ISSN: 0973-7510
E-ISSN: 2581-690X
The most common cause of severe diarrhea in children under five is rotavirus infection. Pediatric comorbidities are integral components that may affect the prognostic outcomes of rotavirus gastroenteritis (RVGE). However, little is known about RVGE severity in the presence of comorbid conditions in children under five years of age. This study aims to compare the clinical outcomes of rotavirus diarrhea in children with and without co-morbidities. Children under five years of age suffering from acute diarrhea and admitted to the Inpatient Department (IPD)/Intensive Care Unit (ICU) were included. ELISA was used to identify the rotavirus antigen in stool samples obtained from each of the study participants. The Vesikari severity score index was used to assess the degree of disease severity. Out of the 76 participants recruited, rotavirus was detected in 13 (17.1%) cases. Comorbidities were present in 42 (55.2%) of the study participants, and eight (10.5%) patients had RVGE. The most common comorbidities observed were severe acute malnutrition (SAM) followed by malignancies. An increased frequency of diarrhea, vomiting, and fever was observed among children under five years of age with rotavirus diarrhea (p < 0.05). A significant difference in the severity of clinical manifestations was observed among RVGE patients with comorbidities (p < 0.05). This study demonstrated that rotavirus diarrhea was still common in hospitalized children under five years old, even though rotavirus vaccination was added to the universal immunization program. The presence of comorbidities further accelerated the deterioration of clinical conditions among RVGE patients highlighting the need for additional measures to prevent rotavirus infection in children with comorbid conditions.
Rotavirus, Co-morbidity, Gastroenteritis, Rotavirus Antigen ELISA, Under 5 children
Rotaviruses continue to cause severe, dehydrating diarrhoea in children under five years old, and they are responsible for over 25 million outpatient visits and over 2 million hospitalizations annually worldwide.1 In 2016, rotavirus diarrhoea accounted for 28.8% (95% UI, 25.0%-32.6%) of fatalities in children under the age of five globally.2 According to the National Rotavirus Surveillance Network, an overall rotavirus prevalence of
36.3% was reported among children younger than five years of age hospitalized for diarrhoea in India.3
Rotavirus infection is highly communicable. Infected individuals excrete large amounts of the virus in their stool, starting two days before the onset of diarrhea and continuing for many days after symptoms appear. In immunocompromised hosts, the period of viral excretion are prolonged.4 Although the disease is self-limiting in many cases, complications such as severe diarrhoea, dehydration, electrolyte imbalance, and metabolic acidosis have been reported among infants and young children.4 Rotavirus-induced diarrhea plays a significant role in the development of long-term nutritional deficiencies, including stunting, wasting, malnutrition, and impaired cognitive development. Rotavirus infection is an independent risk factor for neonatal seizures, and associated with the white matter injury.5 Presence of co-morbidities is an additional risk factor which is to be considered while assessing the prognosis of RVGE patients. Due to the immature immune systems and lower antibody levels, premature neonates are at a higher risk of developing severe disease during the first month of life and early infancy.6 In a study conducted among children with cancer undergoing extensive treatment with chemotherapy, disease severity, complications and length of hospital stay were reported to be higher among RVGE cases. A greater percentage of patients who tested positive for rotavirus required tube feeding and parenteral nourishment.7 Malnutrition or secondary immunosuppression due to high-dose chemotherapy in patients with malignancy or after hematopoietic stem cell transplantation impact both the gut’s physiological integrity and its microbial composition.8 This may increase susceptibility to enteric pathogens including rotavirus and worsen the disease severity and prognosis of RVGE.
In India, the Rotavirus vaccine (RVV) was introduced into the Universal Immunization Program (UIP) in March 2016. It was introduced into Madhya Pradesh in a phased manner, with the second phase commencing in early 2017, as part of UIP. The present study was conducted after RVV implementation to assess the clinical profiles of rotavirus-infected children under five years old. Pediatric comorbidities are integral components that may affect prognostic outcomes of RVGE. Accordingly, special management and decisions regarding additional RVV doses in this special group should be planned by healthcare providers and policymakers. Our study aims to compare the clinical outcomes of rotavirus diarrhea in children under 5 years of age, focusing on those with and without associated comorbidities.
Ethical considerations
The study was initiated after obtaining due approval from the Istitutional Human Ethics Committee. Study participants were recruited upon acquiring the parents’ or guardians’ written informed consent.
Recruitment, clinical data collection and sampling of study participants
This was a prospective, observational study, conducted at the Department of Microbiology and Paediatrics from June 2020 to December 2021 at AIIMS Bhopal, which is a tertiary care teaching hospital located in Central India.
Children <5 years of age suffering from acute diarrhea and admitted to the Inpatient Department (IPD) or Intensive Care Unit (ICU) of our hospital were included. Acute diarrhea was defined as the diarrhea (stools of a less formed character than usual) within a 24 h period, less than 7 days prior to hospital visit.9
Co-morbid conditions were considered as any child suffering from malnutrition (undernutrition or overnutrition), malignancies, immunodeficiency diseases, children admitted in ICU due to any other debilitating conditions, or any other chronic illnesses. These cases were identified based on clinical diagnosis and confirmation by diagnostic tests such as imaging (X-rays, CT scans, MRIs), biopsies, or blood tests including CBC, immunoglobulin levels and tumor markers for childhood malignancies.
The detailed epidemiological and clinical presentations of the study participants were recorded on a predesigned case record form. Details of the study participants, such as age, sex, anthropometric measures, nutritional status, rotavirus immunization status, and associated morbidities were also noted. The modified Kuppuswamy’s socioeconomic status scale based on three parameters, that is, education, occupation, and family income, was used to score the socioeconomic status of the recruited participants.10
The Vesikari severity scoring index was used to assess illness severity in the recruited participants. The illness was graded as mild (score <7), moderate (score 7-10), and severe (score >11) based on the scoring system of clinical parameters such as duration and frequency of diarrhea and vomiting, body temperature, severity of dehydration, and treatment modality required.11
To detect the rotavirus antigen, a stool sample was collected from each participant in a sterile screw-capped container before being transferred to the virology laboratory of the microbiology department. If immediate transport was not possible, the specimen was stored at 2-80 °C and transported to the laboratory under a cold chain.
Rotavirus antigen detection
The samples were processed using the PREMIER® Rotaclone (Meridian Bioscience Inc., Cincinnati, Ohio, USA) ELISA, according to the manufacturer’s instructions. It was a solid phase sandwich type ELISA directed against the product of the sixth viral gene (VP6), a group specific antigen for all known human rotaviruses. Results were interpreted by both the methods as provided with the kit instructions.
a). By visual determination: Any sample with blue color more intense than that of the negative was considered positive. Any sample with color equal to or less intense than the negative control was considered negative.
b). By spectrophotometric determination: Specimens with absorbance units (A450) greater than 0.150 was considered positive. Specimens with absorbance equal to or less than 0.150 was considered negative.
In case of discrepancies in results between the two methods, results of spectrophotometric method was taken into consideration.
Statistical analysis
The statistical program IBM SPSS 24 was utilized to analyze the data. Descriptive statistics were presented as percentages of all categorical variables. Quantitative variables were expressed as means and standard deviations. The correlation was tested using the chi-square test, and a p-value of less than 0.05 was deemed significant. An independent sample t-test was used to identify continuous variables that were significantly different between children with and without associated comorbidities.
Of the 76 participants recruited in the study, rotavirus was detected as the cause of acute infectious diarrhea in 13 (17.1%) cases. Only 1 out of 10 rotavirus-vaccinated participants developed rotavirus diarrhea. The average time between the last dose of the vaccine and sample collection was 10 months. We observed no predisposition to rotavirus diarrhea based on age, gender, socioeconomic status, or a history of RVV (Table 1).
Table (1):
Socio-demographic characteristics of study participants
| Rotavirus Positive (n) | Rotavirus Negative (n) | Total n (%) | p-value by Chi-square test | |
|---|---|---|---|---|
| Gender | ||||
| Male | 8 | 36 | 44 (57.9%) | 0.77 |
| Female | 5 | 27 | 32 (42.1%) | |
| Age | ||||
| Upto 24 months | 10 | 49 | 59 (77.6%) | 0.94 |
| 25-60 months | 3 | 14 | 17 (22.3%) | |
| Rotavirus Vaccination Status | ||||
| Vaccine Received | 1 | 9 | 10 (13.1%) | 0.52 |
| 3 doses | 1 | 5 | ||
| 2 doses | 0 | 2 | ||
| 1 dose | 0 | 2 | ||
| Vaccine Not Received | 12 | 54 | 66 (86.8%) | |
| Socioeconomic status | ||||
| Lower class | 1 | 3 | 4 (5.3%) | 0.53 |
| Upper lower | 6 | 31 | 37 (48.7%) | 0.84 |
| Lower middle | 4 | 22 | 26 (34.2%) | 1.0 |
| Upper middle | 2 | 6 | 8 (10.5%) | 0.61 |
| Upper class | 0 | 1 | 1 (1.3%) | 1.0 |
| Total | 13 | 63 | 76 | – |
Comparing the clinical characteristics of the recruited patients, we observed an increased frequency of diarrhea, vomiting, and fever among patients with rotavirus diarrhea. However, there was no discernible difference in the duration of diarrhea between those with and without rotavirus diarrhea. The Vesikari Severity score index was used to determine the degree of diarrhea. Participants with rotavirus infection did not differ significantly from those without rotavirus infection in terms of disease severity (Table 2).
Table (2):
Details of clinical features among study participants
| Rotavirus Positive (n = 13) | Rotavirus Negative (n = 63) | Total (n = 76) test | p-value by Chi-square | |
|---|---|---|---|---|
| Presence of co-morbidities Clinical Features | 08 | 34 | 42 (55.2%) | 0.6 |
| Frequency of diarrhoea/day | 4.77 ± 2.58 | 3.49 ± 1.39 | 3.71 ± 1.70 | 0.04 |
| Duration of diarrhoea (days) | 4.85 ± 2.85 | 3.95 ± 1.33 | 4.11 ± 1.70 | 0.09 |
| Frequency of vomiting/day | 3.0 ± 1.91 | 1.33 ± 0.86 | 1.62 ± 1.26 | <0.001 |
| Duration of vomiting (days) | 2.62 ± 1.38 | 1.17 ± 0.38 | 1.42 ± 0.85 | <0.001 |
| Fever (%) | 6 (46.2%) | 13 (20.6%) | 19 (25.0%) | 0.05 |
| Severity grading of diarrhoea by the Vesikari severity scoring index | ||||
| Mild | 3 (11.5%) | 23 (88.5%) | 26 (34.2%) | 0.16 |
| Moderate | 5 (14.3%) | 30 (85.7%) | 35 (46.0%) | |
| Severe | 5 (33.3%) | 10 (66.7%) | 15 (19.8%) | |
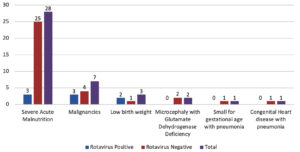
By analyzing the association of different comorbidities among the recruited patients, we found a similar distribution between the two groups. The most common comorbidities observed in our study were severe acute malnutrition (SAM) followed by malignancies. Common malignancies included B-cell lymphoma, acute lymphoblastic leukemia, Burkitt’s lymphoma, neuroblastoma, and Langerhans histiocytosis. Eight rotavirus-positive cases were reported to have associated morbidities, such as B-cell lymphoma (03), low birth weight (02), SAM (01), SAM with acute kidney injury (01), and SAM with thalassemia major (01) (Figure).
Although rotavirus detection was similar between patients with and without comorbidities, we observed significant differences in the frequency and duration of diarrhea, vomiting, and fever among patients with comorbidities. However, no significant differences were observed in severity of dehydration (Table 3).
Table (3):
Comparison of clinical features among rotavirus-positive cases with and without co-morbidities
With Comorbidity (n = 8) |
Without Comorbidity (n = 5) |
p-value by Chi-square test |
|
|---|---|---|---|
Frequency of diarrhoea/day |
6.50 ± 0.92 |
2.0 ± 1.73 |
|
Duration of diarrhoea (days) |
6 ± 0.0 |
1.40 ± 0.54 |
|
Frequency of vomiting/day |
4.25 ± 1.28 |
1.0 ± 0.0 |
|
Duration of vomiting (days) |
3.50 ± 0.92 |
1.20 ± 0.44 |
|
Fever (%) |
6 (75.0%) |
0 |
0.02 |
Severity of dehydration (%) |
6 (75.0%) |
2 (40.0%) |
0.29 |
In this study, we reported a positivity rate of 17% among hospitalized children under five suffering from diarrhea without any predisposition related to age, sex, socioeconomic status, or history of rotavirus vaccination. The severity of manifestations was higher among children with rotavirus diarrhea (Table 1). We also reported an increase in illness severity among patients suffering from various comorbid conditions. Although constrained by the small sample size, our results showed comparable rates of rotavirus diarrhea in children who received vaccination and in those who did not, which suggests ineffectiveness of vaccination in preventing rotavirus infection in the group of hospitalized patients. This could be attributed to either a potential change in the distribution of serotypes among the circulating rotavirus strains or the inability to mount a protective immune response in relatively sick children. Similarly, the severity of rotavirus diarrhea was found to be higher in children with comorbidities, which also suggests an effective immune system in limiting viral pathogenicity in infected children.
Our results are consistent with those of related studies. A similar study conducted by Chaudhry et al. in Madhya Pradesh, following the introduction of the rotavirus vaccine, reported a positivity rate of 18.8%, which is similar to the findings of our study.9 Additionally, Kumar et al. observed a rotavirus antigen positivity rate of 14.58% at a tertiary healthcare referral hospital in Western Uttar Pradesh, India, after the inclusion of the rotavirus vaccine in the Universal Immunization Program (UIP) in the state.12 We observed low immunisation coverage in our geographical region. It may be attributed to the impact of the ongoing COVID-19 pandemic during the study period, which disrupted routine immunization efforts. Another possible reason could be recall bias from the parents of the recruited children regarding rotavirus vaccination.
In agreement with our findings, previous authors have reported a higher severity of clinical manifestations in children with rotavirus diarrhoea. In the present study, a statistically significant association with clinical features such as increased frequency of diarrhea, frequency and duration of vomiting and fever was observed among rotavirus-infected cases (Table 2). As observed in the present study and reported in previous studies,13,14 rotavirus gastroenteritis is associated with fever and vomiting. In a similar study conducted by Selvarajan et al., the severity of diarrhea was found to be statistically significant in children infected with rotavirus.15 The variation in the severity of rotavirus gastroenteritis reported in different studies may be attributed to circulating rotavirus serotypes in the particular geographical areas.
We observed the presence of comorbidities in approximately 10.5% of participants with rotavirus diarrhea in our study. Jain et al. found that 9% of children with SAM had rotavirus infection. When compared with children with severe diarrhea who were anthropometrically normal, the difference was statistically significant (p < 0.033).16 Chissaque et al. conducted a study on Rotavirus A infection in undernourished children <5 years of age and reported a positivity rate of 27.2% for rotavirus A infection.17 In a study of virus-associated diarrhea conducted by Ghosh et al. among immunocompromised cancer patients, rotavirus infection was reported in 14% of lymphoma cases.18 Dennehy et al. found that infants with low birthweight (<2500 g) and RVGE after the first few months of life had a higher chance of being admitted to the hospital (OR, 2.8; 95% CI, 1.6-5.0).19 Rotavirus-infected children with comorbidities had a longer duration and increased frequency of diarrhea and vomiting compared to children without any comorbidities (p < 0.01). Rayani et al. observed that vomiting, diarrhea, and fever were significantly more common in rotavirus-infected pediatric oncology patients than in rotavirus-negative patients (p < 0.001). A significant difference was observed in the severity of dehydration in children with malignancy.7
The use of a standardized protocol, including consistent transport methods and laboratory confirmation of rotavirus diarrhea at a single reference laboratory, has enhanced the strength of the study. However, the study has limitations of small sample size and also failed to characterize the serotype and genotype distribution of rotavirus cases. The detection of other diarrheal pathogens was outside the scope of the study and were not identified in the rotavirus-negative cases.
This study demonstrated a higher prevalence of rotavirus diarrhea in hospitalized children under five years old, despite the rotavirus vaccine being part of the universal immunization program. The presence of comorbidities accelerated the deterioration of clinical conditions among RVGE patients. Therefore, early diagnosis of rotavirus infection in acute diarrhea cases is required for timely intervention, prevention of further complications, and the spread of infection. If validated in larger multicenter studies, this finding hints at the need for additional measures to prevent rotavirus infections in children with comorbid conditions.
ACKNOWLEDGMENTS
The authors would like to thank DHR-ICMR and AIIMS Bhopal for providing Kits and consumables to carry out the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SN conceptualized the study. SM, DG, and BD performed the recruitment of study participants. KM and DG performed laboratory work. SN, DG, KM, DB, SM and BD performed data analysis. SN and DG performed data interpretation. DB and SN supervised the study. SN and DB wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, AIIMS, Bhopal, vide reference number IHECPGRMD015, dated 11/03/2020.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Rotavirus, World Health Organisation. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/rotavirus. Accessed February 28, 2024
- Troeger C, Khalil IA, Rao PC, et al . Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018;172(10):958-965.
Crossref - Kumar CPG, Giri S, Chawla-Sarkar M, et al. Epidemiology of rotavirus diarrhea among children less than 5 years hospitalized with acute gastroenteritis prior to rotavirus vaccine introduction in India. Vaccine. 2020;38(51):8154-8160.
Crossref - Rotavirus. Margaret M. Cortes and Penina Haber. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/rota.pdf Accessed February 28, 2024
- Yeom JS, Park JS, Kim YS, et al. Neonatal seizures and white matter injury: role of rotavirus infection and probiotics. Brain Dev. 2019;41(1):19-28.
Crossref - Shakya P, Adhikari B, Nepal AS, Pandey P, Maheshwari A. Rotavirus Infection in Neonates and Young Infants. Newborn. 2022;1(1):142-150.
Crossref - Rayani A, Bode U, Habas E, et al. Rotavirus infections in paediatric oncology patients: a matched-pairs analysis. Scand J Gastroenterol. 2007;42(1):81-87.
Crossref - Sen T, Thummer RP. The Impact of Human Microbiotas in Hematopoietic Stem Cell and Organ Transplantation. Front Immunol. 2022;13:932228.
Crossref - Chaudhary P, Jain H, Nair NP, Thiyagarajan V. Rotavirus Diarrhea in Hospitalized Under-5 Children in Madhya Pradesh, India and the Prevalent Serotypes After Vaccine Introduction. Indian J Pediatr. 2021;88(Suppl 1):78-83.
Crossref - Wani RT. Socioeconomic status scales-modified Kuppuswamy and Udai Pareekh’s scale updated for 2019. J Family Med Prim Care. 2019;8(6):1846-1849.
Crossref - Shim DH, Kim DY, Cho KY. Diagnostic value of the Vesikari Scoring System for predicting the viral or bacterial pathogens in pediatric gastroenteritis. Korean J Pediatr. 2016 ;59(3):126-131.
Crossref - Kumar A, Pandey A, Singh AK, Dubey A, Singh A, Gaur V. The Current Epidemiology of Rotavirus Infection in Children Less than 5 Years of Age after Introduction Of RV Vaccine in India. J Pure Appl Microbiol. 2022;16(1):471-480.
Crossref - Giri S, Nair NP, Mathew A, et al. Rotavirus gastroenteritis in Indian children < 5 years hospitalized for diarrhoea, 2012 to 2016. BMC Public Health. 2019;19(1):69.
Crossref - Ambhore J, Ahmed M. Incidence of rotaviral and adenoviral diarrhoea amongst children under 5 year of age in a tertiary care centre. Int J Contemp Pediatrics. 2019;6(2):295-298.
Crossref - Selvarajan S, Reju S, Pushpanathan P, et al. Molecular Characterisation and Clinical Correlates of Rotavirus in Children and Adults in a Tertiary Care Centre, Chennai, South India. Indian J Med Microbiol. 2017;35(2):221-227.
Crossref - Jain A, Shah D, Das S, Saha R, Gupta P. Aetiology and outcome of acute diarrhoea in children with severe acute malnutrition: a comparative study. Public Health Nutr. 2020 ;23(9):1563-1568.
Crossref - Chissaque A, Cassocera M, Gasparinho C, et al. Rotavirus A infection in children under five years old with a double health problem: undernutrition and diarrhoea – a cross-sectional study in four provinces of Mozambique. BMC Infect Dis. 2021;21(1):18.
Crossref - Ghosh N, Malik FA, Daver RG, Vanichanan J, Okhuysen PC. Viral associated diarrhea in immunocompromised and cancer patients at a large comprehensive cancer center: a 10-year retrospective study. Infect Dis (Lond). 2017;49(2):113-119.
Crossref - Dennehy PH, Cortese MM, Begue RE, et al. A Case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. Pediatr Infect Dis J. 2006;25(12):1123-1131.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.