ISSN: 0973-7510
E-ISSN: 2581-690X
Biotechnological and industrial processes involve applications of various microorganisms and enzymes, and laccase, as a multifunctional enzyme, is admired for its role in degrading a variety of substances. Laccase is a copper-containing oxidase enzyme that is usually found in insects, plants, and microorganisms including fungi and archaea. Several phenolic substrates are oxidized by laccases, which results in crosslinking. Various research work and industrial solutions have identified the true potential of laccases to degrade various aromatic polymers, and their plausible application in bioremediation and other industries is entirely conceivable. This review focuses on laccases as a multifunctional enzyme and provides an overview of its natural origin, catalytic mechanism, and various methods of production. Further, we discuss the various applications of laccase in the biotechnological arena. We observed that laccase can degrade and detoxify various synthetic compounds. The broad substrate specificity of the same makes it worthy for different fields of industrial applications such as food and bioremediation technology, textile and paper technology, biosensors and nanobiotechnology, biofuel, and various other applications, which are described in this paper. These recent developments in the application of laccase show the multifunctional role of laccase in industrial biotechnology and provide an outlook of laccase as a multifunctional enzyme at the forefront of biotechnology.
Laccase, Enzyme, Multifunctional, Biotechnology, Oxidation
Enzymes are crucial in biotechnological applications due to their multifunctional characteristic. Laccase is an enzyme that is multifunctional and is used in various biotechnological applications.1
Laccases belong to the multi-copper oxidases enzyme group and are classified as benzenediol oxygen reductases. It is also known as urushiol oxidases and p-diphenol oxidases. They are viewed as versatile proteins fit for oxidizing enormous numbers of particles such as non-phenolic and phenolic compounds because of their broad substrate specificity. Laccases, being a flexible biocatalyst and with eco-friendly enzymatic degradation, produces water as a by-product and utilize molecular oxygen as a concluding acceptor of electrons which results in increased biotechnological interest among the science community. Usually, they are present in insects, plants, and microorganisms including fungi and archaea, along with other oxidative enzymes that degrade lignin. The same is used in a vast range of bioremediation processes for protecting ecosystems from damages incurred through effluents of industries. In recent years, researchers are intensively focusing on laccase, much of it due to the large variety of laccases, their usefulness, and their enzymology, which is very important. 2-4
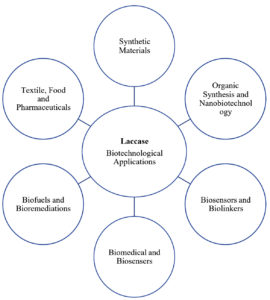
This group of enzymes is used for several purposes, including manufacturing of food additives, in the production process of beverages, for medical uses, and as a pretreatment agent in the biofuel production process. They have also been extensively researched for implantable biosensors and biofuel cells in nanobiotechnology. In addition, their ability to convert complex xenobiotics makes them useful enzymatic bioremediation biocatalysts. The broad substrate specificity, high catalytic constants, and the utilization of atmospheric oxygen as the second substrate offer the basis for the application of laccase in the synthesis of organic compounds, biosensors, and immunoassays.5 Various biotechnological applications of laccase is shown in Figure 1.
Figure 1. Various biotechnological applications of Laccase. The broad substrate specificity and multifunctionality provide enormous opportunities for Laccase in a vast variety of industrial applications.
Laccase was first discovered in 1883 in the latex of the lacquer tree Rhus vernicifera. Both Bertrand and Laborde established for the first time in 1896 that laccase was found in fungi. It is found in higher plants, fungi, bacteria, and in various insects, as well as in archaea.
Catalytic Mechanism of Laccase
Laccases are generally known to be monomeric, but also exist in homodimeric, heterodimeric, and multimeric forms. Some laccases, such as fungal laccases, are glycosylated. The enzyme’s carbohydrate moiety will make up 10-45 pounds (86-91%) of the protein molecule by weight. It includes carbohydrates such as hexosamine, glucose, mannose, galactose, fucose, and arabinose. Depending upon the source of the laccases, the molecular masses can differ and, the probable reason for the variation could be the variation in its structure. Laccases are MCOs and are known to have four copper atoms in special oxidation states: one type-1, one type-2, and two type-3s, together which form the catalytic site.6
Usually, the laccase spectrum shows the property of absorption at 280 and 605 nm but some laccases do not follow the mentioned range. They belong to the group of multicopper oxidase with varying content of glycosylation. Mostly, laccases occur as monomeric and polymeric glycoproteins.
It catalyzes oxidation of a vast range of compounds integrated with 4 electrons reduction of molecular oxygen to water. The biological functionality for laccases depends upon the sources of origin and life stages of the producing organisms. Laccases can oxidize various aromatic compounds including phenol derivatives, amine derivatives, thiols, and also some inorganic compounds such as iodine and ferrocyanide ions.7
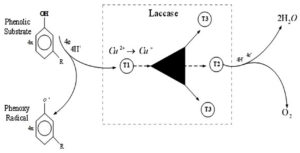
The action mechanism of laccases includes two individual sites that bind the reducing substrate and O2 with four catalytic copper atoms. Two individual sites connecting decreasing substrate and oxygen with 4 catalytic copper atoms form the laccase mechanism of action: the paramagnetic type 1 copper (T1Cu) responsible for the distinctive blue color of the protein in the decreased resting state where the oxidation of the substrate takes place; the T2Cu and the two T3Cu clustered 12Å away from the T1Cu. This trinuclear cluster is where O2 is reduced to two water molecules, receiving four consecutive electrons through a strictly conserved His-Cys- His electron transfer route from four separate mono-oxidation reactions at the T1Cu site. X-ray crystallography resolved approximately 90 laccase structures to date.8,9
The graphical representation of the catalytic mechanism of laccase is shown in Figure 2.
Figure 2. Catalytic mechanism of laccase enzyme. Four monoelectronic oxidations are catalyzed from a substituted phenolic substrate and free radicals are generated. Four copper atoms within the protein transfer and stores electron, and are transferred to molecular oxygen to produce two water molecules.
As a novel industrial enzyme, Laccase received huge attention due to its much higher abilities for catalyzing the oxidation of aromatic compounds that worked on the catalytic activity of laccase hosted in reversed micelles.10 Researchers have successfully heightened the catalytic capacity of the laccase enzyme to degrade the diclofenac through carbon nanotubes.11 Some compounds have been shown to have a capacity to inhibit laccases, such as halides, acetate, and so on.
Laccase production
Laccase is an enzyme released in the extracellular medium by numerous fungi12 through secondary metabolism, however, not every fungal species, such as Chytridiomycetes and Zygomycetes produce laccase.13 Laccase synthesis by soil and some freshwater Ascomycete species is mentioned in the literature.14-18
Various sources of laccase and the organism producing it, are listed in Table 1.
Table (1):
Various sources of laccase and the organism producing it. It is found in higher plants, fungi, bacteria, and in various insects, as well as in archaea.
S. No |
Source |
Organism |
References |
|---|---|---|---|
1. |
Phanerochaete chrysosporium, Theiophora terrestris, Lenzites, betulina |
Fungi |
Viswanath et al. 2008 |
2. |
Streptomyces lavendulae Streptomyces cyaneus, and Marinomonas mediterranea |
Bacteria |
Jimenez-Juarez et al. 2005; Arias et al. 2003; Thakker et al. 1995 |
3. |
Phlebia radiate |
Fungi |
Niku-Paavola et al. 1988 |
4. |
Pleurotus ostreatus |
Fungi |
Palmieri et al. 2000 |
5. |
Trametes versicolor |
Fungi |
Bourbonnais et al. 1995 |
6. |
Trichoderma atroviride, Trichoderma harzianum |
Fungi |
Hölker and Höfer 2002 |
7. |
Trichoderma longibrachiatum |
Fungi |
Velázquez-Cedeño et al. 2004 |
8. |
Monocillium indicum |
Plant |
Thakker et al. 1995 |
9. |
Pycnoporus cinnabarinus, Pycnoporus sanguineus |
Fungi |
Eggert et al. 1996; Vrijmoed 2000 |
10. |
Toxicodendron vernicifluum (Rhus vernicifera) |
Plant |
Takano et al. 2021 |
11. |
Asparagus densiflorus |
Plant |
Watharkar et al. 2018 |
12. |
Gossypium arboreum |
Plant |
Wang et al. 2004 |
13. |
Oryza sativa |
Plant |
Huang et al. 2016 |
14. |
Azospirillum lipoferum |
Bacteria |
Givaudan et al. 1993 |
15. |
Bacillus tequilensis |
Bacteria |
Sondhi et al. 2014 |
16. |
Bacillus subtilis |
Bacteria |
Enguita et al. 2003 |
17. |
Streptomyces, Amycolatopsis, and Nitrosomonas |
Bacteria |
Roberts et al. 2002 |
18. |
Marinomonas mediterranea and Bacillus halodurans |
Bacteria |
Jimenez-Juarez et al. 2005; Ruijssenaars and Hartmans 2004 |
19. |
Drosophila, Lucilia, Manduca, Musca, Orycetes, Papilio, Phormia, Rhodnius, Sarcophaga, Schistocerca, and Tenebrio |
Insects |
Arora and Sharma, 2010 |
Laccase production has also been discovered in Magnaporthe grisea, Ophiostoma novo-ulmi, Gaeumannomyces graminis, Monocillium indicum, Neurospora crassa, and Podospora anserina, Marginella, Melanocarpus albomyces.1 Botryosphaeria develops a real laccase-like dimethoxyphenol oxidising enzyme. Laccase genes that oxidise syringaldazine are found in Ascomycetes that contribute to plant biomass decomposition.19 The Basidiomycete yeast Cryptococcus neoformans generates laccase and oxidises phenols and aminophenols but not tyrosine. Only the Saccharomyces cerevisiae plasma membrane-bound multicopper oxidase has similarity with fungal laccase.
Various well-known species which produce a significant number of laccases in variable quantities are Basidiomycetes and Saprotrophic fungi.20 Laccases produced by Pycnoporus cinnabarinus are known to be a ligninolytic enzymes that can degrade lignin. However, ability of brown-rot fungi for producing laccases is unknown, and no laccases are being purified. Brown-rot Coniophora puteana fungus21 has been discovered to oxidise syringaldazine and assist in oxidation of ABTS in Laetiporus sulphureus.22 Laccase production is influenced by several parameters, including cultivation type (submerging or in state of solid), limitation of carbon and source of nitrogen.23 Various bacteria of various genera are used to produce laccases. Bacillus, Geobacillus, Streptomyces, Rhodococcus, Staphylococcus, Azospirillum, Lysinibacillus, and Aquisalibacillus are examples of Gram-positive bacteria.24,25 For maximum laccase production, literature suggests that each type of fermentation (submerged and solid) is suitable.24
Types of Cultivation
Laccase is produced primarily via submerged and solid-state fermentation methods. In various cultivation techniques (Table 2), wild-type filamentous fungi are employed to produce laccase on a big scale.
Table (2):
Laccase production by various microorganisms as reported in the literature, the cultivation conditions required for laccase production, inducers used, and the laccase activity detected.
Microorganism |
Cultivation Types |
Inducers |
Laccases Activities (U/L) |
|---|---|---|---|
Pycnoporus cinnabarinus |
Submerged |
10mM Veratryl alcohol (VA) |
280 |
Trametes pubescens |
Submerged |
2mM Cu2+ |
333000 |
Neurospora crassa |
Submerged |
1 μMcyclohexamide |
10000 |
T. versicolor |
SSF (Immersion, nylon sponge) |
Tween80 |
229 |
T. versicolor |
SSF (Immersion, barley bran) |
Tween80 |
600 |
T. versicolor |
SSF (Expanded bed, nylon sponge) |
Tween80 |
126 |
T. versicolor |
SSF (Expanded bed, barley bran) |
Tween80 |
600 |
T. versicolor |
SSF (Tray, nylon sponge) |
Tween80 |
343 |
T. versicolor |
SSF (Tray, barley bran) |
Tween80 |
3500 |
T. hirsute |
SSF (Tray, grape seeds) |
– |
18715 |
Streptomyces coelicolor |
Submerged |
– |
350 |
Escherichia coli |
SSF (sawdust) |
– |
41,000 |
Bacillus subtilis |
SSF (agro residue) |
– |
402 |
Solid State Fermentation
Solid state fermentation (SSF) is well suited for enzyme production by using natural substrates such as agriculture residue.26,27 The sugar-rich lignin, cellulose, and hemicellulose enhance the growth of fungus in the fermentor and makes such a process highly cost-effective.28 Many bacteria are common for producing laccases extra-cellularly, on the other hand, many bacteria are not able to secrete laccases outward from the cell.5 Solid-state fermentation is shown as a cost-effective method to produce laccase by bacteria. The nearby absence or absence of water that is free-flowing, causes the process to occur.4 Solid state fermentation requires a moisture content of roughly 15 percent. Sawdust, wheat bran, cereal grains, wood shavings, and a variety of other plants and animal products are the most often utilized solid substrates for SSF.29 Conditions in which microorganisms are cultivated under SSF are comparable to those in their native habitat. The bioreactor design has a severe flaw in that heat and mass transport are narrower. Various bioreactor configurations for laccase production are investigated, including immersion, extended bed, tray, inert (nylon), and non-inert (barley bran) support, with the design of tray yielding the good results.31 Grape seed and orange peels are used as substrates in a comparison of tray and immersion laccase production.32 Rice bran has a higher laccase production rate during both solid state and submerged fermentation than other substrates. Inducers are found to promote laccase production. The addition of veratryl alcohol or MnO2 to the cultures seemed to induce laccase activity production. Cycloheximide is also known to induce laccase biosynthesis. Based on phenolic compounds such as vanillic acid and ferulic acid, rice bran has the ability to induce laccase production. A wide variety of agricultural wastes are also used for laccase production, including seeds of grapes, wastewater, barley bran, cotton stalk, and wheat bran. This is because laccase production was not maximized in either solid-state or submerged fermentation.23
Submerged Fermentation
In submerged fermentation, the microorganism is cultivated in a liquid nutrient medium that contains a high level of oxygen concentration. When it comes to fungal submerged fermentation, the main problem is the viscosity of broth. Because oxygen and mass transfer are limited because of mycelium formation, impeller action is hindered. Diverse strategies have been employed to combat this issue. For maximum efficiency, the bioreactor must run continuously. Trametes versicolor is used to decolorize synthetic dye, and a pulse system was developed for this purpose.33 Cell immobilisation also solves problems with broth viscosity, mass transfer and oxygen. Because Neurospora crassa is immobilised on membrane, Luke and Burton report that regular laccase production occurs for four months without enzyme deactivation. As part of the bioremediation of pentachlorophenol (PCP) and 2,4-dichlorophenol (2,4-DCP) nylon mesh is used to compare free cell cultures of T. versicolor having an immobilised culture of these chemicals.
Multifunctional Biotechnological Applications of Laccase
Due to its multiple signature functions, laccase can be employed as a multifunctional enzyme, relying on the cell type and the conditions under which its isozymes are expressed (intracellular or extracellular). Figure 3 shows the various plausible mechanism for shifting multiple functions of laccases.
Figure 3. Various plausible mechanisms for shifting multiple functions of laccases.
1. Distinct functions at various locations in a cell.
2. Disparate behavior of proteins; intracellularly and intracellularly
3. Discrete functions of proteins when oozed by different cell types.
4. Protein activity actuality is influenced by the binding of substrate, products, or cofactors.
5. Proteins have discrete binding sites for various substrates (Sharma and Kuhad 2008).
As biosensors or bioreporters, multiple oxidoreductases can be added. A miscellaneous and limitless supply of energy sources may be used by enzymatic biofuel cells, which has encouraged scientists to concentrate on small, implantable power devices in living systems.34
Laccase in Food and Bioremediation Technology
Without a costly investment, laccase could improve production, performance, and quality of food products and has the advantage of being a moderate technology. Laccase is one of the most studied enzymes for bioremediation; credited for its ability to decompose phenolic compounds. Laccase helps in the bioremediation of food industry wastewater. Various researchers recorded that laccase has been immobilized on organogel support and was able to extract aqueous suspensions from naturally occurring and xenobiotic aromatic compounds. In a model wastewater solution, with the help of polyethersulphone the laccase was immobilized on it through adsorption which is capable for reducing phenol concentration.35 In food industries, laccase is the choice because of its capability to expedite homo- and hetero- polymerization reactions. It may be used in the stabilization of wine and beer, processing of fruit juices, baking process, improvement of the sensory parameter of food, and gelation of sugar beet pectin.36 Owing to its huge polyphenol contents and dark-blown colors, beer-factory wastewater is a significant environmental concern. The white-rot fungi producer of laccases, Coriolopsis gallica, is used by researchers for degrading its high-tannin-containing wastewater.37
When it comes to the food industry, the major application of laccase is for wine stabilization. While the aroma of wine is caused by alcohol and organic acids, different wine types may be determined through the enrichment of wine with the various compound of phenolic which plays a crucial role in facilitating distinct color and taste to it. To preserve color and flavor in wine, different methods have been used, such as the elimination of polyvinylpolypyrrolidone (PVPP) phenolic groups, alternatively, oxidizers are blocked using sulfur dioxide. Fungal laccase (e.g., derived from Trametes versicolor) is well-suited for polyphenol removal from wines but requires a set of conditions essential for proper functioning, such as medium acid stability with an ideal pH range around 2.5-4.0 and reversible sulfite inhibition.38
Beer polyphenols can be oxidized and removed through the application of laccases (eg. Laccase derived from strains of Aspergillus, Neurospora etc.) ― filtration or other separating processes are preferred for the removal of formed polyphenol complexes. Laccases were also preferred for the removal of O2 from beer at the end of the production process, thus enhancing the storage life of beer.39 Excessive phenolic oxidation has almost always been considered unfavorable for apple and grape juices. By delaying the onset of protein-polyphenol haze formation, laccase has also been used to stabilize fruit juices in several tests.40
Various other applications such as sugar beet pectin gelation, improvement of food sensory parameters, and ascorbic acid determination, where fungal laccases were proved to be an extremely potential and propitious enzyme for the food industries.
The xenobiotic may be oxidized by laccase or Laccase-mediator System (LMS), which may be readily taken away through a practical or mechanical process to produce a product that is less toxic or has greater bioavailability. Laccase has been reported to oxidize numerous hazardous compounds alone or through a redox mediator. Laccase enzyme may be employed for detoxifying pollutants ― the toxins are oxidized into free radicals or quinones, as a result, it undergoes polymerization and partial precipitation. In their insoluble shape, the contaminants are less harmful and can be separated from water through physical procedures.41 To form reactive aryloxy radicals, both laccase and peroxidase oxidize phenolics, which automatically polymerize for forming an insoluble complex. Such complexity may be eliminated with the application of physical methods like precipitations, filtrations, or centrifugations.41 Laccase from Trametes versicolor can potentially decompose pesticides (insecticides, fungicides, and herbicides). While trinitrotoluene does not serve as a substrate for an oxidative enzyme such as laccases, whereas, partial degradation is possible and it does cause the accumulation of reduction of metabolites such as aminodinitrotoluenes (ADNT), azoxies compound, and diamino nitrotoluenes.42
Laccase in Textile and Paper Technology
Laccases are enzymes that have been applied to bleach textiles, modify cloth surfaces, and synthesize dyes in addition to de-colorize textile effluents. Therefore, various chemicals, energies, and water-consuming textile operations could be replaced by laccase-based processes. Laccase-based processes seem to be an appealing alternative due to their ability for degrading dye of various chemical structures, which also include synthetic dyes which are being used in the industry.4, 43
Different aromatic compounds (which includes phenol and aniline) may also be oxidized by laccase to simultaneously facilitate non- enzymatic homo and hetero-coupling reaction, resulting in color palettes of various useful textile dyes (which include phenoxazine and azo dye).44
In the paper pulp industry, laccases are favored over peroxidases (lignin, manganese, and flexible peroxidases) because unlike peroxidases, they utilize O2 instead of H2O2 and the co-substrate does not affect their functioning. There have been records of enzymatic bleaching of flax pulps along with laccase of various fungi and redox mediators of pure and synthetic origin.44 Various other applications are there including some fungal and bacterial laccase-induced grafting of phenol to flax fiber for the production of paper.45, 46, 47
Laccase in Biosensors and Nanobiotechnology
Enzyme immobilization is useful in biomedicine. Enrico et al. (year?) worked with the production of cheaper carriers of nylons 6 film and nanofiber and immobilization on nylon carriers by laccase, which helped to develop new biocatalytic material for industrial use.48
Chauhan et al.5 made a laccase (Coriolus Versicolor) immobilized biosensor on a polyethers sulfon membranes at pH value 4.5, which is added to the base electrode of Universal Sensors. Reduction of the oxidation product of several laccase catalyzed polyphenols was performed at a potential for which the polyphenol of interest has been found to react. Catechol reductions have been found to occur at the potential of -200 mV, sometimes referring to the polyphenolic biosensor literature.49 researched the production of the catechol biosensor, and laccase enzymes were used from two separate sources, namely tree laccases from Rhus vernicifera and fungal laccases from Coriolus hirsutus. Laccase, through glutaraldehyde coupling, is immobilized on amine termination thiol monolayer on the surface which is golden. Based on sensitiveness, stability, reproducibility, and various electrochemical properties of enzyme electrodes and many monolayers which are investigated, Cystamine has been found much optimal49,50 worked to make a novel laccases-modified screen-printing electrodes and these bio nanocomposite sensors were optimized in terms of enzyme loading, pH, and applied potential. It was assessed for Bisphenol A (BPA) determination.50
A laccase biosensor can be used for electrochemical estimation of the polyphenol index in wines using a laccase biosensor. It was reported to be used for rapid and reliable amperometric estimation of the total content of polyphenolic compounds in wines.51 By inter-linking with the glutaraldehyde on a glassy carbon electrode, the enzyme was immobilized. To carry out such an estimation, caffeic and gallic acid were chosen as a compound that is standard.
Laccase in Biofuel and other Applications
Laccase plays a major role in the biodegradation of lignin, and its potential uses as an agent in the development of biofuels and as biocatalysts for the removal of growth of yeast inhibitors (majorly phenolics) for the consecutive enzymatic process is therefore being studied. Laccases are the enzyme, the investigation of which is done not for potential use as a pre-treatment agent in developing biofuels, primarily as enzyme delignify, on the other hand, also as a biotechnological method to extract (mainly phenolic) inhibitors from subsequent enzymatic processes.52 Researchers developed laccase–glucose oxidase biofuel cells with designs and analysis of biofuel cells consisting of an anode basis on glucose oxidase (Aspergillus niger) and laccases-basis of cathodes (Trametes versicolor) using redox polymers basis of osmium as a mediator of electrons transferring by biocatalysts to graphite electrodes surface.53 Laccases-based biocathode for bio electrocatalytic oxygen reduction and its use of advanced electrode material, like nanoparticle and nanowire is researched for commercial applications.54
Laccase is very promising as a biocatalyst in the synthesis of bioactive compounds. Their response requires molecules of oxygen only and as only by-products, releases water. To generate reactive radicals, Laccase catalysis requires abstracting one electron through its substrate. Subsequently, free radical is subjected to homo & hetero-coupling for forming oligomeric, polymeric, dimeric, and cross-coupling products through practical consequences for organic synthesis.55
Laccases can be used in the formulation of hair dyes to substitute H2O2 as an oxidizing agent. The potential for real phenols for forming products beneficial in hair dyeing is explored by laccase-catalyzed polymerization.56 By use of mixtures of two phenolic monomers, 15 phenol test yields colored product after treatment of lacquer, and diversity of color has been achieved. The study shows that for the production of new cosmetic pigments, laccases- catalyze polymerizations of natural phenol are applicable.56
Various industrial applications of laccase and its innumerable usages are listed in Table 3.
Table (3):
Laccase industrial applications and their innumerable usages. The wide range of applications is in the industries of food bioremediation, nanotechnology, textile, pulp, and paper industry, dye synthesis and dye decoloration, petroleum chemistry, biosensor, etc.
Application |
Use |
Reference |
|---|---|---|
Industries of Food |
Stabilization of Wine, Stabilization of Beer, Processing of Fruit Juice, baking (bread), preparing cork stoppers, pectin gelation of sugar beet, improve in parameter of food sensory etc. |
Osma et al. 2010 |
Bioremediation |
Degradation of xenobiotics, degrading Pharma & Products of personal care (PPCPs), degradation of antibiotics, reduce estrogen concentration from municipal waste water treatment plants, removal of pentachlorophenols (PCP) and polychlorinated biphenyls (PCB) from waste water. |
Viswanath et al. 2014 Yang et al. 2017 Strong and Claus 2011 |
Nanotechnology |
Laccase mediator system- As substrate molecule is not able for entering into the active sites of laccases because of size, these mediator react as carrier of electron b/w enzymes and substrates. Mediators mostly used are 3-hydro-xyanthranilic acids (3-HAA), N-hydro-xyphthalimide (NHPI), p-coumaric acid, syringaldehyde, 1-hydroxy benzotriazole (HBT), 2, 2- azinobis-(3-ethylbenzthiazoline-6-sulfonate) (ABTS), and 3 hydroxyanthranilic acid |
Christopher et al. 2014 Li et al. 1999 |
Textile, Pulp and Paper industry |
Discharging treatments of pulps and papers industries, pitch control, Laccases assisting transformations of lignin to value adding aromatic compound, denim finish, cotton bleach, rove scoure, treating of dye contamination of waste water. |
Singh and Arya 2019 |
Dye synthesis and Dye decoloration |
Dyes of old news print & waste papers through laccase mediator system, synthesis of hair dye using plant derived phenols catalyzed by laccase enzymes. |
Singh and Arya 2019 Ibarra et al. 2012 Jeon et al. 2010 |
Petroleum chemistry |
Used for degrading of Polycyclic Aromatic Hydrocarbon (PAH) in petroleum waste produce |
Peixoto et al. 2013 Wang et al. 2018 |
Biosensor |
Laccases-base on biosensor for detecting of phenolic compound |
Rodríguez-Delgado et al. 2015 |
Because of its ability for oxidizing phenolic and non-phenolic lignin relating compounds as well as extremely recalcitrant environmental contaminants, laccases have gained great attention from researchers in recent decades, which make them much beneficial for their application in biotechnological process. These uses include the detoxifying industry’s waste, primarily from paper and pulps, textiles and petrochemicals industry, and the use of herbicides, pesticides, and some explosives in the soil as a method for medical diagnostics and as bioremediation agents. There are numerous applications in food handling, such as heating, gluten-free bread, beverage stability (wine, juice, brew), bioremediation, and so on, and enamel plays a vital role in green food preparation. Future research with laccase will further extend the scope of its multifunctional applicability in biotechnology and various industrial and research field.
Biotechnological applications are likely to be found for laccase due to its high increase potential, which makes it a better choice for biotechnological applications in the food industry. Laccase may make food handling safer for the environment and more prudent. This potential is expected to be fully implemented with a better understanding of laccase creation and its mechanism of activity. Mediators can be used to extend the laccase portion to non-phenolic substrates, as well. Laccase’s infinite occurrence in various organisms ensures its widespread accessibility, particularly among wood decay parasites, also known as white decay growths, which are excellent laccase producers. Laccase overexpression in heterologous frameworks has been shown to increase laccase expression levels and improve catalytic activity. Streamlining the media and selecting the appropriate producers can provide further benefits to increased production with less resource use. Laccases were created using aggressive aquatic cultivation processes, according to scientists. Reduced fermentation, on the other hand, promotes the SSF for modern laccase production. Future efforts to improve the design of SSF bioreactors could make SSF even more unique and competitive.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Shekher R, Sehgal S, Kamthania M, Kumar A. Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011;2011:217861.
Crossref - Mayer AM, Staples RC. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60(6):551-65.
Crossref - Alcalde M. Laccases: biological functions, molecular structure and industrial applications. In Industrial enzymes. Springer, Dordrecht. 2007:461-476.
Crossref - Couto SR, Herrera JL. Industrial and biotechnological applications of laccases: a review. Biotechnology advances. 2006;24(5):500-13.
Crossref - Chauhan PS, Goradia B, Saxena A. Bacterial laccase: recent update on production, properties and industrial applications. 3 Biotech. 2017;7(5):1-20.
Crossref - Yaropolov AI, Skorobogat’Ko OV, Vartanov SS, Varfolomeyev SD. Laccase. Applied Biochemistry and Biotechnology. 1994;49(3):257-80.
Crossref - Claus H. Laccases and their occurrence in prokaryotes. Archives of microbiology. 2003;179(3):145-50.
Crossref - Hakulinen N, Rouvinen J. Three-dimensional structures of laccases. Cellular and Molecular Life Sciences. 2015;72(5):857-68.
Crossref - Moţ AC, Pârvu M, Damian G, et al. A “yellow” laccase with “blue” spectroscopic features, from Sclerotinia sclerotiorum. Process Biochemistry. 2012;47(6):968-75.
Crossref - Michizoe J, Goto M, Furusaki S. Catalytic activity of laccase hosted in reversed micelles. Journal of bioscience and bioengineering. 2001;92(1):67-71.
Crossref - Xu R, Tang R, Zhou Q, Li F, Zhang B. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes. Chemical Engineering Journal. 2015;262:88-95.
Crossref - Agematu H, TSUCHIDA T, Kominato K, Shibamoto N, Yoshioka T, Nishida H, Okamoto R, SHIN T, MURAO S. Enzymatic dimerization of penicillin X. The Journal of antibiotics. 1993;46(1):141-8.
Crossref - Morozova OV, Shumakovich GP, Gorbacheva MA,etal. “Blue” laccases. Biochemistry (Moscow). 2007;72(10):1136-50.
Crossref - Junghanns C, Moeder M, Krauss G, Martin C, Schlosser D. Degradation of the xenoestrogen nonylphenol by aquatic fungi and their laccases. Microbiology. 2005;151(1):45-57.
Crossref - Abdel-Raheem A, Shearer C. Extracellular enzyme production byfreshwater ascomycetes. Fungal Diversity. 2002;11:1-9.
- Banerjee UC, Vohra RM. Production of laccase byCurvularia sp. Folia microbiologica. 1991;36(4):343- 6.
Crossref - Scherer M, Fischer R. Purification and characterization of laccase II of Aspergillus nidulans. Archives of microbiology. 1998;170(2):78-84.
Crossref - Rodríguez-Delgado MM, Alemán-Nava GS, Rodríguez- Delgado JM, et al. Laccase-based biosensors for detection of phenolic compounds. TrAC Trends in Analytical Chemistry. 2015;74:21-45.
Crossref - Lyons JI, Newell SY, Buchan A, Moran MA. Diversity of ascomycete laccase gene sequences in a southeastern US salt marsh. Microbial Ecology. 2003;45(3):270-81.
Crossref - Hattaka A. Biodegradation of lignin. Biopolymers, Lignin, humic substances and coal. 2001.
- Lee KH, Wi SG, Singh AP, Kim YS. Micromorphological characteristics of decayed wood and laccase produced by the brown-rot fungus Coniophora puteana. Journal of Wood Science. 2004;50(3):281-4.
Crossref - Schlosser D, Höfer C. Laccase-catalyzed oxidation of Mn2+ in the presence of natural Mn3+ chelators as a novel source of extracellular H2O2 production and its impact on manganese peroxidase. Applied and Environmental Microbiology. 2002;68(7):3514-21.
Crossref - Muthukumarasamy NP, Jackson B, Joseph Raj A, Sevanan M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochemistry research international. 2015;2015.
Crossref - Rezaei S, Shahverdi AR, Faramarzi MA. Isolation, one-step affinity purification, and characterization of a polyextremotolerant laccase from the halophilic bacterium Aquisalibacillus elongatus and i ts application in the delignification of sugar beet pulp. Bioresource technology. 2017;230:67-75.
Crossref - Couto SR, Sanromán MA. Application of solid-state fermentation to ligninolytic enzyme production. Biochemical Engineering Journal. 2005;22(3):211-9.
Crossref - Brijwani K, Oberoi HS, Vadlani PV. Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochemistry. 2010;45(1):120-8.
Crossref - Couto SR, Toca-Herrera JL. Laccase production at reactor scale by filamentous fungi. Biotechnology advances. 2007;25(6):558-69.
Crossref - Glazer AN, Nikaido H. Microbial biotechnology: fundamentals of applied microbiology. Cambridge University Press; 2007.
- Sharma KK, Kuhad RC. Laccase: enzyme revisited and function redefined. Indian journal of microbiology. 2008;48(3):309-16.
Crossref - Couto SR, Moldes D, Liébanas A, Sanromán A. Investigation of several bioreactor configurations for laccase production by Trametes versicolor operating in solid-state conditions. Biochemical Engineering Journal. 2003;15(1):21-6.
Crossref - Rosales E, Couto SR, Sanromán MA. Increased laccase production by Trametes hirsuta grown on ground orange peelings. Enzyme and Microbial Technology. 2007;40(5):1286-90.
Crossref - Blánquez P, Caminal G, Sarrà M, Vicent T. The effect of HRT on the decolourisation of the Grey Lanaset G textile dye by Trametes versicolor. Chemical Engineering Journal. 2007;126(2-3):163-9.
Crossref - Minussi RC, Pastore GM, Durán N. Potential applications of laccase in the food industry. Trends in Food Science & Technology. 2002;13(6-7):205-16.
Crossref - Lante A, Crapisi A, Krastanov A, Spettoli P. Biodegradation of phenols by laccase immobilised in a membrane reactor. Process Biochemistry. 2000;36(1- 2):51-8.
Crossref - Yagüe S, Terrón MC, González T, Zapico E, Bocchini P, Galletti GC, González AE. Biotreatment of tannin- rich beer-factory wastewater with white-rot basidiomycete Coriolopsis gallica monitored by pyrolysis/gas chromatography/mass spectrometry. Rapid communications in mass spectrometry. 2000;14(10):905-10.
- Servili M, De Stefano G, Piacquadio P, Sciancalepore V. A novel method for removing phenols from grape must. American journal of enology and viticulture. 2000;51(4):357-61.
- Osma JF, Toca-Herrera JL, Rodríguez-Couto S. Uses of laccases in the food industry. Enzyme research. 2010;2010.
Crossref - Strong PJ, Claus H. Laccase: a review of its past and its future in bioremediation. Critical Reviews in Environmental Science and Technology. 2011;41(4):373- 434.
Crossref - Claus H, Bausinger T, Lehmler I, et al. Transformation of 2, 4, 6-trinitrotoluene (TNT) by Raoultella terrigena. Biodegradation. 2007;18(1):27-35.
Crossref - Lacasse K, Baumann W. Textile Chemicals: Environmental data and facts. Springer Science & Business Media; 2012 Dec 6.
- Polak J, Jarosz-Wilkolazka A. Fungal laccases as green catalysts for dye synthesis. Process Biochemistry. 2012;47(9):1295-307.
Crossref - Virk AP, Sharma P, Capalash N. Use of laccase in pulp and paper industry. Biotechnology progress. 2012;28(1):21-32.
Crossref - Singh G, Arya SK. Utility of laccase in pulp and paper industry: A progressive step towards the green technology. International Journal of Biological Macromolecules. 2019;134:1070-84.
Crossref - Fatarella E, Spinelli D, Ruzzante M, Pogni R. Nylon 6 film and nanofiber carriers: Preparation and laccase immobilization performance. Journal of Molecular Catalysis B: Enzymatic. 2014;102:41-7.
Crossref - Gupta G, Rajendran V, Atanassov P. Laccase biosensor on monolayer-modified gold electrode. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis. 2003;15(20):1577- 83.
Crossref - Portaccio M, Di Tuoro D, Arduini F, et al. Laccase biosensor based on screen-printed electrode modified with thionine–carbon black nanocomposite, for Bisphenol A detection. Electrochimica Acta. 2013;109:340-7.
Crossref - Gamella M, Campuzano S, Reviejo AJ, Pingarrón JM. Electrochemical estimation of the polyphenol index in wines using a laccase biosensor. Journal of Agricultural and Food Chemistry. 2006;54(21):7960-7.
Crossref - Adelakun OE, Kudanga T, Parker A, Green IR, le Roes- Hill M, Burton SG. Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. Journal of Molecular Catalysis B: Enzymatic. 2012 ;74(1-2):29-35.
Crossref - Barrière F, Kavanagh P, Leech D. A laccase–glucose oxidase biofuel cellprototypeoperatingina physiological buffer. Electrochimica Acta. 2006;51(24):5187-92.
Crossref - Le Goff A, Holzinger M, Cosnier S. Recent progress in oxygen-reducing laccase biocathodes for enzymatic biofuel cells. Cellular and Molecular Life Sciences. 2015;72(5):941-52.
Crossref - Kudanga T, Nemadziva B, Roes-Hill L. Laccase catalysis for the synthesis of bioactive compounds. Applied Microbiology and Biotechnology. 2017;101(1):13-33.
Crossref - Jeon JR, Kim EJ, Murugesan K, et al. Laccase-catalysed polymeric dye synthesis from plant-derived phenols for potential application in hair dyeing: Enzymatic colourations driven by homo-or hetero-polymer synthesis. Microbial biotechnology. 2010;3(3):324-35.
Crossref - Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G. Fungal laccases and their applications in bioremediation. Enzyme research. 2014;2014.
Crossref - Jimenez-Juarez N, Roman-Miranda R, Baeza A, Sánchez-Amat A, Vazquez-Duhalt R, Valderrama B. Alkali and halide-resistant catalysis by the multipotent oxidase from Marinomonas mediterranea. Journal of biotechnology. 2005;117(1):73-82.
Crossref - Arias ME, Arenas M, Rodríguez J, Soliveri J, Ball AS, Hernández M. Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Applied and Environmental Microbiology. 2003;69(4):1953-8.
Crossref - Thakker GD, Evans CS, Rao KK. Purification and characterization of laccase from Monocillium indicum Saxena. Applied Microbiology and Biotechnology. 1992;37(3):321-3.
Crossref - Niku-Paavola ML, Karhunen E, Salola P, Raunio V. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochemical Journal. 1988;254(3):877-84.
Crossref - Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Applied and environmental microbiology. 2000;66(3):920-4.
Crossref - Bourbonnais R, Paice MG, Reid ID, Lanthier P, Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2, 2′-azinobis(3- ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Applied and Environmental Microbiology. 1995;61(5):1876-80.
Crossref - Hölker U, Dohse J, Höfer M. Extracellular laccases in ascomycetesTrichoderma atroviride and Trichoderma harzianum. Folia microbiologica. 2002;47(4):423-7.
Crossref - Velázquez-Cedeño MA, Farnet AM, Ferre E, Savoie JM. Variations of lignocellulosic activities in dual cultures of Pleurotus ostreatus and Trichoderma longibrachiatum on unsterilizedwheat straw. Mycologia. 2004;96(4):712- 9.
Crossref - Eggert C, Temp U, Eriksson KE. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Applied and environmental microbiology. 1996;62(4):1151-8.
Crossref - Takano M, Nakamura M, Tabata M. Comprehensive analysis of the isozyme composition of laccase derived from Japanese lacquer tree, Toxicodendron vernicifluum. Journal of Wood Science. 2021;67(1):1-0.
Crossref - Watharkar AD, Kadam SK, Khandare RV, et al. Asparagus densiflorus in a vertical subsurface flow phytoreactor for treatment of real textile effluent: a lab to land approach for in situ soil remediation. Ecotoxicology and Environmental Safety. 2018;161:70-7.
Crossref - Wang X, Sun SY, Ni ZJ, Li ZX, Bao J. Degradation of polycyclic aromatic hydrocarbons in contaminated soil by immobilized laccase. Journal of the Serbian Chemical Society. 2018;83(5):549-59.
Crossref - Huang MT, Lu YC, Zhang S, Luo F, Yang H. Rice (Oryza sativa) laccases involved in modification and detoxification of herbicides atrazine and isoproturon residues in plants. Journal of Agricultural and Food Chemistry. 2016;64(33):6397-406.
Crossref - Givaudan A, Effosse A, Faure D, Potier P, Bouillant ML, Bally R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiology Letters. 1993;108(2):205-10.
Crossref - Sondhi S, Sharma P, Saini S, Puri N, Gupta N. Purification and characterization of an extracellular, thermo-alkali- stable, metal tolerant laccase from Bacillus tequilensis SN4. PloS one. 2014;9(5):e96951.
Crossref - Enguita FJ, Martins LO, Henriques AO, Carrondo MA. Crystal structure of a bacterial endospore coat component: a laccase with enhanced thermostability properties. Journal of Biological Chemistry. 2003;278(21):19416-25.
Crossref - Roberts SA, Weichsel A, Grass G, et al. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proceedings of the National Academy of Sciences. 2002;99(5):2766-71.
Crossref - Ruijssenaars HJ, Hartmans S. A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Applied microbiology and biotechnology. 2004;65(2):177-82.
Crossref - Singh Arora D, Kumar Sharma R. Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol. 2010;160(6):1760-88.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.