ISSN: 0973-7510
E-ISSN: 2581-690X
Fifty-one rhizobacteria were isolated from root nodules of the wild legume Lathyrus ochrus, wild legume growing in a polluted soil around the Steel Factory Sider El-Hadjar in the north east of Algeria. These isolates characterized for their ability to dissolve inorganic phosphate and their tolerance to the following heavy metals cadmium, cobalt, chromium, copper, mercury, nickel, lead, iron and zinc. Sixty per cent of the isolates solubilize Ca3 (PO4)2 and CaHPO4 on solid agar media. The solubilization of an Algerian rock phosphate (ARP) carried out on liquid media (National Botanical Research Institute) and the higher concentrations of phosphate released by isolates LnPF46 and LnPF32 are respectively 150.22 and 206.98 mg/L. The test of the tolerance to heavy metals show that isolates LnPF44, LnPG1 and LnPG2 are resistant to high concentrations of iron, zinc, mercury, lead and copper.
ARP, heavy metals, Lathyrus ochrus, Plant Growth Promoting Rhizobacteria (PGPR), phosphate solubilization.
One of the main concerns of the world today is the sustainability of agricultural systems without compromising the quality and conservation of the environment1. Phosphorus (P) is the essential mineral for plant development and growth, it is the second chemical compound used in agriculture in the world2.
One of the main reasons for using PGPRs in agriculture is their secretion of metabolites that directly stimulate plant growth3 and among them solubilization of inorganic phosphate4, and improvement plant resistance to stress, salinity and heavy metals toxicity5-6. The PGPR use several P solubilization mechanisms as, dissolving inorganic phosphate by organic acids7, liberation of extracellular enzymes and phosphate release during substrate degradation8. Some legumes such as Lathyrus species play an important role in the natural ecosystem by occupying various habitats because of their higher adaptability to all types of soils and climatic conditions9.
In Algeria, twenty-one species and subspecies of Lathyrus sp. are recorded10 essentially cultivated for human and animal nutrition11.
The aim of this work is the study of PGPR associated with indigenous Lathyrus ochrus, endemic in the Northeast region of Algeria. The PGPRs isolated were tested for their ability to solubilize inorganic P on solid and liquid media, and their resistance to different concentrations of Cd++, Co++, Cr++, Cu++, Hg++, Ni++, Pb++, Fe++ and Zn++.
Plant material and rhizobacteria trapping tests
The soil samples taken from a polluted area and used for rhizobacteria trapping tests collected at Sidi Amar El-Hadjar locality at the Northeast of Algeria. The origin of the metallic pollution of the soil in this area is the redeposit dusts from Steel Industry (Sider El-Hadjar). Twenty jars were used and filled by soil samples (1 Kg), supplemented with 200g of iron ore. Each jar sown with two germinated seeds of Lathyrus ochrus and a period of two months is required for growth and nodulation of plants. Then the plants carefully uprooted and the root nodules were surface sterilized by immersion 4 min in a sodium hypochlorite solution (2%) and washed six times with sterile distilled water. Root nodules were crushed aseptically with glass rod and the suspension obtained was then streaked on three solid media; YMFP [Yeast Extract Mannitol supplemented with iron ore and ARP (33% of P)], acetamide agar and charcoal medium12.
Screening for phosphate solubilizing bacteria (PSB) on solid media
The aim of this test is the evaluation and appreciation of the ability of the isolates to solubilize various forms of inorganic P. Isolates were tested on five solid media; YMA (yeast extract mannitol agar)13, PVK14, SP (basal Sperber medium)15, MNBRI and NBRIP16. Using the drop method, plates were inoculated with 7 µL of the crushed suspension and incubated at 28°C during 15 days as time growth. The rhizobacterial isolates obtained were tested on three media, NBRIP medium supplemented with 5 g/L of Ca3 (PO4)2 (TCP), CaHPO4 (CHP) and ARP, as insoluble P source.
A comparative test done with MNBRI and NBRIP media with and without yeast extract. The first medium composition is as follow; 20 g/L of glucose, 10 g/L of MgCl2 and 0.1 g/L of yeast extract, and the second medium contains 10 g/L of glucose and 5 g/L of MgCl2, using CHP as the insoluble source of P.
ARP solubilization
Twenty-six selected isolates used to quantify the ARP solubilization on NBRIP and MNBRI liquid media by using Erlenmeyer flasks containing 50mL of the medium. This test done in triplicate with 2mL of rhizobacterial isolate suspensions (≈ 8.108 CFU/mL) and an uninoculated medium used as a control.
The cultures incubated on rotary shaker at 28°C with an agitation speed of 150 rpm for one week. The cultures were harvested by centrifugation at 6000 rpm for 20 min. Phosphate released is estimated by the method described by Chen et al17.
Heavy metals resistance
Twenty-six phosphate-solubilizing isolates selected for heavy metal resistance ability. The concentrations of heavy metals are as follow; [Zn++ ], 12, 16 and 32 mM; [Cd++], 0.8, 1 and 2.5 mM; [Ni++], 0.7, 1, 2.5 mM; [Co++], 1, 2 and 3 mM; [Cu++], 1, 2 and 2.5 mM; [Cr++], 0.2, 0.7 and 1mM; [Fe++], 1.7, 8.95, 17.90, 26.85, 35.8 and 44.75 mM; [Hg++], 1, 2, 5, 8, 15 and 20 mM, [Pb++], 0.4, 0.7, 1, 3, 8 and 10 mM.
Each metal concentration tested in duplicate and the culture incubation is at 28°C for two weeks.
Plant nodulation test
Seeds of Lathyrus ochrus, were germinated on Murashige and Skoog medium under sterile conditions using the method described by Brunet et al18. Three seeds planted in plastic jars containing sterile sand. The inoculation suspensions were prepared with isolates at the exponential growth phase and the growth of the inoculated plants done under natural conditions and watered with the solution of Rigaud and Puppo19. After eight weeks, the plants uprooted and the presence of nodules on the roots recorded. Four replications carried out for inoculated plants and two replications for non-inoculated jars (negative control).
Phenotypic characterization of ARP-solubilizing isolates
Nine isolates were characterized for catalase, oxidase, growth on glucose peptone agar medium (GPA), acid or alkali production and API20NE standardized systems. The isolates tested for their growth on YMA medium supplemented by 1, 2.5 and 5% (w/v) of NaCl, and on TA medium containing 2% (w/v) of NaCl20. Agrobacterium tumefasciens, Burkholderia sp. and Cupriavidus sp. used as reference strains.
Statistical analysis
The Principal Component Analysis (PCA) was performed to examine the relationships between the tolerance of isolates in vitro to high concentrations of heavy metals, ARP solubilization and salinity, and a phenogram was constructed by the Agglomerative Hierarchical Clustering method (AHC). Computations, graphical display and a computer cluster analysis phenotypic variables carried out using the XLSTAT software package (http://www.xlstat.com).
Trapping and rhizobacteria isolation
Forty Lathyrus ochrus plants show a well-developed aerial part and a highly branched root system and the number of nodules is between 24 to 50 nodules with red or white color, and mainly located in the upper part of the roots. Lathyrus ochrus plants are well adapted to soil enriched with iron ore used. The nodulations, in this trapping test, are due to the presence of free rhizobia capable of inducing nodulation.
In this study, fifty-one strains were isolated from nodules of Lathyrus ochrus developed on the mixture of soil and iron ore. These isolates are different in their growth time ranged between 48 h and 5 days (Table 1) and macroscopic characters of colonies as colours (white, red, pink, yellow, and egg yolk); aspect, smooth or rough, translucent or opaque and circular colonies. The diameter of the colonies varied between 1 and 5 mm and even punctuate after 3-7 days of incubation. The isolates did not produce polysaccharide. This variety of morphological characteristics of isolates reflects the diversity of rhizobacteria inside nodules of Lathyrus ochrus.
Table (1):
Growth time of isolates.
Growth time (days) |
Isolates (%) |
Isolates |
|---|---|---|
Group A : 2 |
37.25 |
LnPF2, LnPF3*, LnPF4, LnPF5, LnPF8, LnPF13, LnPF14, LnPF18*, LnPF22*, LnPF26, LnPF42, LnPF44, LnPF46, LnPC1, LnPC2, LnPC3, LnPC4, LnPG1*, LnPG2* |
Group B : 3-4 |
33.33 |
LnPF7, LnPF9, LnPF11, LnPF12, LnPF19, LnPF20, LnPF21, LnPF23, LnPF24, LnPF25, LnPF30, LnPF32, LnPF34, LnPF35, LnPF39, LnPF43, LnPF45 |
Group C : 5 |
29.41 |
LnPF1, LnPF6, LnPF10, LnPF15*, LnPF16, LnPF17, LnPF27, LnPF28, LnPF29, LnPF31*, LnPF33, LnPF36, LnPF37, LnF40, LnPF41. |
*Colonies with metallic reflects
Growth of isolated phosphate-solubilizing rhizobacteria
The solubilization of TCP tested on YMA and the results shows that isolates LnPF3, LnPF12, LnPF14, LnPF20, LnPF23, LnPF25, and LnPG1 exhibit a low solubilization of TCP. This observation could be explained by the effect of carbon sources on solubilization of phosphate; it is known that mannitol is not a good source of C for P mobilization studies21 and generally, the solubilization of P is better when glucose is used as carbon source22. The second solubilization test done with SP medium shows a clear but not complete solubilization of P by isolates LnPC2, LnPF4, LnPF5, LnPF8, LnPF13, LnPF14, LnPF19, LnPF23, LnPF31, LnPF44, LnPF46, LnPG1 and LnPG2.
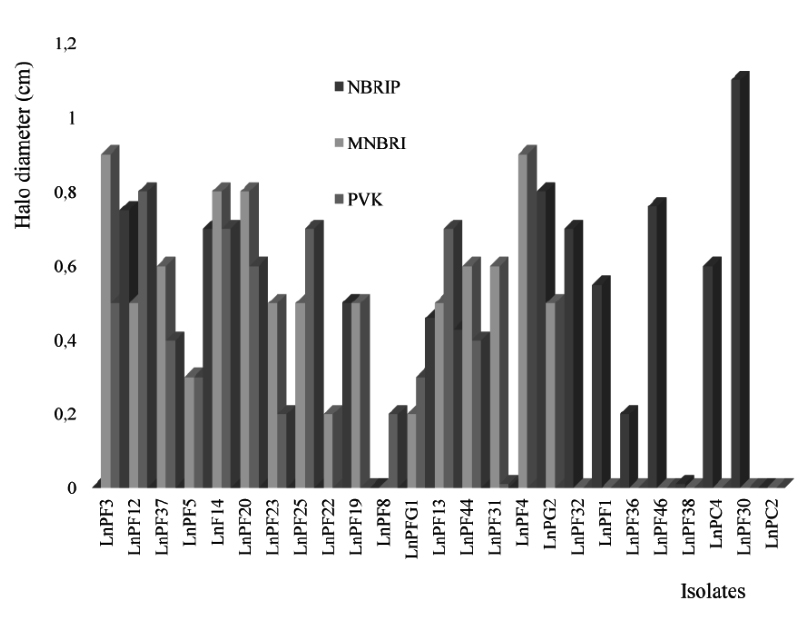
The media NBRIP, PVK and MNBRI, give a better solubilization of P and the number of isolates are respectively 12, 13 and 16 as presented in the Fig. 1. There is a similarity in chemical composition between these three media and the analysis of the results presented in Table 2 shows that the solubilization of TCP on these media is similar with a significant relationship between MNBRI and PVK (P < 0.01).
The isolates solubilize CHP better than TCP on NBRIP medium and no strain could solubilize ARP on solid media. The solubilization of P is also affected by yeast extract; the isolates LnPF5, LnPF20, LnPF25 and LnPF37 required yeast extract for solubilizing P and the others like LnPF19, LnPF28, LnPF31 and LnPF32 did not need yeast extract. Nautiyal16 demonstrate that a concentration higher than 0.5 g/L of yeast extract can inhibit the solubilization of P and in our study, we note that solubilization of P is inhibited with only 0.1 g/L of yeast extract.
The interpretation of the results (Table 2) gives the significant relationship between only NBRIP (with yeast extract) and MNBRI, with P < 0.005. According to these results, we selected MNBRI and NBRIP media without yeast extract to carry out the other tests of solubilization. These results allowed us to select 26 solubilizing phosphate isolates on solid media used.
Table (2):
ANOVA of the solubilization of inorganic P (TCP, CHP, ARP) on the solid media Mean values (cm) are ratio of dissolution halo (DH)/colony diameters (CD).
| Medium | Inorganic P | ||
|---|---|---|---|
| DH/CD | Degree of freedom | Mean square | |
| NBRIP MNBRI PVK |
0.62a 0.55b 0.44b |
2 | 0.094 |
| NBRI(TCP) NBRI(CHP) NBRI(ARP) |
0.44c 0.93d 0e |
2 | 3.69 |
| NBRIP* NBRIP** MNBRI |
0.93f 0.79g 0.73g |
2 | 0.38 |
Mean values (cm) are ratio of dissolution halo (DH)/colony diameters (CD)
*: without yeast extract, **: with yeast extract (0.1mg/L)
Results are mean of three replicates. Means followed by the same letter are significantly at P<0.01
ARP solubilization on liquid media
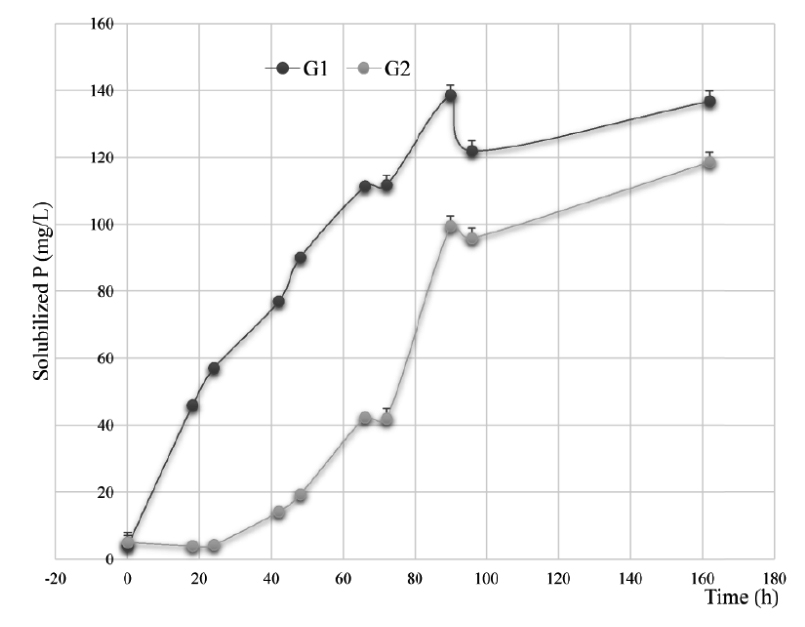
The results of the solubilization of ARP on NBRIP and MNBRI media used in this test, have allowed to select the following nine isolates; LnPF18, LnPF46, LnPF13, LnPF14, LnPF44, LnPF19, LnPG1, LnPG2 and LnPF32. The two groups of rhizobia strains G1 (LnPF13, LnPF14, LnPF19, LnPF44, LnPF46, LnPG1) and G2 (LnPG2, LnPF18 and LnPF32) Fig. 2 released an average of 136.84 and 118.62 respectively of P from ARP. These quantities were significantly (P < 0.005) correlated. According to Fig. 2, the solubilization activity of ARP by all strains tested was significantly (P < 0.05) increased with time and then the higher concentration of P solubilized achieved after 90 hours for G1 and 162 hours for G2.
Fig. 2. Solubilization of ARP in the liquid NBRIP and MNBRI media by bacterial isolates belonging to the following groups: (G1), LnPF13, LnPF14, LnPF19, LnPF44, LnPF46, LnPG1 and (G3), LnPG2, LnPF18 and LnPF32. Error bars are ± standard error (n = 3)
The P solubilization activities by G1 strains are as follow; LnPF13, 115.91 mg/L; LnPF14, 136.64 mg/L; LnPF44, 145.77 mg/L; LnPF46, 150.22 mg/L; LnPF19, 168.23 mg/L; LnPG1, 152.69 mg/L. The G2 strains have better activities of P solubilization. Indeed, the concentrations of phosphate released in the liquid medium are respectively for LnPF32, 206.98 mg/L higher than LnPF18, 112.21 mg/L and LnPG2; 36.69 mg/L.
The release of available P leads to a decrease of the pH values of the liquid media from 7 to 3.88 and 3.97 for LnPF46 and LnPG1 respectively. This inverse relationship observed between the pH and soluble P concentration indicates that organic acids plays a significant role in the acidification of the medium facilitating the P solubilization by these phosphate solubilizing bacteria strains23, such as gluconate, oxalate, citrate, acetate, lactate, tartarate, succinate, ketogluconate and glycolate24. The two groups of rhizobia strains have a strong significant (G1: P < 0.001, G2: P < 0.05) inverse correlations (G1: r = -0.8, G2: r = -0.7) were observed between the pH of the cultures and their soluble P content.
Heavy metals resistance test
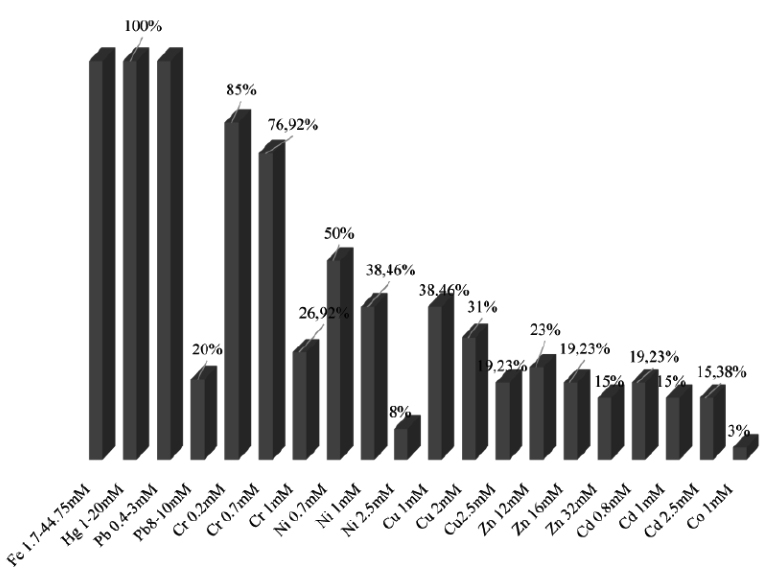
The results shown on Fig. 3 indicate the percentage of isolates resistance at different concentrations of nine heavy metals. We note that all the isolates resist to Fe, Hg, Pb > Cr> Cu > Ni > Cd >Zn.
Fig. 3. Resistance percentage of isolates to different concentrations of heavy metals on solid medium
Isolates LnPF44, LnPF23, LnPF5, LnPF36, LnPG1 and LnPG2 are the most resistant isolates to all heavy metals tested and LnPG1 is the only isolate that resist to Co at 1mM.
Table (3):
Maximal tolerance concentration (MTC) of Zn++, Cu++, Cr++, Co++, Cd++, Ni++, Pb++, Hg++ and Fe++ in (mM) towards isolates solubilizing ARP.
| Isolates solubilizing ARP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MTC (mM) | LnPF13 | LnPF14 | LnPF18 | LnPF19 | LnPF32 | LnPF44 | LnPF46 | LnPG1 | LnPG2 |
| 12≤ [Zn++ ] ≤16 | – | – | – | – | – | + | – | + | + |
| 12≤Zn++≤16 | – | – | – | – | – | – | – | + | + |
| 1≤Cu++≤2 | – | – | – | – | – | + | – | + | + |
| 1≤Cu++≤2.5 | – | – | – | – | – | + | – | – | + |
| Cr++≤0.2 | + | + | – | + | + | + | + | + | + |
| 0.2≤Cr++≤0.7 | + | + | – | + | – | + | + | + | + |
| 0.7≤Cr++≤1 | – | – | – | – | – | + | – | – | – |
| Co++≤1 | – | – | – | – | – | – | – | + | – |
| Cd++≤0.8 | – | – | – | – | – | – | – | + | + |
| Cd++≤2.5 | – | – | – | – | – | – | – | + | |
| 0.7≤Ni++≤1 | + | + | – | + | – | – | + | – | – |
| 0.4≤Pb++≤3 | + | + | + | + | + | + | + | + | + |
| 1≤Hg++≤20 | + | + | + | + | + | + | + | + | + |
| 1.7≤Fe++≤44.5 | + | + | + | + | + | + | + | + | + |
+ : resistant, – : sensitive
The results presented in Table 3 show the resistance of nine isolates to heavy metals on solid media and their maximal tolerance concentration (MTC) described by Al-Enzi and Charrakh25 as the highest metal concentration at which the growth of bacteria was still observed. The isolates tolerate heavy metals according to this order Fe, Hg, Pb > Cr >Ni > Zn, Cu > Cd > Co.
The toxicity of heavy metals evaluated as low for Fe, medium for Zn, Ni, Cu, Co, Cr and high for Cd, Hg, Pb26, and our isolates have resisted to these three degrees of heavy metal toxicity.
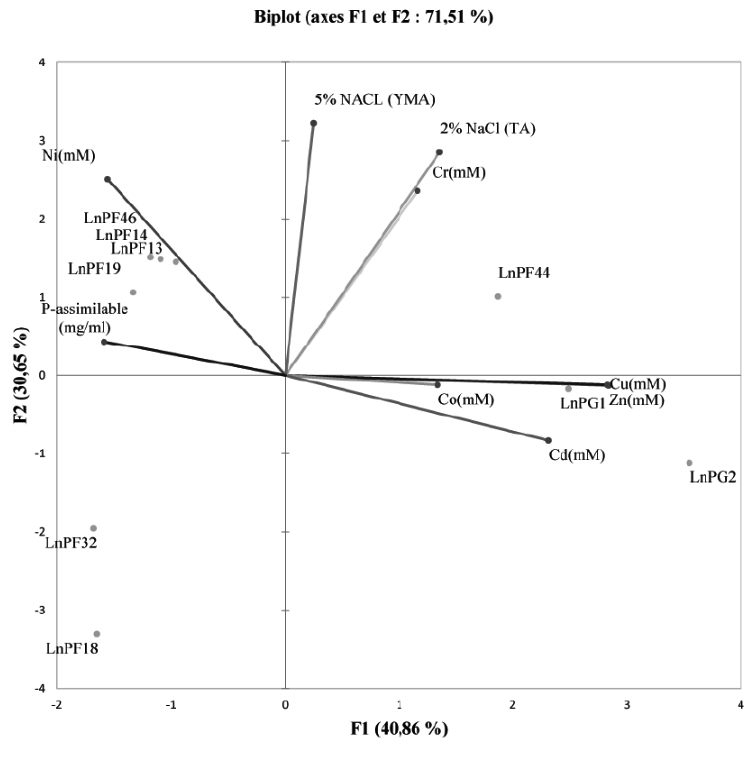
Fig. 4. PCA showing the relationship between heavy metals, ARP solubilisation and tolerance to salinity on liquid media
The PCA graphs in Fig. 4 showed that the direction of the axis variables reflects the strong relationship between resistance to heavy metals and solubilization of ARP. This relation was confirmed by Seob et al27 and reported that all PSB strains were tolerant to heavy metals (Cd, Pb and Zn). In addition, Burkholderia sp. resistant to heavy metals isolated from contaminated soil with heavy metals is capable of solubilizing inorganic P28.
According to the correlation study between the parameters using the Pearson test revealed a significant correlation between all the parameters studied. The ARP solubilization activity negatively correlated with the resistance to Zn, Cu, Cd, and Co. On the other hand, the tolerance to salinity significantly correlated with the resistance to Ni and Cr.
For the behavior of the isolates with regard to the resistance to heavy metals, the ARP solubilization activity and the tolerance to the salinity, we can classify them into three classes:
-The first group contains LnPF44, LnPG1 and LnPG2 isolates with high resistance to the majority of heavy metals tested and tolerate 5% of NaCl,
-The second group comprises LnPF14, LnPF19, LnPF46 and LnPF13 isolates having a high tolerance to NaCl 5% and resistant to Ni and Cr but they do not resist to Co, Zn and Cd,
-The third group includes LnPF32 and LnPF18 isolates that are poorly resistant to metals (not resistant to Co, Zn, Cd and Ni) and tolerate only 2.5% of NaCl.
In this study, we note that fast growing isolates were generally more tolerant to high NaCl concentrations and more resistant to heavy metals than slow growing one.
Nodulation test
Fifty seven per cent of the isolates have induced nodulations in the host plant Lathyrus ochrus. The observation of nodules showed that nodules are white and their number varied from 4 to 26. Experiments carried out on the mutant of Rhizobium meliloti unable to synthesize the ´-aminolevulnic acid (ALA) precursor of the heme produced white nodules incapable of fixing nitrogen29, which may be due to the metallic pollution or absence of genes Fix (bacteria nod+ and fix-) which produces white nodules unable to fix atmospheric nitrogen.
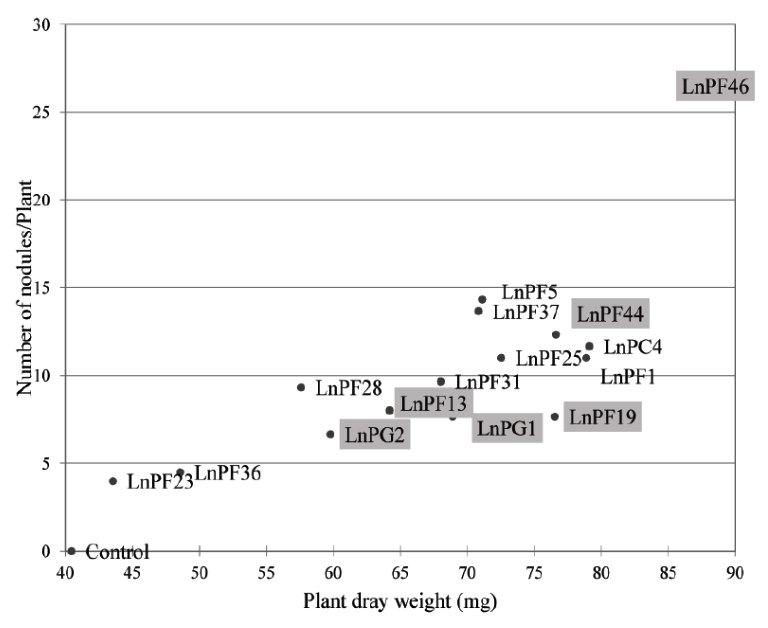
Fig. 5. The number of nodules induced by Lathyrus ochrus isolates versus plant dry matter, framed isolates correspond to those solubilize the P on liquid medium
The Fig. 5 shows the relationship between dry matter (dry weight of the aerial parts of the nodulated plants) and the number of nodules. We observed that the dry matter increased proportionally with the number of nodules, this explain that these bacteria were able to develop the aerial part of Lathyrus ochrus and thus increase its growth.
Among nine ARP-solubilizing isolates, LnPF13, LnPF19, LnPF44, LnPF46, LnPG1 and LnPG2 isolates are able to infect their host plant and LnPF46 isolate produce the highest number of nodules up to 26 nodules.
Phenotypic characterization for isolates solubilizing ARP
The nine isolates solubilizing ARP were Gram-negative, Catalase and oxidase-positive. Furthermore, the majority of colonies obtained in YMA-CR were retained their initial color and/or absorbed little CR and color change of pH indicator to yellow was detected of the majority of isolates tested except LnPF18 and LnPF32 which have alkalized the medium
The results obtained of API20NE, osmotolerance and all presumptive tests were used for phenotypic characterization with Burkholderia sp., Cupriavidus sp. and Agrobacterium tumefasciens as reference strains.
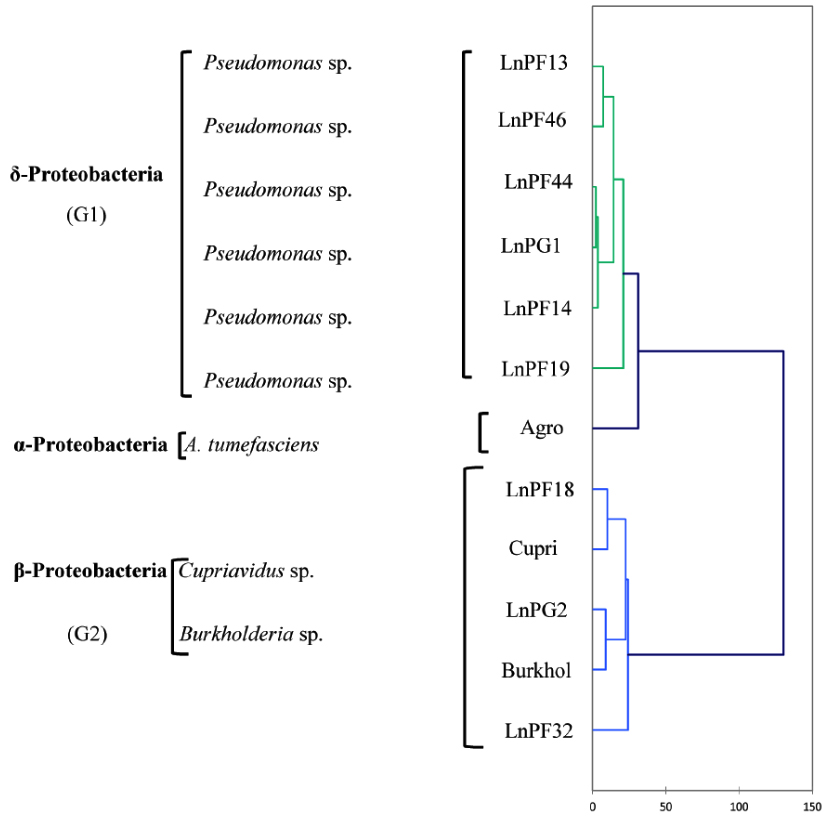
Fig. 6. AHC dendrogram of phenotypic characters of isolates solubilizing ARP and their belonging genus
The analysis of phenotypic characters presented as a dendrogram of dissimilarity HAC in Fig. 6, and the results showed three separate groups (G1, G2 and G3). G1 group comprise LnPF13, LnPF14, LnPF19, LnPF44, LnPF46 and LnPG1 isolates and belong to gamma-Proteobacteria (Pseudomonas sp.). The reference species Agrobacterium tumefasciens C58 constitute a separate group then the isolates do not belong to the genera Agrobacterium. G2 group comprise LnPG2, LnPF18, LnPF32 and reference species Burkholderia sp. and Cupriavidus sp.; this group belong to beta-Proteobacteria. According to the study carried out by Shiraishi et al30, Pseudomonas sp. and Burkholderia sp. form nodules on Black locust and developed differentiated nodule tissue. A phylogenetic analysis of nodA and nodC, nifH and nif HD genes revealed that these symbiotic genes of Pseudomonas sp. and Burkholderia sp. have high similarities with rhizobial species. These results indicate the importance of horizontal gene transfer for establishing symbiotic interactions in the rhizosphere.
Lathyrus sp. are commonly nodulated by Rhizobium leguminosarum viciae31, but the presence of R. tropici were found in L. sylvestris and L. vernus, the nodules of L. palustris were shown to contain bacteria similar to Agrobacterium sp. All bacteria isolated from Gmelinlathyrus (L. gemelinii Fritsh) were schown to be similar to Phyllobacterium myrsincearum by the 16SrDNA gene sequence32. Kuznetsova et al33, they found the presence of Phyllobacterium and Burkholderia sp and Bosea lathyri in Lathyrus humilis nodules, also De Meyer and Willems12 have a gain mentioned the presence of Bosea lathyri in a nodules of Lathyrus latifolius.
In conclusion, we showed that the Lathyrus ochrus developed in the mixture of soil contaminated with redeposit dusts from Steel Industry (Sider El-Hajar) from the Northeast of Algeria and iron ore, represent one of the best host traps to isolate rhizobacterial strains which have an important PGPR characteristics, solubilization of ARP, resistance to heavy metals and osmotolerance. The phenotypic characteristics studied shows that these rhizobacterial strains are outside the traditional rhizobia and/or belong to fast growing rhizobia; may represent new genospecies that need further characterization to assess for their taxonomical status.
- Khalid,A., Arshad, M., Shaharoona, B., Mahmood, T.: Plant growth promoting Rhizobacteria and sustainable agriculture. In: Microbial strategies for crop improvement (ed). Berlin: Heidelberg Springer-Verlag, 2009; pp.133-160.
- Hameeda, B., Harini, G., Rupela, O.P., Wani, S.P., Reddy, G. Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol. Res., 2008; 163: 234-242.
- Esitken, A., Yildiz, H.E., Ercilis, S., Donnez, M.F., Turan, M., Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown Strawberry. Sci. Hortic., 2010; 124: 62-66.
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Hindawi. Pub Corp. sci., 2012; 2012: 1-15.
- Ahemad, M. and Kibret, M. Mechanisms and application of plant growth promoting rhizobacteria: Current perspective. J. King. Saud. Univ. Sci., 2014; 26: 1-20.
- Wekesa, C., Okun, D., Juma, K., Shitabule, D., Okhoth, P., Nyongesa, P., Katoo, A., Mulama, S., Wamalwa, E., Mahalo, C., Koyo, M., Rotich, A., Kawaka, F., Muoma, J. Abundance and symbiotic potential of common bean (Phaseolus vulgaris) nodule associated bacteria in Western Kenya soil. MAYFEB. Journal of Agricultural Science., 2016; 1:1-9.
- Ivanova, R., Bojinova, D., Nedialkova, K. Rock phosphate solubilization by soil bacteria. J. Chem. Technol. Metall., 2006; 41: 297-302.
- Sharma, S.B, Sayyed, R.Z, Trivedi, M.H, Gobi, T.A. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soil. Springer plus., 2013; 2: 587.
- Campbell, C.G.: Grass pea. Lathyrus sativus L. Promoting the conservation and use of underutilized and neglected crops. 18. In: Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute. Rome: Italy, 1997; pp 1-91.
- Quezel, P. and Santa, S.:New flora of Algeria and the southern desert regions, 1 and 2 tomes. Paris: CNRS, 1962; pp1-1170.
- Second National Report on the State of Plant Genetic Resources. National Institute of Agronomic Research of Algeria. United Nations Food and agriculture.,2006.
- De Meyer, S.E. and Willems, A. Multilocus sequence analysis of Bosea species and description of Bosea Lupini sp.nov., Bosea Lathyri sp.nov. and Bosea robiniae sp.nov., isolated from legumes. Int.J. Sys. Evol. Microbiol., 2012; 62: 2505-2510.
- Vincent, J.M. (ed): A manual for the practical study of root nodule bacteria. Oxford: IBP handbook 15 Blackwell Scientific Publications, 1970; pp1-164.
- Pikovskaya, R.I.: Mobilization of phosphorus in soil connection with the vital activity of some microbial species., J. Microbiol., 1948; 17: 362-370.
- Sperber, J.I. The incidence of apatite by solubilizing organisms in the rhizosphere and soil. Aust. J. Agri. Res., 1958; 9: 778-781.
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS. Microbiol. Lett., 1999; 170: 265-270.
- Chen, P.S., Toriba, T.Y., Warner, H. Microdetermination of phosphorus. Anal. Chem., 1956; 28: 1756-1758.
- Brunet, J., Reppellin, A., Varrault, G., Terryn, N., Zwily-Fodil, Y. Lead accumulation in the roots of grass pea (Lathyrus sativus L.): a novel plant for phytoremediation Systems. C. R. Biol., 2008; 331: 859-864.
- Djedidi, S., Yokoyama, T., Ohkama-Ohtsu, N., Chandra-Prasad-Risal, C., Abdelly, C., Hitoshi-Sekimoto H. Stress tolerance and symbiotic and phylogenic features of root nodule bacteria associated with Medicago species in different bioclimatic regions of Tunisia. Microbes. Environ., 2011; 26: 36–45.
- Thami-Alami, I., Elboutahiri, N., Udupa, S.M. Variability in natural population of Sinorhizobium meliloti in Morocco. Options. Méditerr. Série. A. Méditerr. Semin., 2010; 92:265–269
- Alikhani, H.A., Saleh-Rastin, N., Antoun, H. Phosphate solubilization activity of rhizobia native to Iranian soils. Plant. Soil., 2006; 287: 35-41.
- Silva Filho, G.N. and Vidor, C. Phosphate solubilization by microorganisms in the presence of different carbon sources. Rev. Bras. Ciênc. Solo,. 2000; 24: 311-319.
- Chen, Y.P. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil. Ecol., 2006; 34: 33-41
- Walpola, B.C. and Yoon, M.H. Phosphate solubilizing bacteria: Assessment of their effect on growth promotion and phosphorus uptake of mung bean (Vigna radiate [L.] R.Wilczek). Chil. J. Agri. Res., 2013; 73: 275-281.
- Al-Enzi, R.M.J. and Al-Charrakh, A.H.: Heavy metal resistant bacteria Pseudomonas aeruginosa as a model. In: LAP LAMBERT Academic Publishing, 2012; pp 1-93.
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbial. Biotechnol., 1999; 51:730-750.
- Seob, J., Walpola, B.C., Chung, D.Y., Yoon, M.H. Heavy Metal Resistant Phosphate Solubilising Bacteria. Korean. J. Soil. Sci. Fertil., 2012; 45: 817-821.
- Zaidi, A., Khan, M.S., Ahemed, M., Oves, M. Plant Growth Promotion by Phosphate Solubilizing Bacteria. Acta. Microbiol. Immunol. Hung., 2009; 56: 263–284.
- Hopkins, W. G. and Hüner N. P. A.: Introduction to plant physiology. New York: University of Western Ontario, Jhon Wily and sons.; 2008; pp 1-503.
- Shiraishi, A., Matsuhita, N., Hougestsu, T. Nodulation in balck locust by the Gamma-proteobacteria Pseudomonas sp. and the Beta-proteobacteria Burkholderia sp. Syst. Appl. Microbiol., 2010; 33(5): 269-274.
- Mazur, A., De Meyer, S.E., Tian, R., Wielbo, J., Zebrachi, K., Seshadri, R., Reddy, T., Markowitz, V., Ivanova, N.N., Pati, A., Woyke, T., Kyrpides, N.C., Reeve, W. High-quality permanent draft genome sequence of Rhizobium leguminosarum bv.viciae strain GB30; an effective microsymbiont of Pisum sativum growing in Poland. Genomic. sci., 2015; 10:36.
- Baymiev, A.K., Ptitsyn, K.G., Muldashev, A.A., Baymiev, A.K. Genetic Description of Root Nodule Bacteria of Lathyrus species growing on the Territory of the Republic of Bashkortostan. Russ. J. Genet., 2012; 2: 122-126.
- Kuznetsova, I.G., Sazanova, A.L., Safronova, V.I., Pinaev, A.G., Verkhozina, A.V., Tikhomirova, N.Yu., Osledkin, Yu.S., Belimov, A.A. Genetic diversity among microsymbionts of Lathyrus, Vicia, Oxytropis and Astragalus legume species from Baikal region. Agricultural Biology., 2015; 50: 345-352.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.