D, et al. The Clinical Management and Outcome of Nail Salon – Acquired Mycobacterium fortuitum Skin Infection. Clin Infect Dis. 2004;38(1):38-44.
Crossref - Shallcross LJ, Hayward AC, Johnson AM, Petersen I. Incidence and recurrence of boils and abscesses within the first year: a cohort study in UK primary care.
Br J Gen Pract. 2015;65(639):668-676.
Crossref - Silverberg B. A Structured Approach to Skin and Soft Tissue Infections (SSTIs) in an Ambulatory Setting. Clin Pract. 2021;11(1):65-74.
Crossref - Calabrese EJ. X-Ray treatment of carbuncles and furuncles (boils): A historical assessment. Hum Exp Toxicol. 2013;32(8):817-827.
Crossref - Lin HS, Lin PT, Tsai YS, Wang SH, Chi CC. Interventions for bacterial folliculitis and boils (furuncles and carbuncles). Cochrane Database Syst Rev. 2021;2(2):CD013099.
Crossref - Wang W, Chen W, Liu Y, et al. Antibiotics for uncomplicated skin abscesses: systematic review and network meta-analysis. BMJ Open. 2018;8(2):e020991.
Crossref - Prygiel M, Polak M, Mosiej E, Wdowiak K, Forminska K, Zasada AA. New Corynebacterium Species with the Potential to Produce Diphtheria Toxin. Pathogens. 2022; 11(11):1264.
Crossref - Pickering LK, Baker CJ, Freed GL, et al. Immunization Programs for Infants, Children, Adolescents, and Adults: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(6):817-840.
Crossref - Badenschier F, Berger A, Dangel A, et al. Outbreak of imported diphtheria with Corynebacterium diphtheriae among migrants arriving in Germany, 2022. Euro Surveill. 2022;27(46):2200849.
Crossref - Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30:B26-B36.
Crossref - Cartwright K. Pneumococcal disease in western Europe: burden of disease, antibiotic resistance and management. Eur J Pediatr. 2002;161(4):188-195.
Crossref - Vitek CR, Wharton M. Diphtheria in the Former Soviet Union: Reemergence of a Pandemic Disease. Emerg Infect Dis. 1998;4(4):539-550.
Crossref - Hadfield TL, Mcevoy P, Polotsky Y, Tzinserling VA, Yakovlev AA. The Pathology of Diphtheria. J Infect Dis. 2000;181:116-120.
Crossref - Seth-smith HMB, Egli A. Whole Genome Sequencing for Surveillance of Diphtheria in Low Incidence Settings. Front Public Health. 2019; 7:1-13.
Crossref - Groman NB. Conversion by corynephages and its role in the natural history of diphtheria.
J Hyg (Lond). 1984;93(3):405-417.
Crossref - Parveen S, Bishai WR, Murphy JR. Corynebacterium diphtheriae: diphtheria toxin, the tox operon, and its regulation by Fe2+-activation of apo-DtxR. Microbiol Spectr. 2021;7(4).
Crossref - Collier RJ. Diphtheria Toxin: Mode of Action and Structure. Bacteriol Rev. 1975;39(1):54-85.
Crossref - Wagner KS, White JM, Lucenko I, Mercer D, Crowcroft NS, Neal S, Efstratiou A. Diphtheria in the Postepidemic, Europe, 2000–2009. Emerg Infect Dis. 2012;18(2):217-225.
Crossref - Efstratiou A, Engler KH, Mazurova IK, Glushkevich T, Vuopio-Varkila J, Popovic T. Current Approaches to the Laboratory Diagnosis of Diphtheria. J Infect Dis. 2000;181(Suppl 1):138-145.
Crossref - Graham BS, Ambrosino DM. History of Passive Antibody Administration for Prevention and Treatment of Infectious Diseases. Curr Opin HIV AIDS. 2016;10(3):129-134.
Crossref - Hoefer A, Herrera-León S, Domínguez L, et al. Zoonotic Transmission of Diphtheria from Domestic Animal Reservoir, Spain. Emerg Infect Dis. 2022;28(6):1257-1260.
Crossref - Alberto C, Osdoit S, Villani AP, et al. Cutaneous ulcers revealing diphtheria: A re-emerging disease imported from Indian Ocean countries? Ann Dermatol Venereol. 2021;148(1):34-39.
Crossref - Gower CM, Scobie A, Fry NK, et al. The changing epidemiology of diphtheria in the United Kingdom, 2009 to 2017. Eurosurveillance. 2020;25(11).
Crossref - Dureab F, Al-Sakkaf M, Ismail O, et al. Diphtheria outbreak in Yemen: The impact of conflict on a fragile health system. Confl Health. 2019;13(1).
Crossref - Griffith J, Bozio CH, Poel AJ, et al. Imported Toxin-Producing Cutaneous Diphtheria-Minnesota, Washington, and New Mexico, 2015-2018. Morb Mortal Wkly Rep. 2019; 68(12):281–284.
Crossref - Rane MS, Rohani P, Halloran ME. Association of Diphtheria-Tetanus-Acellular Pertussis Vaccine Timeliness and Number of Doses with Age-Specific Pertussis Risk in Infants and Young Children. JAMA Netw Open. 2021.
Crossref - Winkler NE, Dey A, Quinn HE, et al. Australian vaccine preventable disease epidemiological review series: diphtheria 1999-2019. Commun Dis Intell (2018). 2022;46.
Crossref - Will RC, Ramamurthy T, Sharma NC, et al. Spatiotemporal persistence of multiple, diverse clades and toxins of Corynebacterium diphtheriae. Nat Commun. 2021;12(1).
Crossref - Truelove SA, Keegan LT, Moss WJ, et al. Clinical and epidemiological aspects of diphtheria: A systematic review and pooled analysis. Clin Infect Dis. 2020;71(1):89-97.
Crossref - Green M, Feschuk AM, Kashetsky N, Maibach HI. Clinical characteristics and treatment outcomes of Pityrosporum folliculitis in immunocompetent patients. Arch Dermatol Res. 2022;1-13.
Crossref - Romero-Mate A, Arias-Palomo D, Hernandez-Nunez A, Cordoba-Guijarro S, Borbujo-Martínez J. Chronic non-scarring scalp folliculitis. Retrospective 34 case-series study. J. Am. Acad. Dermatol. 2019;81(4):1023-1025.
Crossref - Nunzi E, Parodi A, Rebora A. Ofuji‘s disease : High circulating titers of IgG and IgM directed to basal cell cytoplasm. J Am Acad Dermatol. 1985;12:268-273.
Crossref - Kaye KS, Petty LA, Shorr AF, Zilberberg MD. Current Epidemiology, Etiology, and Burden of Acute Skin Infections in the United States. Clin Infect Dis. 2019;68(Suppl 3):193-199.
Crossref - Vakilzadeh F, Suter L, Knop J, Macher E. Eosinophilic pustulosis with pemphigus-like antibody. Dermatologica. 1981;162(4):265-272.
Crossref - Nervi SJ, Schwartz RA, Dmochowski M. Eosinophilic pustular folliculitis: A 40 year retrospect. J Am Acad Dermatol. 2006;55(2):285-289.
Crossref - Hillier A, Lloyd DH, Weese JS, et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet Dermatol. 2014;25(3):163-e43.
Crossref - Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40(6):419-423.
Crossref - Alexis A, Heath CR, Halder RM. Folliculitis Keloidalis Nuchae and Pseudo folliculitis Barbae Are Prevention and Effective Treatment Within Reach ? Dermatol Clin. 2014;32(2):183-191.
Crossref - Sun KL, Chang JM. Special types of folliculitis which should be differentiated from acne. Dermatoendocrinol. 2018;9(1):4-7.
Crossref - Laureano AC, Schwartz RA, Cohen PJ. Facial bacterial infections: Folliculitis. Clin Dermatol. 2014;32(6):711-714.
Crossref - Ravaioli GM, Starace M, Alessandrini AM, Guicciardi F, Piraccini BM. Pressure alopecia in pediatric and adult patients: Clinical and trichoscopic findings in 12 cases. J Am Acad Dermatol. 2019;81(4):1021-1023.
Crossref - Miguel-Gomez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: Effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79(5):878-883.
Crossref - Durdu M, Ilkit M. First step in the differential diagnosis of folliculitis: Cytology. Crit Rev Microbiol. 2013;39(1):9-25.
Crossref - Prindaville B, Belazarian L, Levin NA, Wiss K. Pityrosporum folliculitis: A retrospective review of 110 cases. J Am Acad Dermatol. 2018;78(3):511-514.
Crossref - Pereira, L. B. Impetigo – review. An. Bras. Dermatol. 2014; 89(2): 1-7.
Crossref - Bowen AC, Mahé A, Hay RJ, et al. The Global Epidemiology of Impetigo: A Systematic Review of the Population Prevalence of Impetigo and Pyoderma. PLoS One. 2015;10(8):e0136789.
Crossref - Schachner LA, Lynde CW, Kircik LH, et al. Treatment of Impetigo and Antimicrobial Resistance. J Drugs Dermatol. 2021;20(4):366-372.
Crossref - Galindo E, Hebert AA, Galindo E. Expert Opinion on Drug Safety A comparative review of current topical antibiotics for impetigo A comparative review of current topical antibiotics for impetigo. Expert Opin Drug Saf. 2021;1-7.
Crossref - Motswaledi MH. Impetigo in children: a clinical guide and treatment options, S Afr Fam Pract. 2011; 53(1): 44-46.
Crossref - Gahlawat G, Tesfaye W, Bushell M, et al. Emerging Treatment Strategies for Impetigo in Endemic and Nonendemic Settings: A Systematic Review. Clin Ther. 2021;43(6):986-1006.
Crossref - Coskey MDRJ, Coskey BALA. Diagnosis and treatment of impetigo. J. Am. Acad. Dermatol. 1987; 17(1):62-63.
Crossref - Mora M, Bensi G, Capo S, et al. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc Natl Acad Sci USA. 2005;102(43):15641-15646.
Crossref - Duggal SD, Bharara T, Jena PP, Kumar A, Sharma A, Gur R, Chaudhary S. Staphylococcal bullous impetigo in a neonate. World J Clin Cases. 2016; 4(7): 191-194.
Crossref - Galli L, Novelli A, Ruggiero G, Stefani S, Fortina AB. Pediatric impetigo: an expert panel opinion about its main controversies. J Chemother. 2022;34(5):279-285.
Crossref - Gupta D. Bacterial Skin and Soft Tissue Infections in Children. Pediatic Infectious Diseases. 2021;3(4).
Crossref - Darmstadt GL, Lane AT. Impetigo: an overview. Pediatr Dermatol. 1994;11(4):293-303.
Crossref - Rosen T, Albareda N, Rosenberg N, et al. Efficacy and Safety of Ozenoxacin Cream for Treatment of Adult and Pediatric Patients With Impetigo: A Randomized Clinical Trial. JAMA Dermatol. 2018;154(7):806-813.
Crossref - Loadsman MEN, Verheij TJM, van der Velden AW. Impetigo incidence and treatment: A retrospective study of Dutch routine primary care data. Fam Pract. 2019;36(4):410-416.
Crossref - Abrha S, Tesfaye W, Thomas J. Intolerable burden of impetigo in endemic settings: A review of the current state of play and future directions for alternative treatments. Antibiotics. 2020;9(12):1-15.
Crossref - D’Cunha NM, Peterson GM, Baby KE, Thomas J. Impetigo: A need for new therapies in a world of increasing antimicrobial resistance. J Clin Pharm Ther. 2018;43(1):150-153.
Crossref - Bradley SF. Methicillin-resistant Staphylococcus aureus in nursing homes. Epidemiology, prevention and management. Drugs Aging. 1997;10(3):185-198.
Crossref - Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285-292.
Crossref - Criddle P, Potter J. Exploring patients’ views on colonisation with meticillin-resistant Staphylococcus aureus. Br J Infect Control. 2006;7(2):24-28.
Crossref - Nandhini P, Kumar P, Mickymaray S, Alothaim AS, Somasundaram J, Rajan M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics (Basel). 2022;11(5):606.
Crossref - Neyra RC, Frisancho JA, Rinsky JL, Resnic C. Multidrug-Resistant and Methicillin-Resistant Staphylococcus aureus (MRSA) in Hog Slaughter and Processing Plant Workers and Their Community in North Carolina (USA). Environ. Health Perspect. 2014; 122(5).
Crossref - Köck R, Mellmann A, Schaumburg F, Friedrich AW, Kipp F, Becker K. The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Germany. Dtsch Arztebl Int. 2011;108(45):761-767.
Crossref - Romero DV, Treston J, O’Sullivan AL. Hand-to-hand combat: Preventing MRSA infection. Adv Skin Wound Care. 2006;19(6):328-335.
Crossref - Ryan MO, Haas CN, Gurian PL, Gerba CP, Panzl BM, Rose JB. American Journal of Infection Control Application of quantitative microbial risk assessment for selection of microbial reduction targets for hard surface disinfectants. Am J Infect Control. 2014;42(11):1165-1172.
Crossref - Monecke S, Coombs G, Shore AC, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6(4):e17936.
Crossref - Boyce JM. MRSA patients: proven methods to treat colonization and infection. J Hosp Infect. 2001;48: S9-S14.
Crossref - Harbarth S, Hawkey PM, Tenover F, Stefani S, Pantosti A, Struelens MJ. Update on screening and clinical diagnosis of meticillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents. 2011;37(2):110-117.
Crossref - Emge DA, Bassett RL, Duvic M, Huen AO. Methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen in erythrodermic cutaneous T-cell lymphoma (CTCL) patients. Arch Dermatol Res. 2020;312(4):283-288.
Crossref - Lee AS, de Lencastre H, Garau J, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4:18033.
Crossref - Craft KM, Nguyen JM, Berg LJ, Townsend SD. Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. Medchemcomm. 2019;10(8):1231-1241.
Crossref - Holmes NE, Howden BP. What’s new in the treatment of serious MRSA infection? Curr Opin Infect Dis. 2014;27(6):471-478.
Crossref - Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203-218.
Crossref - Rodríguez-Zúñiga MJM, Heath MS, Gontijo JRV, Ortega-Loayza AG. Pyoderma gangrenosum: a review with special emphasis on Latin America literature.
An Bras Dermatol. 2019;94(6):729-743.
Crossref - Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23(9):1008-1017.
Crossref - Monari P, Moro R, Motolese A, et al. Epidemiology of pyoderma gangrenosum: Results from an Italian prospective multicentre study. Int Wound J. 2018;15(6):875-879.
Crossref - Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73(4):691-698.
Crossref - Wollina U. Pyoderma gangrenosum–a systemic disease?. Clin Dermatol. 2015;33(5):527-530.
Crossref - Reese AM, Haag CK, Jung E, Nauta AC, Swerlick RA, Ortega-Loayza AG. Pyoderma gangrenosum underrepresentation in non-dermatological literature. Diagnosis (Berl). 2020;8(1):85-90.
Crossref - Skopis M, Bag-Ozbek A. Pyoderma Gangrenosum: A Review of Updates in Diagnosis, Pathophysiology and Management. J. 2021; 4(3):367-375.
Crossref - Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): An updated review. J Am Acad Dermatol. 2015;73(4):691-698.
Crossref - Clayman E, Marcet K, Kuykendall L, Atisha D. Pyoderma Gangrenosum Following Bilateral Deep Inferior Epigastric Perforator Flaps. J Reconstr Microsurg Open. 2016;01(02):125-127.
Crossref - Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: Challenges and solutions. Clin CosmetInvestig Dermatol. 2015;8:285-293.
Crossref - Marchegiani A, Fruganti A, Bazzano M, Cerquetella M, Dini F, Spaterna A. Fluorescent Light Energy in the Management of Multi Drug Resistant Canine Pyoderma: A Prospective Exploratory Study. Pathogens. 2022;11(10).
Crossref - George C, Deroide F, Rustin M. Pyoderma gangrenosum – a guide to diagnosis and management. Clin Med (Lond). 2019;19(3):224-228.
Crossref - Gavioli EM, Casias M, Ngo L. Pyoderma gangrenosum and superimposed infection: A case report. Adv Skin Wound Care. 2020;33(6):1-3.
Crossref - Kayiran MA, Akdeniz N. Diagnosis and treatment of urticaria in primary care. North Clin Istanb. 2019;6(1):93-99.
Crossref - Compston A, Coles A. Multiple sclerosis. The Lancet. 2008;372(9648).
Crossref - Powell RJ, Leech SC, Till S, et al. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45(3):547-565.
Crossref - Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. 2010;35(8):869-873.
Crossref - Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract.;2014:674709.
Crossref - Asero R, Tedeschi A, Marzano AV, Cugno M. Chronic urticaria: a focus on pathogenesis. F1000Res. 2017;6:1095.
Crossref - Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133(5):1270-1277.
Crossref - Ansotegui IJ, Bernstein JA, Canonica GW, et al. Insights into urticaria in pediatric and adult populations and its management with fexofenadine hydrochloride. Allergy Asthma Clin Immunol. 2022;18(41):1-17.
Crossref - Brar AK, Grace J. Urticaria and HIV Infection: A Case Report. Cureus. 2022;14(9):e29662.
Crossref - Ensina LF, Cusato-Ensina AP, Cardona R. Advances in the pathogenesis representing definite outcomes in chronic urticaria. Curr Opin Allergy Clin Immunol. 2019;19(3):193-197.
Crossref - Ryan D, Tanno LK, Angier E, et al. Clinical review: The suggested management pathway for urticaria in primary care. Clin Transl Allergy. 2022;12(10):e12195.
Crossref - Olasode OA, Henshaw EB. Prevalence of skin infections, infestations, and papular urticaria among adolescents in secondary schools in Calabar, Nigeria. Ghana Med J. 2019;53(4):287-293.
Crossref - Minciullo PL, Cascio A, Barberi G, Gangemi S. Urticaria and bacterial infections. Allergy Asthma Proc. 2014;35(4):295-302.
Crossref - Bahrami F, Babaei E, Badirzadeh A, Riabi TR, Abdoli A. Blastocystis, urticaria, and skin disorders: review of the current evidences. Eur J Clin Microbiol Infect Dis. 2020;39(6):1027-1042.
Crossref - Kolkhir P, Gimenez-Arnau AM, Kulthanan K, Peter J, Metz M, Maurer M. Urticaria. Nat Rev Dis Primers. 2022;8(1).
Crossref - Chopra H, Islam MA, Sharun K, Emran TB, Al-Tawfiq JA, Dhama K. Recent advances in the treatment of biofilms induced surgical site infections. Int J Surg. 2023;109(1):65-67.
Crossref - Sharma A, Chopra H, Singh I, Emran TB. Physically and chemically crosslinked hydrogels for wound healing applications. Int J Surg. 2022;106:106915.
Crossref - Chopra H, Tsagkaris C, Matthews L, Gautam RK, Kamal MA. Novel Approaches for the Application of Herbs for Skin Care. Curr Pharm Biotechnol. 2023;24(1):164-187.
Crossref - Chopra H, Kumar S, Singh I. Bioinks for 3D printing of artificial extracellular matrices. In: Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering. Elsevier; 2020:1-37.
Crossref - Chopra H, Kumar S, Singh I. Biopolymer-based Scaffolds for Tissue Engineering Applications. Curr Drug Targets. 2021;22(3):282-295.
Crossref - Elsadek NE, Nagah A, Ibrahim TM, et al. Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials (Basel). 2022;15(5):1934.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.