ISSN: 0973-7510
E-ISSN: 2581-690X
Reproductive tract infections in antenatal women are frequently occurring public health concern affecting the quality of life in the infected women and further affects the neonatal outcome negatively. Vaginal infections are predominantly caused due to the disturbance in the normal vaginal microflora. Group B Streptococcus (GBS) is a major peritoneal pathogen leading to morbidity and mortality in both mother and neonates leading to complications like preterm labour, chorioamnionitis and Preterm rupture of membrane (PROM). Further, Vulvovaginal candidiasis (VVC) is a frequently encountered gynaecological disease causing morbidity in 3/4th of women at least once in their life span. It leads to infertility in non-pregnant women. It results in adverse pregnancy outcomes such as chorioamnionitis and congenital abnormalities in the neonates. The aim of this study is to screen for potential bacterial and yeast infections with focus on GBS and Candida infections and its neonatal outcome. Candida species and GBS were identified by routine culture-based tests. HiChrome agar was used for speciation of Candida species. CAMP test was performed for GBS; further identification was done using Streptococcal grouping kit. GBS was found in 15% of the antenatal women and Candida Species was found to be most common with an infection rate of 50%. Lower segment Cesarean section (LSCS) rate in GBS positive women was 60% and in Candida Non albicans positive women was found to be 65.38%. 33% of the neonates born to GBS positive mothers had respiratory distress. Infection in antenatal women negatively influenced the mode of delivery and the mean body weight of the neonates born to infected mothers were comparatively less.
Antenatal Women, Candida species, Group B Streptococcus, Neonatal Outcome, Vaginal Infections
Infections of the reproductive tract pose a public health threat and it is said to have 5% annual prevalence and about 40 million cases of new infections occur per year.1 Vaginitis can be caused by infective sources and might also be non-infectious in nature. The microbial factors involved in causing vaginitis act by dominating the normal vaginal flora and further leading to the deficiency of Lactobacilli species which has a crucial role in maintaining the balance of healthy vaginal microflora.2
Streptococcus agalactiae (Group B Streptococcus) continues to be a crucial peritoneal pathogen causing morbidity and mortality in both mothers and their infants.3 GBS is often associated with negative pregnancy outcomes, including preterm labour, chorioamnionitis and premature rupture of membrane (PROM).4 In pregnancy, GBS colonization causes asymptomatic bacteraemia or Urinary tract infections (UTI). GBS colonization increases the risk of attaining GBS infection in the subsequent pregnancy. Hence, GBS colonization requires appropriate treatment during labour.5 S.pyogenes was thought to be the most important pathogen to cause puerperal sepsis until Fry in 1937 reported 7 cases of puerperal fever with 3 deaths associated with GBS infection.4 GBS infection in neonates presents with meningitis, septicaemia, pneumonia and is considerably fatal.6 Other infections by GBS include respiratory infections, endocarditis, peritonitis, conjunctivitis, arthritis and osteomyelitis.7
Furthermore, more than 70% of women will experience morbidity from the common gynaecological disease vulvovaginal candidiasis (VVC) at a certain point in their lives.8 Vulvovaginal candidiasis is more common in pregnant women and there is an increased chance of recurrence. Candidiasis usually does cause any risk to the foetus during normal pregnancy. But, VVC can have negative effects on pregnancy. Untreated vaginal candidiasis causes chorioamnionitis which further leads to abortion and prematurity in pregnant women. It might lead to congenital infection of the neonate. Non pregnant women have an increased risk of attaining Pelvic inflammatory disease (PID) leading to infertility. Antibiotic treatment, diabetes mellitus and pregnancy are the most common predisposing factors leading to Candida infections.9-13 Some studies suggest that vulvovaginitis has shot up in the past three decades as the antifungal resistance in the Candida species is increasing and further leading to change in women’s health quality.12,14-16
This prospective study was made on 150 symptomatic and asymptomatic women of third trimester who were attending the OPD of the tertiary care centre in Potheri, Tamil Nadu, India. Women participating in the study were willing to deliver in the same institution. The study participants were followed up since the period of admission for complaints of vaginal discharge, fever and urinary tract infection. Neonates were followed up for a period of 90 days post-delivery for late onset infections like sepsis, pneumonia and meningitis. Informed consent was taken from the participants with high vaginal swabs (HiCulture collecting device, Himedia). A detailed case history was taken which includes their Name, age, address and clinical diagnosis. The high vaginal swabs were processed according to the routine biochemical and cultural tests.
Identification of Candida species
Identification of Candida species were done using standard microbiological procedures. KOH wet mount was performed to identify the yeast cells microscopically, followed by germ tube formation, chlamydospore formation, sugar fermentation and assimilation tests. Further, identification of Candida species was further done with CHROM agar, Hi-Chrome agar (Himedia). Briefly, the colonies from SDA were streaked onto Chrome agar plates and were incubated for 24-28 hours at 25°C. After incubation, the colour formed by the colonies were noted. (Light green-Candida albicans, Dark green- Candida dubliniensis, Cream coloured-Candida parapsilosis, Metallic blue-Candida tropicalis; Pink-Candia krusi).17
Identification of GBS infections
The high vaginal swabs were inoculated in a selective medium (Todd Hewitt broth) supplemented with nalidixic acid and gentamicin and were incubated for 18-24 hours at 37°C. The isolates were obtained by further subculture on 5% blood agar, incubated at 37°C or 24 hours. Catalase negative, gram positive cocci showing narrow zone of beta hemolysis is indicative of GBS.18,19
CAMP reaction
Staphylococcus aureus is inoculated on the blood agar and S. agalactiae is streaked perpendicular to S.aureus. Formation of the zone of haemolysis after 24-48 hours of incubation indicates a positive CAMP reaction.19
Serotyping of GBS
Serotyping was done by a commercially available Streptococcal grouping kit. There is a group specific antigen in Streptococcus species which is polysaccharide in nature. The antigen once extracted by a specific enzyme is capable of agglutinating with the latex particles which is coated with corresponding antibody. In the absence of the group specific antigen, the latex won’t agglutinate and will remain as a smooth suspension. Positive reaction is indicated by visible agglutination of the latex particles.20
A total of 150 high vaginal swabs were collected from antenatal women belonging to the age group of 19-38 years and gestational age of 35-37 weeks at the time of sampling. 79 among the 150 participants were asymptomatic and 71 of them were symptomatic for vaginal infections. 69% of the symptomatic cases suffered vaginal discharge, 6.7% reported itching, 3.3% had burning sensation and another 3.3% had draining per vagina. 1.3% of them suffered from fever in labor. 13.33% of the discharge were mucoid in nature, 3.3% was watery, 8.0% were blood stained and 8.0% was thick purulent discharge (Table 1). Most of the participants belonged to the age group of 21-25 years.
Table (1):
Antenatal women and the Maternal symptoms
Maternal symptoms |
Number (n=150) |
Percentage (%) |
|---|---|---|
White mucoid discharge |
20 |
13.33 |
Itching |
10 |
6.7 |
Burning |
5 |
3.3 |
Blood-stained discharge |
12 |
8 |
White purulent discharge |
12 |
8 |
Watery discharge |
5 |
3.3 |
Draining per vagina |
3 |
2 |
Fever in labour |
2 |
1.3 |
Preterm labour |
3 |
2 |
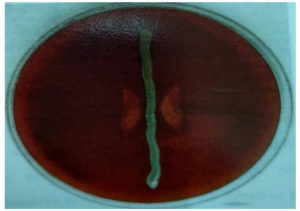
This study also analysed the socio-economic status of the women who were enrolled in the study and it was found that the largest proportion of patients belonged to the lower socio-economic status. Of the 150 women, 95 (63.3%) were positive for potential pathogens. Among the positives, most common isolate was C.albicans 28 (29.47%), followed by Candida non albicans 26 (27.36%), S. aureus 22 (23.15%), S. agalactiae 15 (15.78%), E. coli (2.10%), K. pneumonia 2 (2.10%)(Table 2). Candida species and S. agalactiae were identified according to the standard microbial techniques. S. agalactiae showed CAMP positive results (Figure). While CROM agar aided in species identification of Candida species presumptively.
Table (2):
Mode of delivery, neonatal distress, and the mean baby weight
Organisms isolated |
Number of antenatal women (n=150) |
Percentage |
Caesarean section |
Normal vaginal delivery |
Neonatal distress |
Mean Baby weight |
|---|---|---|---|---|---|---|
Total number of organisms |
55 |
36.7 |
47 |
8 |
5 |
3 |
S.aureus |
22 |
14.7 |
4 |
18 |
6 |
2.8 |
C.albicans |
28 |
18.6 |
4 |
24 |
– |
3.2 |
Candida Non albicans |
26 |
17.3 |
17 |
9 |
15 |
2.5 |
K.pneumoniae |
2 |
1.3 |
– |
2 |
– |
2.8 |
Str.agalactiae |
15 |
10 |
9 |
6 |
13 |
2.08 |
E.coli |
2 |
1.3 |
– |
2 |
– |
2.4 |
Moreover, the data was analysed according to the mode of delivery (Caesarean section / Normal Vaginal delivery), neonatal distress and mean baby weight (Table 2).
In this study, 63.3% (95) of pregnant women were diagnosed with vaginal infection in the last trimester. In a previous study made by Benedetto et al., it was reported that 44.3% of the pregnant women were positive for cervicovaginal culture.21 Pathological vaginal discharge in pregnancy was found to be 48.05% and positive vaginal culture was found to be 52.5% in pregnant women belonging to India in another study conducted in the year 2021.22 Another study made in the year 2022, reported that 50.00% of pregnant women suffered from vaginal discharge, followed by symptoms of itching and burning sensation.23 The most frequently isolated microorganisms were Candida species(44%), GBS (15%) and bacterial vaginosis causing microorganisms were isolated from 11% of pregnant women.21 The most frequently isolated microorganisms in the present study were Candida species (56.54%), GBS (15.76%) and Bacterial vaginosis causing bacteria (28.3%) which correlates with the previous studies made.24
GBS is less frequently isolated compared to Candida speciesbut is clinically significant as it leads to complications like inflammation of lungs, meningitis and bacteraemia in neonates.24 The occurrence of GBS infection in pregnant women is relatively less. Maternal age and BMI might act as risk factors.25,26 The infection can spread vertically from the infected mothers to the neonates. While most of the neonates are not affected by the bacteria, a very small number (2-4%) will develop GBS infection, which might lead to severe problems mentioned above. It also leads to urinary tract infections, Chorioamnionitis, Endometritis and sepsis in pregnant women.27 GBS colonization was found to be highly prevalent in pregnant women visiting a tertiary care hospital during the 3rd trimester of pregnancy.28 Moreover, GBS colonization was found to be significantly associated with the presence of vaginal discharge.28 In this study, the carriage rate of GBS was found to be 10% (15.78) amongst pregnant women.
It was found that the mean birth weight of babies born to culture positive mothers was 2.77 kg and to culture negative mothers was 2.95 kg. Further the rate of cesarean section was found to be 78.75% in culture positive mothers and 21.425 in culture negative mothers. LSCS rate in women positive for GBS was 60% and Candida non albicans was 65.38%. Further, 33.3 % neonates had respiratory distress in mothers positive for GBS. 30% of women positive for GBS had urinary tract infections. 3 patients presented with draining per vagina in labour of which one mother was GBS culture positive.
The differences in the vaginal carriage rates of GBS depend on the particular population and laboratory method to detect GBS. Some studies in India document vaginal colonization rates of GBS between 5%-6%.24 10% of the neonates born to GBS infected women had respiratory distress. Mode of delivery 9(60%) was by caesarean section 6(40%) was normal vaginal delivery 13 were CRP positive which is significant. The rate of GBS varies with geographical distribution. Blood culture for all neonates with Streptococcus B positive mother had no growth in the study. In this study 30% 3 women of 10 positive for GBS infection had urinary tract infection. Creixems et al in his study in 1997 got 20% urinary tract infections in Streptococcus agalactiae positive mothers.29 Draining per vagina was reported by 3 participants of which one mother was positive for GBS and all the 3 participants presented with preterm labour.
Approximately 75% of all pregnant women experience one episode of vaginal candidiasis during their lifetime and 50% of them suffer recurrent infection. Recurrent VVC imposes a widespread problem and has multifactorial cause, which includes imbalance in vaginal microflora, increasing antifungal drug resistance and genetic predisposition.30 VVC is not fatal but affects the women adversely by decreasing their quality of life. One dose managements of fungal infections and long term overuse of over the counter drugs are important contributing factors for VVC. VVC is believed to be the second common cause of vaginal discharge only after bacterial vaginosis.31,32 Although it is believed that Candida albicans which is a dimorphic opportunistic fungal pathogen is involved in most of the VVC infections, there is also increasing evidence of non albicans causing VVC. A study made in the year 2019 on the population of pregnant women reported VVC prevalence of 36.5%.33 Another study found 30.7% prevalence of VVC in pregnant women, where more than 70% of the affected women were infected by Candida non-albicans species.34 VVC was found to be more prevalent in pregnant women than non-pregnant women.35,36 Highest prevalence of Candida species was observed in pregnant women belonging to the age group of 26-28 years (56.7%) in a study made by Christopher et al.23 It was further reported that 24% of VVC were caused by Candida non-albicans species and the causative non-albicans were C. glabarata, C. parapsilosis and C. lusitaniae.37 The prevalence of vaginal candidiasis is usually doubled during the third trimester of pregnancy. Yeast may colonize the reproductive tract but it may not necessarily cause clinical infection and thus most of the women remain as symptomatic carriers.38 In a study made by Pei Pei Chong in 2003, most frequently reported Candida species were C. albicans (70%) followed by C. glabarata (14.9%), C. krusi (2.3%) and C. parapsilosis (2.35%).39 Our present study reported 51.85% of C.albicans and 48.15% of Candida non albicans among the 54 Candida species isolated which correlates with the study made in 2022 on non-pregnant women with VVC where C. albicans was the most common yeast isolated followed by C. glabrata, C. krusei and C. parapsilopsis.40
Vaginal infections account for enormous public health problems. The findings of this study highlight the prevalence of GBS and Candida infections. More than 15% of the clinical isolates were found to be GBS and over 50% of the clinical isolates belonged to Candida species. Candida Non albicans were found almost equal to the C. albicans in this study. The vaginal infection negatively influenced the mode of delivery where the LSCS cases were increased in women with infections. The mean body weight of the infants was also significantly less in the culture positive mothers compared to the infants born to culture negative mothers. Thus, it is important to screen for potential pathogens in the antenatal women to reduce the pregnancy related risks and outcomes.
ACKNOWLEDGMENTS
The authors would like to thank SRM Medical College Hospital and Research Centre for providing the necessary facilities and support for carrying out the research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The study was approved by the Institutional Ethical Committee, SRM Medical College Hospital & Research Centre, Chennai, India, with ethics clearance number 240/IEC/2012.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Thulkar J, Kriplani A, Agarwal N, Vishnubhatla S. Aetiology & risk factors of recurrent vaginitis & its association with various contraceptive methods. Indian J Med Res. 2010;131:83-87.
- Razzak MSA, Al-Charrakh AH, Al-Greitty BH. Relationship between lactobacilli and opportunistic bacterial pathogens associated with vaginitis. N Am J Med Sci. 2011;3(4):185-192.
Crossref - Rausch AV, Gross A, Droz S, Bodmer T, Surbek D v. Group B Streptococcus colonization in pregnancy: prevalence and prevention strategies of neonatal sepsis. J Perinat Med. 2009;37(2):124-129.
Crossref - Fry R. Fatal infections by hqmolytic Streptococcus group B. Lancet. 1938;231(5969):199-201.
Crossref - Turrentine MA, Ramirez MM. Recurrence of group B streptococci colonization in subsequent pregnancy. Obstetrics and Gynecology. 2008;112(2 Pt 1):259-264.
Crossref - Pass MA, Gray BM, Dillon HC. Puerperal and perinatal infections with group B streptococci. Am J Obstet Gynecol. 1982;143(2):147-152.
Crossref - Konrad G, Hauch S, Pylypjuk C. Prevention of neonatal group B streptococcal infection: approaches of physicians in Winnipeg, Man. Can Fam Physician. 2007;53(2):290-289:e.
- Yano J, Sobel JD, Nyirjesy P, et al. Current patient perspectives of vulvovaginal candidiasis: incidence, symptoms, management and post-treatment outcomes. BMC Womens Health. 2019;19(1):48.
Crossref - Oviasogie F, Okungbowa F. Candida species amongst pregnant women in Benin city, Nigeria: Effect Of Predisposing Factors. Afr J Clin Exp Microbiol. 2009;10(2).
Crossref - Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstetrics and Gynecology. 1998;92(5):757-765.
Crossref - Baby, Kansal P, Jindal N, Bansal R, Faujdar SS. Study of Epidemiology, Risk Factors and Clinical Spectrum for Neonatal Candidemia at Tertiary Care Hospital in North India. Int J Curr Microbiol App Sci. 2021;10(01):264-270.
Crossref - El Ahmed HH, Canadas-De la Fuente GA, Fernandez-Castillo R, Gonzalez-Jimenez E, Cantero-Hinojosa J, Lardon-Fernandez M. [Generalized cutaneous candidiasis in newborn at term]. Biomedica. 2012;32(2):170-173.
- Panwar S, Faujdar SS. Prevalence, distribution, risk factors and antifungal susceptibility profiles of Candida species in a tertiary care hospital. Int J Curr Microbiol Appl Sci. 2016;5(4):329-337.
Crossref - Olowe OA, Makanjuola OB, Olowe R, Adekanle DA. Prevalence of vulvovaginal candidiasis, trichomoniasis and bacterial vaginosis among pregnant women receiving antenatal care in Southwestern Nigeria. Eur J Microbiol Immunol (Bp). 2014;4(4):193-197.
Crossref - McGregor JA, French JI. Bacterial vaginosis in pregnancy. Obstet Gynecol Surv. 2000;55(5 Suppl 1):S1-19.
Crossref - Mohanty S, Xess I, Hasan F, Kapil A, Mittal S, Tolosa JE. Prevalence & susceptibility to fluconazole of Candida species causing vulvovaginitis. Indian J Med Res. 2007;126(3):216-219.
- Odds FC, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32(8):1923-1929.
Crossref - Baker CJ, Clark DJ, Barrett FF. Selective broth medium for isolation of group B streptococci. Appl Microbiol. 1973;26(6):884-885.
Crossref - Rosa-Fraile M, Spellerberg B. Reliable Detection of Group B Streptococcus in the Clinical Laboratory. J Clin Microbiol. 2017;55(9):2590-2598.
Crossref - Slotved HC, Elliott J, Thompson T, Konradsen HB. Latex assay for serotyping of group B Streptococcus isolates. J Clin Microbiol. 2003;41(9):4445-4447.
Crossref - Benedetto C, Tibaldi C, Marozio L, et al. Cervicovaginal infections during pregnancy: epidemiological and microbiological aspects. J Matern Fetal Neonatal Med. 2004;16(Suppl 2):9-12.
Crossref - Prasad D, Parween S, Kumari K, Singh N. Prevalence, Etiology, and Associated Symptoms of Vaginal Discharge During Pregnancy in Women Seen in a Tertiary Care Hospital in Bihar. Cureus. 2021;13(1):e12700.
Crossref - Christopher MA, Nyoyoko VF, Antia UE, Eyo IE. Prevalence of vulvovaginal candidiasis in pregnant women attending antenatal clinic in Abak, South-South Nigeria. Microbes Infect Dis. 2022;3(3).
- Dechen TC, Sumit K, Ranabir P. Correlates of Vaginal Colonization with Group B Streptococci among Pregnant Women. J Glob Infect Dis. 2010;2(3):236-241.
Crossref - Khalil M, Hartvigsen C, Thorsen P, Moller J, Uldbjerg N. Maternal age and body mass index as risk factors for recto-vaginal colonization with Group B Streptococci. Int J Gynaecol Obstet. 2023;161(1):303-307.
Crossref - Jiao J, Wu W, Shen F, Liu Z, Zhou H, Fan G, Zhou Y. Clinical Profile and Risk Factors of Group B Streptococcal Colonization in Mothers from the Eastern District of China. J Trop Med. 2022;5236430.
Crossref - Heath PT, Jardine LA. Neonatal infections: group B streptococcus. BMJ Clin Evid. 2014;28;2014.
- Wasim Z, Afsar M, Hussain S, Riaz T, Noor N, Shafique S. Prevalence of Group B Streptococcus Colonization in Pregnant Women. Pak J Med Health Sci. 2022;16(4).
Crossref - Munoz P, Llancaqueo A, Rodriguez-Creixems M, Pelaez T, Martin L, Bouza E. Group B streptococcus bacteremia in nonpregnant adults. Arch Intern Med. 1997;157(2):213-216.
Crossref - Ratushniak N, Sukhanova A. Recurrent vulvovaginal candidiasis: current issues (a review). World Science. 2022.
Crossref - Shanmugam N, Balasundharam A, Thomas I, a, R, James J. A Cross-Sectional Clinical Investigation of Organisms Causing Vaginal Discharge in Patients in Rural Tamil Nadu, India. Cureus. 2023;15(1):e33979.
Crossref - Sadaf R, Kishwar N, Tabassum S, Rauf B, Javed S, Khan M. Frequency of Microorganisms in High Vaginal Swabs Obtained from women in OPD presenting with Vaginal Discharge. Pak J Med Health Sci. 2023;17(2).
Crossref - Konadu DG, Owusu-Ofori A, Yidana Z, et al. Prevalence of vulvovaginal candidiasis, bacterial vaginosis and trichomoniasis in pregnant women attending antenatal clinic in the middle belt of Ghana. BMC Pregnancy Childbirth. 2019;19(1):341.
Crossref - Waikhom SD, Afeke I, Kwawu GS, et al. Prevalence of vulvovaginal candidiasis among pregnant women in the Ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth. 2020;20(1):266.
Crossref - Chatzivasileiou P, Vyzantiadis TA. Vaginal yeast colonisation: From a potential harmless condition to clinical implications and management approaches-A literature review. Mycoses. 2019;62(8):638-650.
Crossref - Goodfellow L, Verwijs MC, Care A, et al. Vaginal bacterial load in the second trimester is associated with early preterm birth recurrence: a nested case-control study. BJOG. 2021;128(13):2061-2072.
Crossref - Kennedy MA, Sobel JD. Vulvovaginal Candidiasis Caused by Non-albicans Candida Species: New Insights. Curr Infect Dis Rep. 2010;12(6):465-470.
Crossref - Aguin TJ, Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep. 2015;17(6):462.
Crossref - Chong PP, Lee YL, Tan BC, Ng KP. Genetic relatedness of Candida strains isolated from women with vaginal candidiasis in Malaysia. J Med Microbiol. 2003;52(8):657-666.
Crossref - Intra J, Sala MR, Brambilla P, Carcione D, Leoni V. Prevalence and species distribution of microorganisms isolated among non-pregnant women affected by vulvovaginal candidiasis: A retrospective study over a 20 year-period. J Med Mycol. 2022;32(3):101278.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.