ISSN: 0973-7510
E-ISSN: 2581-690X
Forty streptomycetes were isolated from the soils of farms in Riyadh. Only three isolates (St-2, St-9, and St-25) exhibited cellulolytic-ligninolytic activity, with the St-9 isolate exhibiting the highest activity and identified as Streptomyces lazureus. The optimum environmental and nutritional conditions for maximum cellulolytic-ligninolytic activity were determined as fermentation batch of pH of 7.5, inoculum size of 200 µL of bacterial suspension, incubation period of 7 d, and incubation temperature of 30°C. In addition, the fermentation batch contained peptone and yeast extract as the best nitrogen and carbon sources, respectively. Cellulase and ligninase were purified via gel filtration column chromatography. The accumulated end-product of the fermentation process was glucose powder, which was subjected to a partial characterization process. The glucose powder appeared white, melted at 146°C, was highly soluble in water, slightly soluble in ethanol, and insoluble in ethyl ether. The glucose solution appeared clear without precipitates and had a low electric conductivity of 15 µS.cm-1.
Biodegradation, Cellulose, Glucose, Ligninase, Streptomycetes
Sawdust is a waste material generated from the timber industry and is composed of fine wood particles. Sawdust may be an environmental hazard with misuse; some countries burn huge quantities of sawdust, causing considerable air pollution. Moreover, in tropical regions there is a danger of sawdust piles igniting at high temperatures.1 Sawdust is also a common waste product of conifers and in Mexico and USA, is used as biomass during bioremediation in biofuel production.2,3 Sawdust bioremediation and briquette production reduce air pollution and provide a useful and popular commodity.4 Sawdust is converted to biofertilizers by composting, which ties up nitrogen for 180 d in soil.5
Sawdust is composed of carbon (60.8%), hydrogen (5.2%), oxygen (33.8%), nitrogen (0.9%), and polysaccharides (5%–10%).6 It is a non-degradable organic waste because of its low nitrogen content and high lignin and cellulose content. Some microorganisms, especially Actinobacteria, effectively decompose sawdust with nitrogen supplementation.7,8 Sawdust decomposition depends on the complete hydrolysis of lignin and cellulose by ligninase and cellulase, respectively. Lignin and cellulose are more complex polysaccharides with a high molecular weight, and cellulose has approximately 2×103 to 104 glucose monomers and a molecular weight ranging from 2×105 to 2.4×106 mole.9,10
Actinobacteria are gram-positive bacteria, with high guanine and cytosine content in DNA. They are widely prevalent in diverse habitats, even in extreme habitats, owing to their effective metabolites, including hydrolyzing enzymes that degrade complex organic substances. Actinobacteria are saprophytes that decompose recalcitrant organic substances, such as plant and animal debris. They are high producers of bioactive metabolites, including hydrolyzing enzymes.11,12 According to Bergey’s Manual of Systematic Bacteriology,13,14 Streptomyces is an Actinobacteria genus belonging to the family Actinobacteriaceae. Different species of Streptomyces, such as S. purpureus, S. albidoflavus, S. antibioticus, S. albus, and S. cyaneus can grow on potato dextrose agar and Sabouraud agar containing different concentrations of sawdust (0.5, 1.0, and 1.5 mg/100 mL) as the sole carbon source.15 Sawdust biodegradation depends on the release of a wide array of hydrolyzing enzymes, including cellulase and ligninase, which hydrolyze cellulose and lignin, respectively.16,17 Environments rich in polysaccharides, such as soil, are a favorable source of sawdust-decomposing microorganisms, where effective hydrolyzing enzymes are continually being released.18 This research aimed to convert sawdust to glucose during fermentation using Streptomyces, a high producer of cellulase and ligninase, which were subjected to extraction and purification.
Collection of Soil Samples
Only five samples were collected from farm soils in Riyadh city. The soil was collected at a depth of 30 cm after the superficial layer was removed. Soil samples were placed in sterile polyethylene bags, transported to the laboratory, and left in air overnight at room temperature to dry. Dry soil (100 g) was added to 1 L of sterile distilled water, and the mixture was filtered through a sterile gauze to remove soil particles. The soil extract was diluted in sterile distilled water using the serial dilution method (10-1–10-5).
Isolation of Streptomyces Bacteria
Streptomycetes were isolated using inorganic salt starch agar medium (International Streptomyces Project medium 4 [ISP 4]) containing nystatin (50 µg.mL-1) to prevent fungal contamination. The agar plates were inoculated with diluted fractions of soil extract (10-1 to 10-5) using the spreading method. The plates were incubated at 30°C for 14 d, and each colony was streaked on the same medium, followed by incubation at 30°C for 7 d.
Screening Test of Cellulolytic-ligninolytic Streptomyces Bacteria
Pretreatment of Sawdust Waste
Sawdust heaps were collected from various timber industry sites in Riyadh. Metals were eliminated from sawdust heaps using a magnetic separator.19 The sawdust heap was thoroughly ground to form a soft powder capable of acid hydrolysis,20 and then left exposed for 7 d to be completely dried. It was then stored in polyethylene bags away from humidity, which was not allowed to exceed 14%.21
Acid Hydrolysis
The treated sawdust heap was hydrolyzed using 100 mL of absolute HCl and H2SO4 (0.6, 6, and 11 M) at 25°C. The treated sawdust (100 g) was added to 100 mL of different concentrations of absolute HCl and H2SO4 and left for 48 h.21-23 The mixtures were then placed in a conical flask (1 L) and supplemented with distilled water to neutralize the acidity, after which they were filtered through filter paper (Whatman 1; Sigma-Aldrich). Glucose was detected in each filtrate using Benedict’s reagent. Activated charcoal (50 g) was added to the filtrate containing glucose and left for 24 h to detoxify the toxic compounds formed during the reaction and to inhibit microbial activity.24 The pH of each filtrate was adjusted to be in the range of 4.8–6 using 9 M NaOH.25
Cellulolytic Activity
The liquid medium (ISP 4) was inoculated with 1 mL of spore suspension of Streptomyces isolate, and incubated accompanied by shaking at 120 rpm at 30°C for 7 d. Each bacterial suspension (10 mL) was centrifuged at 5,000 × g for 20 min at 4°C. A clear upper layer containing the enzyme was used for enzymatic activity assays. Carboxymethylcellulose activity was assayed according to the method described by Mandels et al.26 The reducing sugars produced were assayed using a standard, 3,4 dinitrosalicylic acid with glucose (Sigma-Aldrich). Hydrogen peroxide (50 mmol, 200 μL) was added to initiate the reaction, which was assayed at 510 nm per minute using a UV spectrophotometer (Beckman Coulter). The absorption coefficient 21.647 M-1.cm-1 was used to calculate the enzyme activity.27
Lignolytic Activity
Ligninase activity was assayed according to the method detailed in Kizhekkedathu et al.28 using a standard, 2,4-dichlorophenol (Sigma-Aldrich). The reaction mixture (1 mL) was supplemented with 200 μL potassium phosphate buffer (0.1 M, pH 7), 25 mmol 2,4- dichlorophenol, 16 mmol 4-amino-antipyrine, and culture filtrate. Hydrogen peroxide (50 mmol, 200 μL) was added to initiate the reaction, which was assayed at 510 nm per minute using a UV spectrophotometer (Beckman Coulter). The absorption coefficient 21.647 M-1.cm-1 was used to calculate enzyme activity.
Identification of the most Potent Streptomyces Isolate (St-9)
Classical Method
The most potent cellulolytic-ligninolytic Streptomyces isolate (St-9) was identified according to the method described by Shirling and Gottlieb.29 The isolate was cultured on agar medium (ISP 4) for 7 d in preparation for scanning electron microscopy. The medium was streaked with bacterial spores and incubated for 7 d at 35°C. Bacterial growth was collected, fixed in glutaraldehyde (2.5% v/v), washed with water, and refixed in osmium tetraoxide (1% w/v) for an hour. The specimen was washed twice with water, dehydrated in ethanol, dried in a dryer (Polaron E3000), coated with gold, and examined using a JEOLISM 541OLV scanning electron microscope at 15 kV.
Genetic Method
The 16S rRNA sequencing gene was carried out using a thermocycler (Perkin Elmer Cetus Model 480; PerkinElmer, Inc.) and universal primers 27f (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1525r (5′-AAG GAG GTG ATC CAG CC-3′). The reaction conditions were as follows: 94°C for 5 min; 35 cycles of 94°C for 60 s, 55°C for 60 s, 72°C for 90 s; and 72°C for 5 min. The product was sequenced using a BigDye Terminator Cycle Sequencing Kit (PE Applied Biosystems, USA) on an ABI 310 automated DNA sequencer (Applied Biosystems). The homology of the 16S rRNA sequence of the isolate was analyzed using the Basic Local Alignment Search Tool program of the GenBank database.30
Optimization of Maximum Activity of Cellulase and Ligninase
The environmental conditions were optimized according to the method described by Isenberg31 and Van Derzant and Splittstoesser32 to yield the maximum activity of cellulase and ligninase enzymes.
Effect of Inoculum Size
The ISP 4 liquid medium was prepared, dispensed into conical flasks (100 mL/flask), and autoclaved. Each autoclaved conical flask was inoculated with a specific inoculum (20 to 220 µL) of the filtrate of the most potent Streptomyces isolate (St-9). The flasks were incubated with shaking at 120 rpm and 30°C for 7 d. Cellulase and ligninase activities were assayed for each inoculum size.
Effect of Incubation Period
ISP 4 liquid medium was prepared (100 mL), autoclaved, inoculated with a filtrate of Streptomyces isolate St-9, and incubated with shaking at 120 rpm and 30°C for different periods (5–21 d). Cellulase and ligninase activities were assayed for each incubation period.
Effect of Incubation Temperature
ISP 4 liquid medium was prepared and dispensed into conical flasks (100 mL/flask), autoclaved, inoculated with a filtrate of Streptomyces isolate St-9, and incubated with shaking at 120 rpm and different temperatures (20–45°C) for 7 d. Cellulase and ligninase activities were assayed at each incubation temperature.
Effect of pH Value
ISP 4 liquid medium was prepared and dispensed into conical flasks (100 mL/flask), and the pH of each flask was adjusted to a specified value ranging from 6.5–9, after which the flasks were autoclaved. Each autoclaved conical flask was inoculated with the best inoculum size of the filtrate of the most potent Streptomyces isolate St-9, placed in a shaker 120 rpm, and incubated at the best incubation temperature for the best incubation period. Cellulase and ligninase activities were assayed as described above for each pH value.
Effect of Nitrogen Source
Potassium nitrate-free ISP 4 liquid medium was prepared and dispensed into conical flasks (100 mL/flask). Potassium nitrate was substituted with a specific nitrogenous substance in each flask, such as peptone, urea, casein, and beef extract. The amount of the alternative nitrogenous substance was calculated using the following equations:
M = W × % of N in an alternative nitrogenous substance / 100
% of N in an alternative nitrogenous substance = [ Molecular mass / Molecular weight ] × 100
where M is the required amount of an alternative nitrogenous substance and W is the weight of the original nitrogenous substance.
The optimal pH value was adjusted for all flasks, which were then autoclaved. Autoclaved flasks were inoculated with the best inoculum size of the filtrate of Streptomyces isolate St-9, placed in a shaker at 120 rpm, and incubated at the best incubation temperature for the best incubation period. Cellulase and ligninase activities were assayed as described above for each nitrogenous substance.
Effect of Carbon Source
Soluble starch-free ISP 4 liquid medium was prepared and dispensed into conical flasks (100 mL/flask). Soluble starch was substituted with a specific carbonaceous substance in each flask, such as yeast extract, molasse, rice straw powder, and oat powder. The amount of the alternative carbonaceous substance was calculated using the following equations:
M = W × % of N in an alternative carbonaceous substance / 100
% of N in an alternative carbonaceous substance = [ Molecular mass
Molecular weight ] × 100
where M is the required amount of an alternative carbonaceous substance and W is the weight of the original carbonaceous substance.
The optimal pH value was adjusted for all flasks, which were then autoclaved, after which they were inoculated with an appropriate inoculum size of Streptomyces isolate St-9 filterate, and placed in a shaker at 120 rpm, for the appropriate time at the appropriate temperature. Cellulase and ligninase activities were assayed as described above for each carbonaceous substance.
Submerged Fermentation of Sawdust Wastes
Process
Sawdust heap (50 g) was placed in a conical flask (1 L) containing 500 mL of tap water. Equivalent weights of peptone and yeast extract were added, and the pH was adjusted to 7.5. The fermentation batch was inoculated with 200 µL.mL-1 of bacterial suspension and incubated with shaking at 120 rpm and 30°C for 7 d.
Preparation of Cell Free Extract
The fermentation batch was filtered through a sterile gauze. The filtrate was centrifuged at 5,000 × g for 10 min, and the supernatant containing cellulase and ligninase was collected in a sterile glass test tube and stored at 4°C.
Extraction and Purification of Cellulase and Ligninase
Enzyme Precipitation
A wide range of saturated ammonium sulfate (10%–90%) was used to precipitate cellulase and ligninase. The fraction was incubated for 2 h at 4°C and centrifuged at 5,000 × g for 20 min at 4°C. The precipitate was then dissolved in phosphate buffer (10 mL; pH 7.5) and centrifuged. The pellet was dissolved in phosphate buffer (10 mL, pH 7.5). Cellulolytic and ligninolytic activities were assayed for each fraction. The active fraction was dialyzed overnight in distilled water.
Estimation of Total Protein Content
One milliliter of fraction containing the enzyme was placed in a sterile glass test tube supplemented with 5 mL of alkaline copper reagent, and incubated at room temperature for 15 min.33 The reaction fraction was mixed with diluted folin reagent (0.5 mL) and incubated at room temperature for 30 min. The optical density of the reaction was measured at 280 nm using a UV-spectrophotometer (Beckman Coulter) with a mixture of a copper reagent (5 mL) and diluted folin reagent (0.5 mL) as the blank.
Gel Filtration
Cellulase and ligninase enzymes were purified using the gel filtration method, wherein Sephadex G-200 (10 g) was dissolved in of phosphate buffer pH 7.5 (400 mL), boiled in a water bath for 6 h, cooled to 50°C, and packed in a column (2.5×50 cm). The active fractions were pooled, dialyzed, and gently poured into a Sephadex G-200 chromatography column, which was eluted with phosphate buffer (pH 7.5) at a flow rate of 5 mL/25 min.34
Partial Characterization of Purified Glucose
The glucose powder was partially characterized according to the method described by Shendurse and Khedkar.35
Detection of Glucose
Only two or three drops of an extract containing glucose were added to 5 mL of boiling Fehling’s reagent, and a red precipitate indicated a positive result.
Appearance Test
A small amount of glucose was examined to discriminate the color of the solid substance, and consistency was detected.
Clarity and Color Test
Test solution: Glucose (10 g) was dissolved in 15 mL of water by heating in a water bath, and the solution was cooled to room temperature. Glucose was completely dissolved in water, and a clear solution was observed. The optical density of the glucose solution was measured and found to be equal to that of the control solution. Control solution: Di-cobalt chloride (2.5 mL), tri-iron chloride (6 mL), and di-copper sulfate (1 mL) were thoroughly mixed and diluted to 1000 mL with diluted hydrochloride solution (1:10).
Melting Point Test
A small amount of glucose was introduced into a capillary tube, which was attached to the stem of a thermometer centered in a heating bath that was heated slowly, and the melting point was observed at the beginning and completion of melting.
Solubility Test in Water
Glucose powder (1 g) was added to 10 mL of water. The mixture was then stirred at room temperature. A positive result was indicated by complete and easy dissolution of glucose in water, that is, a very clear solution with no precipitate.
Solubility Test in Ethanol
Glucose powder (1 g) was supplemented with 20 mL of ethanol, and the solution was boiled in a reflux condenser. A positive result was indicated by a clear solution.
Solubility Test in Ethyl Ether
Glucose powder (1 g) was supplemented with 20 mL of ethyl ether and boiled in a reflux condenser. A positive result was indicated by a clear solution.
Conductivity Test
Glucose powder (20 g) was dissolved in freshly boiled and cooled distilled water to a final volume of 100 mL. The conductivity of the solution was measured using a conductivity meter at 25°C with gentle stirring using a magnetic stirrer. The acceptable value of conductivity should not exceed 20 µS.cm-1.
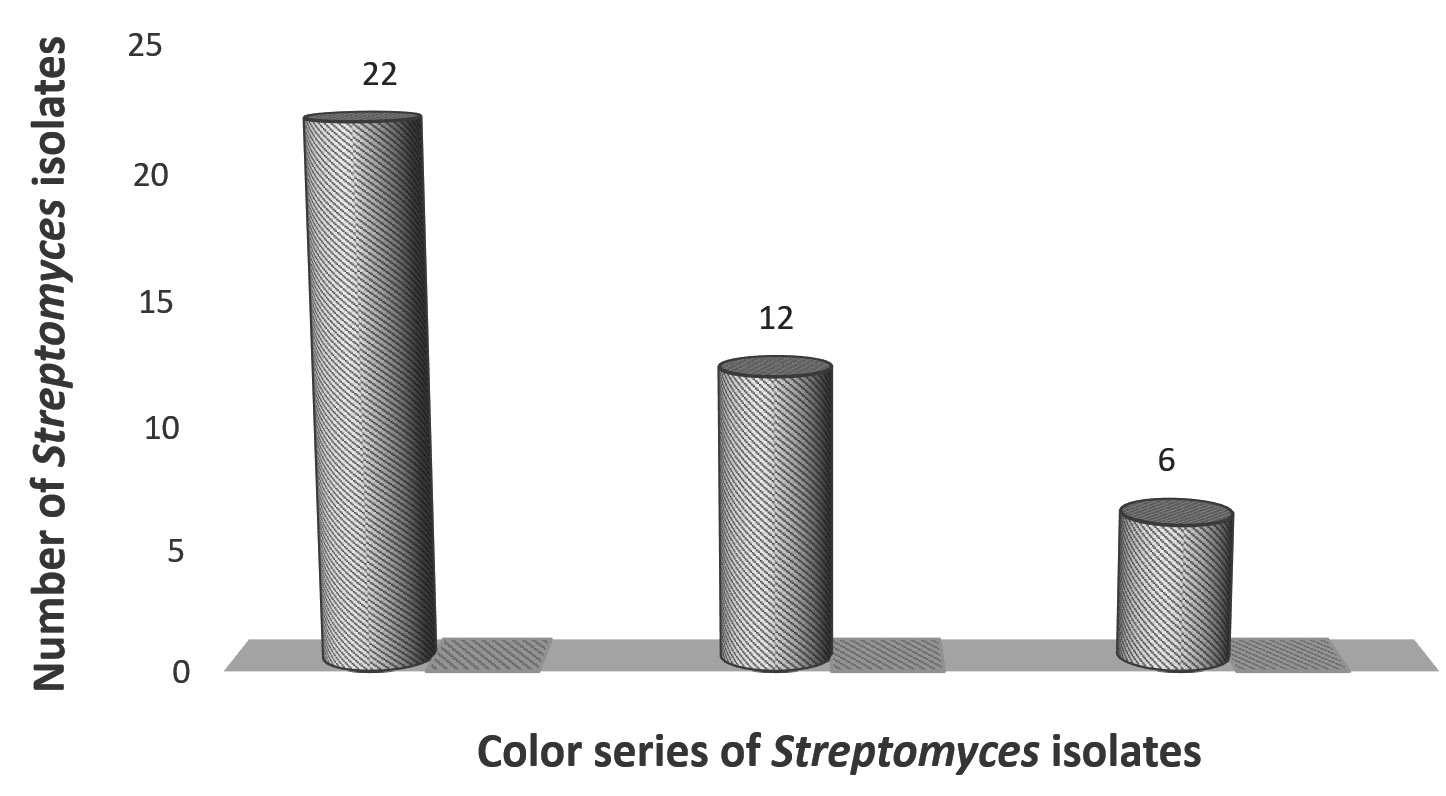
Forty unrepeated Streptomyces isolates were obtained from five soil samples. Three color series of Streptomyces isolates with discriminative cultural characteristics were observed (Figure 1). These isolates were subcultured on agar plates and agar slopes and stored at 4°C.
Forty streptomycetes were screened for production cellulolytic-ligninolytic activities. Only three isolates exhibited cellulolytic-ligninolytic activity: two had gray aerial mycelia indicating St-2 and St-9, and one had green spore mass indicating St-25. The St-9 isolate was the most potent isolate. Eight isolates did not exhibit cellulolytic or ligninolytic activity. Cellulolytic activity was produced by 19 isolates, while ligninolytic activity was produced by 10 isolates (Table 1).
Table (1):
Screening test for releasing cellulolytic-ligninolytic activity.
| Isolate | Activity (mg/ml) | Color series | Isolate | Activity (mg.ml-1) | Color series | ||
|---|---|---|---|---|---|---|---|
| Cellulase | Ligninase | Cellulase | Ligninase | ||||
| St-1 | 0.412 | None | Grey | St-21 | 0.311 | None | Grey |
| St-2 | 0.325 | 0.274 | Grey | St-22 | 0.305 | None | Grey |
| St-3 | 0.357 | None | Grey | St-23 | 0.408 | None | Green |
| St-4 | None | None | Grey | St-24 | 0.125 | None | Green |
| St-5 | 0.215 | None | Grey | St-25 | 0.415 | 0.342 | Green |
| St-6 | 0.294 | None | Grey | St-26 | None | 0.102 | Green |
| St-7 | 0.354 | None | Grey | St-27 | None | 0.147 | Green |
| St-8 | 0.425 | None | Grey | St-28 | None | 0.271 | Green |
| St-9 | 0564 | 0.482 | Grey | St-29 | None | 0.232 | Green |
| St-10 | None | 0.211 | Grey | St-30 | None | None | Green |
| St-11 | None | 0.384 | Grey | St-31 | None | None | Green |
| St-12 | None | None | Grey | St-32 | 0.353 | None | Green |
| St-13 | None | None | Grey | St-33 | None | 0.211 | Green |
| St-14 | None | 0.388 | Grey | St-34 | None | 0.202 | Green |
| St-15 | None | 0.247 | Grey | St-35 | None | None | Yellow |
| St-16 | 0.335 | None | Grey | St-36 | None | None | Yellow |
| St-17 | 0.247 | None | Grey | St-37 | None | None | Yellow |
| St-18 | 0.415 | None | Grey | St-38 | 0.348 | None | Yellow |
| St-19 | 0.259 | None | Grey | St-39 | 0.314 | None | Yellow |
| St-20 | 0.349 | None | Grey | St-40 | 0.254 | None | Yellow |
The most potent isolate, St-9, was identified to be S. lazureus. The isolate was cultivated on seven recommended culture media according to the ISP to determine its cultural characteristics (Table 2). It had approximately the same cultural characteristics on both tryptone yeast extract broth and glycerol asparagine agar media, in which good growth of a light grey spore mass and yellowish-brown substrate mycelia were observed; however, a yellowish-brown diffusible pigment was observed only on tryptone yeast extract broth medium, and no diffusible pigment was observed on glycerol asparagine agar medium. Poor growth of a light grey spore mass, greyish-yellow substrate mycelia, and no diffusible pigment were observed on yeast–malt extract agar medium. Good growth of light grey spore mass, greyish-brown substrate mycelia, and diffusible pigment were observed on oat-meal extract agar medium. Good growth of pinkish-grey spore mass, yellowish-brown substrate mycelia, and no diffusible pigment were observed on inorganic salt starch agar medium. Moderate growth of a white spore mass, yellow substrate mycelia, and light grey diffusible pigment were observed on peptone yeast extract iron agar medium. Good growth of white spore mass, yellowish-brown substrate mycelia, and yellowish-brown diffusible pigment were observed on tyrosine agar medium.
Table (2):
Discriminative cultural characteristics of Streptomyces isolate St-9.
| Medium | Growth rate | Color | ||
|---|---|---|---|---|
| Aerial mycelia | Substrate mycelia | Diffusible Pigment | ||
| TYEB | Good | Light grey | Yellowish brown | Yellowish brown |
| YMEA | Poor | Light grey | Grayish yellow | None |
| OMEA | Good | Light grey | Grayish brown | Grayish brown |
| ISSA | Good | Pinkish grey | Yellowish brown | None |
| GAA | Good | Light grey | Yellowish brown | None |
| PYEIA | Moderate | White | Yellow | Light grey |
| TA | Good | White | Yellowish brown | Yellowish brown |
TYEB; Tryptone Yeast Extract Broth, YMEA; Yeast-Malt Extract Agar, OMEA; Oat-Meal Extract Agar, ISSA; Inorganic Salt Starch Agar, GAA; Glycerol Asparagine Agar, PYEIA; Peptone Yeast Extract Iron Agar, TA; Tyrosine Agar.
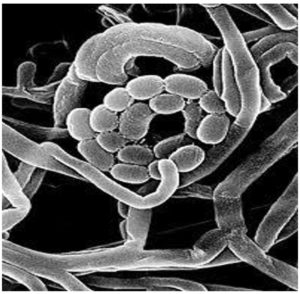
A spiral spore chain composed of smooth-surfaced ellipsoidal spores was observed on the scanning electron micrograph (Figure 2). Grey spore masses of the nonmotile isolates were observed (Table 3). The cell wall contained LL-diaminopimelic acid, and no sugar pattern was detected. Amylase, protease, lipase, cellulase, ligninase, pectinase, and catalase were produced, whereas lecithinase and nitrate reductase were not. Melanoid pigment was produced on tryptone yeast extract broth, peptone yeast extract iron agar, and tyrosine agar media. Hydrogen sulfide was not produced, and neither xanthin nor esculin was degraded. The isolate was sensitive to both streptomycin and amoxicillin. Rhamnose, xylose, arabinose, and inositol were not fermented, whereas glucose, fructose, galactose, sucrose, lactose, mannose, mannitol, raffinose, and starch were. In addition, cysteine, proline, valine, alanine, lysine, leucine, tyrosine, and phenylalanine were fermented. Good growth of the isolate was observed over a wide range of NaCl concentrations (1%–10%). For genetic identification, the sequence of the 16S rRNA gene was determined via polymerase chain reaction, which showed that the Streptomyces isolate St-9 and S. lazureus with accession number NBRC13384/AB184368 in GenBank are 97% similar.
Table (3):
Morphological and physiological characteristics of Streptomyces isolate St-9.
| Item | Character | Result |
|---|---|---|
| Morphological characteristics | Spore mass | Grey |
| Motility | Non-Motile | |
| Cell wall hydrolysis | Diaminopimelic acid (DAP) | LL-DAP |
| Sugar pattern | Not detected | |
|
Physiological characteristics |
Amylase, protease, lipase, cellulase, ligninase, pectinase and catalase | Positive |
| Lecithinase and nitrate reductase | Negative | |
| Melanoid pigment | Positive | |
| H2S production | Negative | |
| Degradation of xanthin and esculin | Negative | |
| Streptomycin and amoxicillin resistance | Negative | |
| Utilization of glucose, fructose, galactose, sucrose, lactose, mannose, mannitol, raffinose and starch | Positive | |
| Utilization of rhamnose, xylose, arabinose and inositol | Negative | |
| Utilization of cysteine, proline, valine, alanine, lysine, leucine, tyrosine and phenylalanine | Positive | |
| NaCl tolerance (1 – 10%) | Positive |
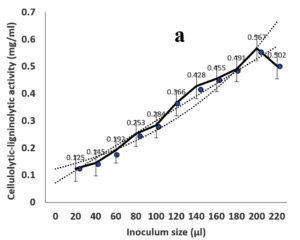
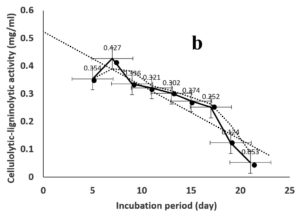
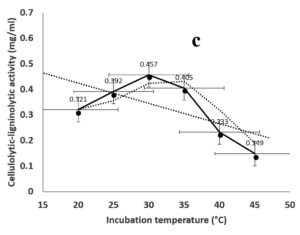
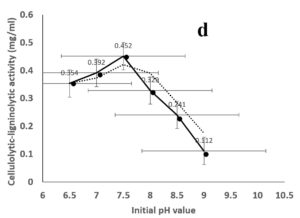
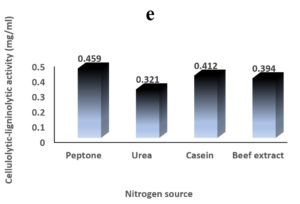
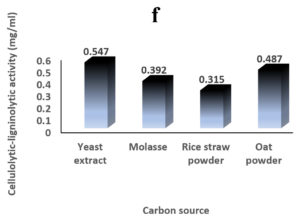
Environmental and nutritional factors were optimized to yield the maximum cellulolytic-ligninolytic activity of Streptomyces isolate St-9. Different inoculum sizes (20–220 µL.mL-1) of the bacterial filtrate were screened, and the best inoculum size was recorded at 200 µL, at which maximum activity (0.567 µL.mL-1) was measured (Figure 3a). The best incubation period was recorded on day 7, at which the highest activity (0.427 µL.mL-1) was measured (Figure 3b). The best incubation temperature was found to be 30°C, at which the highest activity (0.457 µL.mL-1) was measured (Figure 3c). Meanwhile, the best pH value was 7.5, at which the highest activity (0.452 µL.mL-1) was measured (Figure 3d). Peptone was found to be the best nitrogen source, at which maximum activity (0.459 µL.mL-1) was measured (Figure 3e), while yeast extract was found to be the best carbon source, at which maximum activity (0.547 µL.mL-1) was measured (Figure 3f).
Figure 3. Determination the optimum environmental and nutritional factors to yield a maximum cellulolytic-ligninolytic activity by Streptomyces isolate St-9
The fermentation liquid medium containing cellulase and ligninase was filtered and centrifuged to obtain a clear supernatant. The enzyme was precipitated using different concentrations of saturated ammonium sulfate (10–90%). Total protein content (mg), enzymatic activity (mm), and total activity (u) were measured for each fraction. The active fractions were pooled and dialyzed overnight at 4°C. Cellulase was precipitated at 40% and 50% saturated ammonium sulfate (Table 4), where a specific activity of 43.9 and 35.5 u.mg-1, purification fold of 39.1 and 31.6, and yield percentage of 97.2 and 86.4, respectively, were observed. Meanwhile, ligninase was precipitated at 40% and 50% saturated ammonium sulfate (Table 5), where a specific activity of 44.4 and 39.4, purification fold of 36.5 and 32.4, and the yield percentage of 94.1 and 90.5, respectively, were observed. Accordingly, cellulase and ligninase were abundantly produced with high activity by Streptomyces isolate St-9, enabling the degradation of sawdust waste and liberation of glucose monomers as end products.
Table (4):
Purification parameters of cellulase produced by Streptomyces isolate St-9.
% (NH4)2SO4 |
Activity (mm) |
TA (u) |
TPC (mg) |
SA (u/mg) |
PF |
% of yield |
|---|---|---|---|---|---|---|
Filtrate |
40.0 |
185 |
165 |
1.121 |
1.0 |
100 |
10% |
0.0 |
0.0 |
2.4 |
0.0 |
0.0 |
0.0 |
20% |
0.0 |
0.0 |
2.9 |
0.0 |
0.0 |
0.0 |
30% |
0.0 |
0.0 |
3.6 |
0.0 |
0.0 |
0.0 |
40% |
35.0 |
180 |
4.1 |
43.9 |
39.1 |
97.2 |
50% |
25.0 |
160 |
4.5 |
35.5 |
31.6 |
86.4 |
60% |
0.0 |
0.0 |
4.9 |
0.0 |
0.0 |
0.0 |
70% |
0.0 |
0.0 |
5.1 |
0.0 |
0.0 |
0.0 |
80% |
0.0 |
0.0 |
5.4 |
0.0 |
0.0 |
0.0 |
90% |
0.0 |
0.0 |
5.9 |
0.0 |
0.0 |
0.0 |
TA; Total Activity, TPC; Total Protein Content, SA; Specific Activity, PF; Purification Fold
Table (5):
Purification parameters of ligninase produced by Streptomyces isolate St-9.
% (NH4)2SO4 |
Activity (mm) |
TA (u) |
TPC (mg) |
SA (u/mg) |
PF |
% of yield |
|---|---|---|---|---|---|---|
Filtrate |
32.0 |
170 |
140 |
1.214 |
1.0 |
100 |
10% |
0.0 |
0.0 |
2.0 |
0.0 |
0.0 |
0.0 |
20% |
0.0 |
0.0 |
2.3 |
0.0 |
0.0 |
0.0 |
30% |
0.0 |
0.0 |
2.8 |
0.0 |
0.0 |
0.0 |
40% |
25.0 |
160 |
3.6 |
44.4 |
36.5 |
94.1 |
50% |
20.0 |
154 |
3.9 |
39.4 |
32.4 |
90.5 |
60% |
0.0 |
0.0 |
4.6 |
0.0 |
0.0 |
0.0 |
70% |
0.0 |
0.0 |
4.8 |
0.0 |
0.0 |
0.0 |
80% |
0.0 |
0.0 |
5.4 |
0.0 |
0.0 |
0.0 |
90% |
0.0 |
0.0 |
5.7 |
0.0 |
0.0 |
0.0 |
TA; Total Activity, TPC; Total Protein Content, SA; Specific Activity, PF; Purification Fold
Table (6):
Partial characterization of purified glucose.
Characters |
Results |
|---|---|
Appearance |
White powder |
Clarity of solution |
Clear |
Color of solution |
Colorless |
Melting point |
146°C |
Solubility in water |
Very soluble |
Solubility in ethanol |
Slightly soluble |
Solubility in ethyl ether |
Insoluble |
Conductivity |
15 µS.cm-1 |
The solid-released glucose was partially characterized (Table 6). A white powder was observed, which melted at 146°C, and the solution was clear with no precipitates. The powder was highly solubilized in water owing to the high polarity and formation of hydrogen bonds and was slightly solubilized in ethanol owing to its low polarity; however, it was insoluble in ethyl ether owing to the lack of polarity and formation of hydrogen bonds. A low electrical conductivity (15 µS.cm-1) of the glucose solution was measured because of the inability of glucose to ionize during dissolution in water; therefore, the glucose solution was considered to be a nonelectrolyte.
Cellulose, hemicellulose, and lignin are the main components of the plant cell wall. The two polysaccharides are nondegradable owing to their rigidity and complexity. Some microorganisms, particularly actinobacteria and Streptomyces, effectively degrade these recalcitrant substances in vitro and in vivo.36 Biodegradation of these polysaccharides by Streptomyces and others enhances the maintenance of the ecosystem and the carbon cycle.37 In particular, Actinobacteria and Streptomyces completely degrade recalcitrant substances due to the presence of encoded genes (CAZy) for hydrolyzing enzymes.38,39
Lignin is more resistant to biodegradation than other polysaccharides of the plant cell wall because its structure contains polyphenols, which inhibit the biodegradation effect.40 Some microorganisms other than Streptomyces effectively degrade polysaccharides, such as Sphingomonas paucimobilis SYK-6, Serratia, Citrobacter, Klebsiella, Paenibacillus, Aneurinibacillus, and Bacillus spp.41,42 Soil aromatic-degrading bacteria, such as Pseudomonas putida and Rhodococcus sp. RHA1, efficiently degrade lignin.43 Air pollution resulting from sawdust burning was the main motivation to eliminate it through the biodegradation process.44 Lignin biodegradation leads to the release of sugars, biofuels, and other economic products.45
Identification of Streptomyces isolates was based on the determination of cultural, morphological, physiological, biochemical, and genetic characteristics. The cultural characteristics were represented by the growth rate and colors of spore mass, substrate mycelia, and diffusible pigment on different culture media recommended in ISP29 and by Pridham et al.46 Streptomyces was differentiated from other similar anaerobic Actinobacteria and aerobic Nocardia and Rhodococcus; it was documented as aerobic filamentous, gram positive, and partially acid-fast stain negative.
The removal of sawdust from the environment must be carried out safely using saprophytic microorganisms. Owing to the high cellulose and lignin content in sawdust biomass, its degradation depends on the production of cellulase and ligninase. Actinobacteria, especially Streptomyces spp., are distributed in different polysaccharide-rich habitats, such as wood processing plants because of the secretion of hydrolyzing enzymes. Lignocellulosic biomass represents a complex ecosystem in which environmental conditions influence living organisms. Thus, it is very urgent to achieve the strategic goals of prevention of environmental pollution and production of valuable products, especially pharmaceuticals, intended to meet medical field requirements.47 The lignocellulose-Streptomyces bacteria efficiently released sugars. Ventorino et al.48 reported that the most potent lignocellulosic hydrolyzing strains were S. argenteus because they are highly adapted to lignocellulosic ecosystems. Different studies have shown that Actinobacteria, such as Cellulomonas, Thermobifida, and Microbispora, are high producers of cellulase.49 A set of cellulases (endoglucanases, exoglucanases, and cellobiases), hemicellulase, and ligninase are produced by Streptomyces bacteria, making them the most efficient and commonly used enzymes in lignocellulose biomass degradation.50,51 Streptomyces producing cellulases saccharify different lignocellulose biomasses, such as rice straw and cellulose-rich organic materials.52
Alam et al.53 reported that Streptomyces omiyaensis exhibited maximum activity for lignocellulose biomass degradation after 5 d of incubation, while George et al.54 showed that Streptomyces noboritoensis SPKC1 produced the highest lignocellulolytic activity after 8 d of incubation. Jang and Chen55 revealed that Streptomyces T3-1 produced the highest lignocellulolytic activity at 50°C. The best nitrogen source for producing lignocellulolytic activity by Streptomyces sp. BRC2 was found to be ammonium phosphate. The highest lignocellulolytic activity of Streptomyces sp. was observed at pH 6.5–7.5.56 Kunamneni et al.57 showed that a high inoculum size lead to a reduction in enzyme yield due to nutrient limitations. Therefore, a low inoculum size may require a longer time to produce the desired enzyme. Conversely, a high inoculum size provided rapid proliferation of microbial biomass. Therefore, the balance between proliferating biomass and substrate utilization yielded maximum enzyme activity.58
Abdulkhair et al.59 reported that seven marine Streptomyces isolates exhibited antidiabetic activity due to the production of α-glucosidase inhibitory protein, which was measured spectrophotometrically at 400 nm. The most potent isolate was identified as Streptomyces coelicolor, which showed stable activity for 5 d at 37°C. The highest activity and stability of the antidiabetic compound were determined under optimum environmental conditions, including inoculum size (10 cfu.mL-1.300 µL-1), incubation period (14 d), agitation speed (160 rpm), incubation temperature (30°C), and pH (8.5).
Actinobacteria can grow on a sawdust waste and produce different bioactive compounds.60 Although the useful properties of sawdust and other lignocellulose wastes, they have a hazard effect on the environment and public health.61 Different microorganisms including actinobacteria inhabit all environments especially the soil, where they degrade the organic polymers during fermentation process.62
This study showed that S. lazureus biodegraded sawdust waste rich in cellulose and lignin and released glucose monomers. S. lazureus exhibited the highest lignocellulolytic activity under optimum environmental and nutritional conditions. Sawdust biodegradation and glucose production are considered strategic and economic goals, with the prevention of environmental pollution resulting from sawdust burning and the production of glucose as a valuable pharmaceutical product at a low cost.
ACKNOWLEDGMENTS
The authors would like to thank Head and Members of Shaqra University and Head and Members of Science and Humanities College for their great support to this work.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Fregoso-Madueno JN, Goche-Telles JR, Rutiaga-Quinones JG, Gonzalez-Laredo RF, Bocanegra-Salazar M, Chavez-Simental JA. Alternative uses of sawmill industry waste. Rev Chapingo Ser Ciencias For Ambient. 2017;23(2):243-260.
Crossref - Arroyo-Vinueza JS, Reina-Guzman WS. Exploitation of biomass resources in the form of wood waste for steam boiler. Ingenius Rev Ciencias Tec. 2016;16:20-29.
Crossref - Gwenzi W, Ncube RS, Rukuni T. Development, properties and potential applications of high-energy fuel briquettes incorporating coal dust, biowastes and post-consumer plastics. SN Appl Sci. 2020;2(1006):1-14.
Crossref - Diaz-Artigas IJ, Diaz-Concepcinn A, Rodriguez-Pinero AJ, Alfonso-Alvarez A, Tamayo-Mendoza JE. Energy briquettes with sawdust and pine bark. Ing Energetica. 2020;41:1-6.
- Olayinka A, Adebayo A. Effect of pre-incubated sawdust-based cow dung on the growth and nutrient uptake of Zea mays L. and on soil chemical properties. Biol Fert Soil. 1989;7:176-179.
Crossref - Horisawa S, Sunagawa M, Tamai Y, Masuoka Y, Miura T, Terazawa M. Biodegradation of non-lignocellulosic substances II: physical and chemical properties of sawdust before and after use as artificial soil. J Wood Sci. 1999;45(6):492-497.
Crossref - Erikson KE, Blanchette RA, Ander P. Microbial and enzymatic degradation of wood and wood components. In Timell TE (eds.), Wood Decomposition, 1st Ed. Springer: Berlin, Heidelberg, New York. 1990:41-62.
Crossref - Adeline SY, Ka LP. Indigenous actinomycetes from empty fruit bunch compost of oil palm: Evaluation of enzymatic and antagonistic properties. Biocat Agr Biotechnol. 2014;3(4):310-315.
Crossref - Ververis C, Georghiou K, Danielidis D, et al. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Biores Technol. 2007;98:296-301.
Crossref - Kulic GJ, Radojicic VB. Analysis of cellulose content in stalks and leaves of large leaf tobacco. J Agr Sci. 2011;56(3):207-215.
Crossref - Ul-Hassan A, Wellington EM. Encyclopedia of Microbiology, 3rd Ed. Washington, D.C., American Society for Microbiology, USA. 2009.
- Dhakal D, Pokhrel AR, Shrestha B, Sohng JK. Marine rare actinobacteria: Isolation, characterization, and strategies for harnessing bioactive compounds. Front Microbiol. 2017;8(1106):1-13.
Crossref - Boone DR, Castenholz RW, Garrity GM. The archaea and the deeply branching and phototrophic bacteria. In David RB, Richard W (eds.), Bergey’s Manual of Systematic Bacteriology, 2nd Ed. Springer-Verlag, New York. 2001:21-166.
Crossref - Garrity GM, Bell JA, Lilburn TG. Taxonomic outline of the prokaryotes. In George MG, Julia AB, Timothy GL (eds.), Bergey’s Manual of Systematic Bacteriology, 2nd Ed. Springer, New York. 2004:1-401.
- Bassima AA, Essra GA, Anmar AA. Test the inhibition activity of local Streptomyces spp. by used sawdust as a carbon source. Conf Pap Sci Coll Mosul Univ. 2019;4:301-309.
- Sukumaran RK, Singhania RR, Pandey A. Microbial cellulases production, applications and challenges. J Sci Ind Res. 2005;64(11):832-844.
- Wen Z, Liao W, Chen S. Production of cellulase/β-glucosidase by the mixed fungi culture Trichoderma reesei and Aspergillus phoenicis on dairy manure. Proc Biochem. 2005;40(9):3087-3094.
Crossref - Maki M, Leung KT, Qin W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci. 2009;5(5):500-516.
Crossref - Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 2009;48(8):3713-3729.
Crossref - Leustean I. Bioethanol from lignocellulosic materials. J Agro-aliment Proc Technol. 2009;15:94-101.
- Hayes DJ. An examination of bioreining processes, catalysts, and challenges. Catal Today. 2009;145:138-151.
Crossref - Nwakaire JN, Ezeoha SL, Ugwushiwu BO. Production of cellulosic ethanol from wood sawdust. Agric Eng Int CIGR J. 2013;15(3):136-140.
- Olayemi S, Ibikunle A, Olayemi J. Production of ethanol from cassava and yam peels using acid hydrolysis. Am Sci Res J Eng Technol Sci. 2019;52(1):67-78.
- Cao G, Ximenes E, Nichols NN, Zhang L, Ladisch M. Biological abatement of cellulase inhibitors. Bioresour Technol. 2013;146:604-610.
Crossref - Mishra A, Ghosh S. Bioethanol production from various lignocellulosic feedstocks by a novel “fractional hydrolysis” technique with different inorganic acids and co-culture fermentation. Fuel. 2019;236:544-553.
Crossref - Eveleigh DE, Mandels M, Andreotti R, Roche C. Measurement of saccharifying cellulase. Biotechnol Biofuels, 2009;2: 21. doi: 10.1186/1754-6834-2-21.
- Miller GL. Use of dinitro-salicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426-428.
Crossref - Kizhekkedathu NN, Parukuttyyamma P. Mangrove actinomycetes as the source of lignolytic enzymes. Actinomycetologica. 2005;19(2):40-47.
Crossref - Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int Syst Bact J. 1966;16:313-340.
Crossref - Prapagdee B, Kuekulvong C, Mongkolsuk S. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int Boil Sci J. 2008;4(5):330-337.
Crossref - Isenberg HD. Clinical Microbiology Procedures Handbook, 3rd Ed. Washington, D.C., American Society for Microbiology, USA. 1992.
- Van derzant C, Splittstoesser D. Compendium of Methods for the Microbiological Examination of Foods, 3rd Ed. Washington, D.C., American Public Health Association, USA. 1992.
- Lowry OH, Rosebrough J, Farr AL, Randall RJ. Protein measurements with the folin phenol reagent. Biol Chem J. 1951;193(1):265-275.
Crossref - Andrews P. Estimation of the molecular weight of proteins by sephadex gel filtration. Biochem J. 1964;91(2):222-223.
Crossref - Shendurse AM, Khedkar CD. Glucose: properties and analysis. In Caballero B, Finglas P, Toldra F (eds.), Encyclopedia of Food and Health, 1st Ed. Elsevier, Academic Press, Oxford. 2015:239-247.
Crossref - Matulich KL, Martiny JBH. Microbial composition alters the response of litter decomposition to environmental change. Ecology. 2015;96(1):154-163.
Crossref - Chater KF, Biro S, Lee KJ, Palmer T, Schrempf H. The complex extracellular biology of Streptomyces. FEMS Microbiol Rev. 2010;34(2):171-198.
Crossref - Berlemont R, Martiny AC. Phylogenetic distribution of potential cellulases in bacteria. Appl Environ Microbiol. 2013;79(5):1545-1554.
Crossref - Doroghazi JR, Metcalf WW. Comparative genomics of actinomycetes with a focus on natural product biosynthetic genes. BMC genomics. 2013;14(1):6-11.
Crossref - Chandra R, Singh R. Decolorization and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem Engin J. 2012;61:49-58.
Crossref - Masai E, Katayama Y, Fukuda M. Genetic and biochemical investigations of bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem. 2007;71(1):1-15.
Crossref - Chandra R, Singh S, Reddy MMK, Patel DK, Purohit HJ, Kapley A. Isolation and characterization of bacterial strains Paenibacillus sp. and Bacillus sp. for Kraft lignin decolorization from pulp paper mill waste. J Gen Appl Microbiol. 2008;54(6):399-407.
Crossref - Ahmad M, Taylor CR, Pink D, et al. Development of novel assays for lignin degradation: Comparative analysis of bacterial and fungal lignin degraders. Mol Bios. 2010;6(5):815-821.
Crossref - Buraimoh OM, Ilori MO, Amund OO. Characterization of lignocellulolytic bacterial strains associated with decomposing wood residues in the Lagos lagoon, Nigeria. Malays J Microbiol. 2015;11(2015):273-283.
Crossref - Chen F, Dixion RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25(7):759-761.
Crossref - Pridham TG, Anderson PE, Foley LA, Lindenfelser E, Hesseltine W, Benedict RG. A selection of media for maintenance and taxonomic study of Streptomyces. Antibiot Ann. 1956;1957:947-953.
- Dawkins K, Esiobu N. Emerging insights on Brazilian pepper tree (Schinus terebinthifolius) invasion: the potential role of soil microorganisms. Front Plant Sci. 2016;7:712.
Crossref - Ventorino V, Ionata E, Birolo L, et al. Lignocellulose-adapted endo-cellulase producing Streptomyces strains for bioconversion of cellulose-based materials. Front Microbiol. 2016;7:2061.
Crossref - Saini A, Aggarwal NK, Sharma A, Yadav A. Actinomycetes: a source of lignocellulolytic enzymes. Enzym Res. 2015;2015:279-381.
Crossref - Amore A, Pepe O, Ventorino V, Birolo L, Giangrande C, Faraco V. Cloning and recombinant expression of a cellulase from the cellulolytic strain Streptomyces sp. G12 isolated from compost. Microb Cell Fact. 2012;11(1):164.
Crossref - Raweesri P, Riangrungrojana P, Pinphanichakarn P. Alpha-L-arabinofuranosidase from Streptomyces sp. PC22: purification, characterization and its synergistic action with xylanolytic enzymes in the degradation of xylan and agricultural residues. Bioresour Technol. 2008;99(18):8981-8986.
Crossref - Prasad P, Bedi S, Singh T. In vitro cellulose rich organic material degradation by cellulolytic Streptomyces albospinus (M0TCC8768). Malays J Microbiol. 2012;8(3):164-169.
Crossref - Alam MZ, Manchur MA, Anwar MN. Isolation, purification, characterization of cellulolytic enzymes produced by the isolate Streptomyces omiyaensis. Biol Sci. 2004;7(10):1647-1653.
Crossref - George J, Arunachalam R, Paulkumar K, Wesely EG, Shiburaj S, Annadurai G. Characterization and phylogenetic analysis of cellulase producing Streptomyces noboritoensis SPKC1. Interdiscip Sci. Comput Life Sci. 2010;2(2):205-212.
Crossref - Jang HD, Chen KS. Production and characterization of thermostable cellulases from Streptomyces transformant T3-1. World J Microbiol Biotechnol. 2003; 19(3): 263-268.
Crossref - Hossain FMM, Rahman MM, Choudhury N, Malek MA. Extracellular carboxy methyl cellulase and cellobiase of some aerobic bacterial isolates. Bangladesh J Microbiol. 1998;15:17-26.
- Kunamneni A, Permaul K, Singh S. Amylase production in solid state fermentation by the thermophilic fungus Thermomyces lanuginosus. J Biosci Bioeng. 2005;100(2):168-171.
Crossref - Ramachandran S, Patel AK, Nampoothiri KM, et al. Coconut oil cake-a potential raw material for the production of α-amylase. Bioresour Technol. 2004;93(2):169-174.
Crossref - Abdulkhair WM, Walaa SA, Bahy RH. Genetic improvement of antidiabetic alpha-glucosidase inhibitor producing Streptomyces sp. Int J Pharm Pharma Sci. 2018;10(5):77-84.
Crossref - Ekaterina VP, Maria ED, Maria MM, et al. The Use of Baikal psychrophilic actinobacteria for synthesis of biologically active natural products from sawdust waste. Fermentation. 2022;8(213):1-20.
Crossref - Hajam ME, Plavan GI, Kandri NI, et al. Evaluation of softwood and hardwood sawmill wastes impact on the common carp “Cyprinus Carpio” and its aquatic environment: An oxidative stress study. Environ Toxicol Pharmacol. 2020;75:103327.
Crossref - Amin DH, Abdallah NA, Abolmaaty A, Tolba S, Wellington EMH. Microbiological and molecular insights on rare actinobacteria harboring bioactive prospective. Bull Natl Res Cent. 2020;44:5.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.