ISSN: 0973-7510
E-ISSN: 2581-690X
Cotton, a key cash crop vital to the economy, suffers major yield loss due to Tobacco streak virus (TSV). TSV symptoms are frequently mistaken for physiological disorder, highlighting the need for accurate and timely diagnosis to enable effective disease control. Traditional diagnostics are time-consuming, relying on antibodies and specialized equipment. We developed a rapid, accurate RT-LAMP assay to diagnose TSV in cotton. The TSV inoculum was successfully maintained in Vigna unguiculata, Chenopodium amaranticolor and cotton in glasshouse. Molecular analysis through coat protein resulted in a 929 bp amplicon and the sequences were deposited in GenBank (PQ331227, PQ331228). The RT-LAMP assay employing a newly designed primer set efficiently amplified a 240 bp target region and yielded a distinct ladder-like banding pattern on gel electrophoresis confirming the virus. Colorimetric detection using hydroxy naphthol blue (HNB) dye allows visual identification of the virus with distinct color changes from violet to sky blue. A comparative sensitivity assay revealed that RT-LAMP has 10-fold higher sensitivity (0.05 pg/µl). These findings establish RT-LAMP as a rapid on-site diagnosis technique in comparison to RT-PCR for TSV diagnosis in cotton, facilitating early detection.
Cotton, Tobacco Streak Virus, RT-LAMP, RT PCR
Cotton (Gossypium spp.), a member of the Malvaceae family is a vital agricultural commodity widely utilized for textiles and garments. It is a crucial cash crop that influences the Indian economy in multiple ways.1 The cotton crop contributes 2%-2.3% to India’s GDP which accounts for 12%-13% of industrial production and makes up 12% of total export, with cotton yarn, fabrics and handloom product exports reaching $10.4 billion, an 8.7% year-over-year increase.2
India has become one of the few nations that grows all the four primary cotton species, viz. Gossypium hirsutum, G. barbadense, G. herbaceum and G. arboretum, across multiple states including Gujarat, Maharashtra, Telangana, Andhra Pradesh, Haryana, Madhya Pradesh and Tamil Nadu. In 2021, 32 million hectares of cotton were harvested globally, producing 24.1 million metric tons. India, China, U.S., Brazil and Pakistan are the leading cotton producers, that contribute a majority of global production.3
Cotton cultivation is vulnerable to multiple abiotic and biotic stress. Abiotic stress in cotton involves adverse environmental factors such as temperature extreme, drought, salinity, heavy metal and radiation.4 Biotic stresses including pathogens, pests and weeds reduce cotton yield by 10%-30% annually. Among these biotic factors, Tobacco streak virus (TSV), causing cotton stem necrosis become a major threat to crop cultivation. TSV, a member of the Ilarvirus genus within the Bromoviridae family is responsible for cotton necrosis disease, leading to substantial yield loss. It is a’+’ sense ssRNA with a tripartite genome that infects pulses, field crops, horticultural crops and various weed species. The genome contains three segments: RNA 1 for replicase protein, RNA 2 encodes RNA-dependent RNA polymerase and RNA 3 encodes the movement (MP) and coat protein (CP), with the latter expressed from subgenomic RNA.5 The coat protein is essential for Alfamovirus and Ilarvirus genome replication.6 TSV genome encode protein that facilitate systemic infection. The CP aids virion assembly and vector transmission (e.g., Thrips), while the MP modifies plasmodesmata, enabling cell-to-cell spread. Viral RNA-dependent RNA polymerase hijacks host ribosomes to reduce the photosynthetic efficiency. TSV is transmitted primarily through pollen, sap and several species of thrips.7,8 Parthenium pollen act as a key reservoir for TSV inoculum, which is primarily transmitted to cotton plant through feeding wound by Thrips spp. Field epidemic show direct correlation with thrips population density. Effective management requires removal of weed host combined with targeted insecticide application to suppress vector population. TSV was initially reported in sunflower and groundnut by Rao9 and Reddy,10 respectively. Cotton crop affected by Tobacco Streak Virus (TSV) suffer an average yield loss of 60%.11 Similarly, Telangana had the highest TSV incidence (51.11%) in the hybrid RCH659 across central and southern India.12 Valarmathi and Dhamayanthi reported TSV incidence ranging from 5.81% to 26.60% in G. barbadense germplasm in Coimbatore between 2017 and 2019. Recently, TSV outbreak in Andhra Pradesh, Kurnool and Nandyal district have alarmed farmers, damaging 10-15% of cotton.13 Field surveys in Guntur, Krishna and Kurnool revealed TSV incidence varying from 0-30% among different hybrid.14 In Brazil, soybean crops have suffered up to 100% yield loss due to TSV.15 The virus causes characteristic symptoms such as purple necrotic lesions on leaves and bolls, stem necrosis, drying of squares and stunted growth.16 However, TSV symptoms often resemble those caused by nutritional deficiencies, complicating field-level diagnosis.17 For example, necrosis/yellowing resembles K/Mg deficiency but TSV shows irregular vein-limited streaks. Stunted growth was due to Zn deficiency, but Zn affect new leaves first; TSV is systemic. Leaf falling down can look like N deficiency, but TSV causes patchy, rapid shedding.
Managing plant virus is inherently challenging and farmers rely primarily on synthetic chemicals to control vector population and prevent virus spread between the field. However, this approach is limited by improper pesticide use, misdiagnosis of viral infections and potential environmental concern. To effectively manage TSV in cotton, sustainable non-chemical approaches are essential. These include using resistant varieties, biological control like beneficial insects, cultural practices such as crop rotation and physical barriers. However, their success depends entirely on early virus detection through advanced diagnostics. Timely identification allows for prompt intervention, preventing severe outbreak and reducing yield loss while minimizing reliance on harmful chemical. Early detection is therefore the cornerstone of effective eco-friendly management. TSV is currently diagnosed via techniques such as enzyme-linked immunosorbent assay (ELISA), dot blot immunobinding assay (DIBA) and reverse transcription polymerase chain reaction (RT PCR).10,11,18 The development of an RT-LAMP assay for TSV detection addresses critical limitations of conventional RT-PCR, particularly for field application. However, despite its reliability, RT-PCR requires sophisticated laboratory infrastructure, thermal cycling equipment and trained personnel, making it less feasible for on-site or field-level applications. Loop-mediated isothermal amplification (LAMP), an advanced nucleic acids amplification method has emerged shortly.19 It is a highly efficient diagnostic method, offering exceptional sensitivity and rapid detection.20,21 The LAMP assay involves six primer sets which have been specifically developed to target six different regions within the target gene. Amplification was done using Bst DNA polymerase, which possesses chain-displacement operation, ensuring efficient and consistent amplification at an even temperature from 60 °C to 65 °C.22 Several studies have successfully employed RT-LAMP as effectively diagnosis to the prompt identification of RNA viruses across different crop. Notable examples include its application in detecting Tomato chlorosis virus,23 Potato leafroll virus,24 Tomato yellow leaf curl virus,25 Cucumber mosaic virus,26 Cucumber green mottle mosaic virus,27 Citrus yellow mosaic virus,28 Citrus tristeza virus29 and Tobacco streak virus.17
The objective of this investigation is to design a rapid and effective tool for identifying TSV in cotton. Traditionally, RT PCR has been the widely adopted molecular technique to recognize TSV because of its outstanding precision and accuracy. In contrast, RT-LAMP is a quick tool for diagnosis that can be performed in a minimal laboratory setting, offering a practical solution for resource-limited environment. According to the present research, the accuracy of RT-LAMP has been critically evaluated against the performance of the standard RT PCR method to assess its diagnostic efficiency in detecting TSV in infected cotton samples. RT PCR requires expensive equipment (thermocycler, electrophoresis) and takes 2-4 hours to complete. In contrast, RT-LAMP provides 10-fold greater sensitivity, delivers results in 30-60 mins using only a simple heat block, and enables visual detection through colorimetric dyes like HNB. Though RT-PCR maintains advantages for research applications, RT-LAMP rapidity, portability and cost-effectiveness position it as the superior choice for on-site TSV diagnosis and outbreak management.
TSV maintenance and propagation
Roving survey was conducted and 10-20 symptomatic TSV samples were collected from infected cotton field from Department of Cotton, Pulses, Central Institute of Cotton Research, wet land cotton growing area of Coimbatore district, since this area have more Parthenium weed intensity and thrips population. Local lesion and conventional PCR was performed to confirm the TSV before cryopreserved at -80 °C. The typical symptom includes chlorotic and dark purple necrotic lesions on leaves and marginal necrosis. The virus was propagated in cowpea (CO 7), chenopodium and cotton (CO 17) under insect-proof glasshouse condition by mechanical sap inoculation. In this assay, we have maintained four plants for each indicator host with three replications. The viral inoculum was prepared by macerating infected plant tissues in phosphate buffer with 0.1% mercaptoethanol.30 Seedlings at the two-leaf stage were dusted with carborundum before mechanical inoculation using a pestle. After three minutes, leaves were washed with water and symptoms were monitored for expression and lesion formation.
Molecular confirmation
RNA extraction was performed from infected cotton, cowpea local lesions, indicator hosts, pathogenic cotton and healthy leaf sample using the TRIzol reagent method, as described by Chomczynski and Sacchi,31 with slight modification. Specifically, RNA concentration and purity were measured using NanoDrop spectrophotometer and RNA integrity was confirmed by 0.8% agarose gel electrophoresis. Furthermore, we included both infected and healthy control during RNA extraction and cDNA synthesis to ensure the accuracy and reliability of our result. Take 2-3 µg of RNA was converted through the reverse transcription (RT) reagent, following the manufacturer protocol. The resulting cDNA was processed by PCR amplification with the gene encoding coat protein32 under the following conditions: initial denaturation at 95 °C for 2 min; 40 cycles of 94 °C for 30 sec, 59 °C for 45 sec and 72 °C for 1 min and a final extension at 72 °C for 5 min. The amplification of the results of PCR was verified using gel electrophoresis with 1% agarose. After that, the products of amplification were analyzed and submitted to gene bank. Multiple sequence alignment was executed with CLUSTAL OMEGA and a phylogenetic tree was generated with MEGA 11 using the neighbor-joining approach, which uses a 1000-bootstrap value. The similarities between the isolates were then compared.
RT-LAMP assay for TSV detection
Primer design
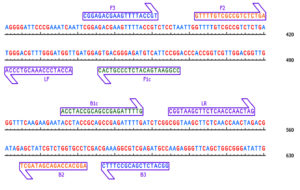
The design of LAMP primers was guided by the nucleotide sequence of the TSV coat protein detected in diverse host plants, including cotton, sunflower and groundnut. These sequences were retrieved from the NCBI GenBank and analyzed through multiple sequence alignment and similarity analysis was performed to identify conserved regions. The primers were designed with Primer Explorer V4 software and custom oligonucleotides were synthesized by Priority Life Science. Table 1 and Figure 1 provide detailed primer sequences and graphical representations of their positions on the target sequence. The assay mixture was fine-tuned and optimized as described in Table 2. The final reaction mixture was kept at 65 °C for 60 minutes, followed by heat inactivation at 80 °C for 2 mins. Because hydroxy naphthol blue dye interact with magnesium, sky blue colour which appears after incubation denotes a positive result. Results were further confirmed using 2% agarose gel, revealing a distinct ladder-like band pattern.
Table (1):
Primers and its information
| Primer Name | Primer Sequence 5′ —> 3′ | No. of Bases |
|---|---|---|
| RT-LAMP | ||
| TSVF3 | CGGAGACGAAGTTTTACCGT | 20 |
| TSVB3 | GGCATCTCGACGCCTTTC | 18 |
| TSVFIP | CCGGAATGACATCTCCCGTCACGTTT TGTCGCCGTCTCTGA | 41 |
| TSVBIP | ACCTACCGCAGCCGAGATTTTGAGGC ACCAGACGATAGCT | 40 |
| TSVLF | ACCATCCCAAACGTCCCA | 18 |
| TSVLB | CGGTAAGCTTCTCAACCAACTAG | 23 |
| RT-PCR with Coat protein specific primers | ||
| TSVCPF | AGATAAGTCGCTTCTCGGAC | 20 |
| TSVCPR | TGCTCGCATGGGTCATAGAC | 20 |
Table (2):
LAMP assay reaction mixture for TSV detection
Components |
Reaction Volume |
|---|---|
10x Thermopol buffer |
2.5 µl |
MgSO4 (8 mM) |
1.5 µl |
dNTPs (10 mM) |
3.5 µl |
TSV-F3 and TSV-B3 primers (0.2 µl) |
1 µl |
TSV-FIP and TSV-BIP primers (1.4 µl ) |
1 µl |
TSV-LF and TSV-LB primers (0.4 µl) |
1 µl |
Betaine 5M |
4 µl |
Bst DNA polymerase |
1 µl |
cDNA (50 ng/µl) |
1 µl |
Nuclease free water |
5.5 µl |
HNB Dye (120 µl) |
1 µl |
Total |
25 µl |
Figure 1. Pictorial representation and position of our synthesised primers located on the subjected sequence
Optimization of primer concentration and amplification precision
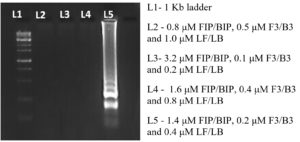
The effect of primer concentration on RT-LAMP accuracy was evaluated by testing varying concentrations for each primer pair. The tested conditions included (A) 0.8 µM FIP/BIP, 0.5 µM F3/B3 and 1.0 µM LF/LB (B) 3.2 µM FIP/BIP, 0.1 µM F3/B3 and 0.2 µM LF/LB (C) 1.6 µM FIP/BIP, 0.4 µM F3/B3 and 0.8 µM LF/LB (D) 1.4 µM FIP/BIP, 0.2 µM F3/B3 and 0.4 µM LF/LB. The specificity of the RT-LAMP assay was assessed by incorporating 50 ng of cDNA template derived from plants infected with TSV, Papaya ringspot virus (PRSV) and Groundnut bud necrosis virus (GBNV) into reaction mixture. As negative control, cDNA templates were obtained from healthy plant.
Validation and sensitivity analysis for TSV detection
For validate and confirm the results obtained from the RT-LAMP assay, conventional RT-PCR was performed with coat protein-encoding gene Rajamanickam18 and F3/B3 primer (Table 1) designed for the present study. To confirm the diagnosis accuracy, cDNA obtained from total RNA isolated from TSV-infected cotton leaves was diluted into nanogram, picogram and femtogram was used in the reaction mixture.
Collection and maintenance of TSV inoculum
Cotton leaves with dark purple necrotic lesions were collected from various fields in the Coimbatore district. Different kinds of symptoms such as small circular necrotic lesions, terminal necrosis, stem necrosis and drying of young buds were observed (Figure 2). Cowpea inoculated with virus sap showed circular necrotic local lesions on the leaves and stem necrosis by 5 days after inoculation (DAI) at 25 ± 2 °C, 60%-70% RH and a 16:8 h light:dark cycle, suitable for host growth and TSV propagation. Subsequently, veinal necrosis and systemic infection are also observed. In the present study, TSV inoculum was mechanically transferred into cowpea, Chenopodium and cotton to observe its efficiency in establishment. The inoculated plant exhibited an excellent host for TSV inoculum maintenance (Figure 3). Symptom severity varied, cowpea showed necrotic lesion, chenopodium had chlorotic spots and cotton exhibited brown necrotic lesion.
Figure 2. Collection of Tobacco streak virus-infected cotton sample
A. Chlorosis with small necrotic spot; B. Purple necrotic spot occurs irregularly; C. Marginal necrosis; D. Young bud drying
Figure 3. Sap inoculation for TSV maintenance and multiplication
(a). Cotton to cowpea (Local lesion); (b). Cowpea to cowpea (Circular necrotic lesion); (c). Cowpea to Chenopodium (Chlorotic spot); (d). Cowpea to cotton (Necrotic lesion)
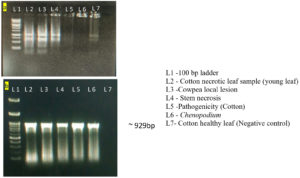
Genomic confirmation of TSV with RT PCR
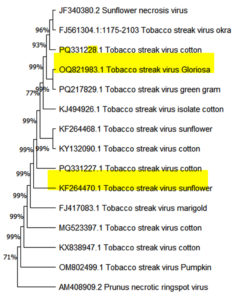
RNA extracted from TSV-infected cotton, cowpea local lesion, indicator hosts and pathogenicity proved cotton leaves was confirmed with specific coat protein. Each of the sample was processed successfully, having an amplicon size of nearly 929 bp (Figure 4). The PCR results were sequenced and submitted to GenBank, which provides the accession number PQ331227 and PQ331228. The sequence was analyzed and comparison with the sequences of established TSV isolates from the NCBI database. The nucleotide sequence of the present cotton sample exhibited a homology from 98.8% to 99.78% with Sunflower, Cotton, Green gram, Pumpkin, Marigold and Gloriosa (Figure 5).
Figure 4. Total RNA Quality check and RT-PCR amplification using coat protein gene
(a). Gel picture for raw RNA; (b). Gel image of RT-PCR products for the confirmation of TSV
(b)
Figure 5. Sequence alignment and Phylogenetic tree construction
(a). Multiple sequence alignment; (b). Phylogenetic tree
The phylogenetic analysis was performed with MEGA 11.0 using neighbour joining method. Tree branch support was determined using 1000 replications. Boot strap values are displayed at the nodes.
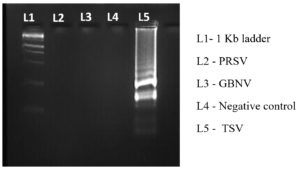
Optimization of primer concentration and diagnostic accuracy for TSV
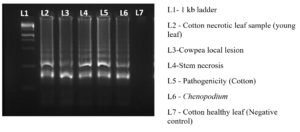
To optimize the reaction conditions, we determined the effect of different primer concentrations. The maximum amplification was achieved at 1.4 µM FIP/BIP, 0.2 µM F3/B3 and 0.4 µM LF/LB (Figure 6). The specificity of the designed primers was validated, confirming amplification of the target virus only, with no cross-reactivity to non-target (Figure 7). The reaction using cDNA from TSV-infected samples was incubated and amplified products displayed a characteristic ladder-like pattern, confirming the presence of TSV in the infected sample (Figure 8).
Validation and sensitive detection of TSV
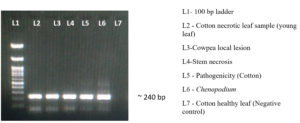
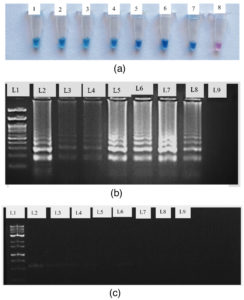
The presence of TSV in the infected sample was confirmed by the RT-LAMP, which efficiently amplified the target sequence under ideal reaction conditions and produced a distinctive ladder-like pattern on a 2% agarose gel. The resulting products were amplified at 929 bp and 240 bp with TSV CPF/CPR and F3/B3, respectively (Figure 9). The sensitivity of viral detection was assessed and comparative experiments indicate that RT-LAMP is capable of reliably detecting the virus even at low titre concentrations (Figure 10).
Figure 10. Compare the RT-LAMP sensitivity detection with RT-PCR
(a) Detection by using HNB dye (1 to 7 – Serially diluted cDNA template 8 – Negative Control); (b) 2% Agarose gel for RT – LAMP assay (L1 – 100 bp ladder; L2 – 50 ng/µl; L3 – 12 ng/µl; L4 – 8 ng/µl; L5 – 50 pg/µl; L6 – 5 pg/µl; L7 – 0.5 pg/µl; L8 – 0.05 pg/µl; L9 – negative control); (c). Conventional RT-PCR on 1% Agarose (L1 – 100 bp ladder; L2 – 50 ng/µl; L3 – 12 ng/µl; L4 – 8 ng/µl; L5 – 50 pg/ µl; L6 – 5 pg/µl; L7 – 0.5 pg/µl; L8 – 0.05 pg/µl; L9 – negative control)
The present study successfully established an efficient protocol for the collection, maintenance and genomic confirmation of TSV in cotton and assay host. Our finding revealed that cowpea, chenopodium and cotton serve as suitable host for sustaining the TSV inoculum which is in agreement with previous report.33,34 The ability of these plant to develop characteristic necrotic lesions upon mechanical inoculation reinforce their role as effective diagnostic hosts for TSV. Genomic confirmation through RT-PCR provided robust evidence of TSV infection in the tested samples. The amplification of a 929 bp fragment using coat protein-specific primer align with previous studies35,36 confirming the reliability of RT-PCR for TSV detection across different hosts. The high sequence homology (98.8%-99.78%) observed between our isolates and known TSV strain from sunflower, black gram and cucumber further substantiate the identity and genetic stability of the virus. To improve diagnostic efficiency, we optimized the concentration of the synthesized primers to achieve more precise and reliable amplification of 1.4 µM FIP/BIP, 0.2 µM F3/B3 and 0.4 µM LF/LB. The test specificity was validated by its exclusive amplification in TSV-infected sample with no cross-reactivity observed in GBNV, PRSV and healthy plant. This conclusion is similar to other research showing that LAMP-based investigations for plant viral infections have significant accuracy.16 Similarly, developed Neutral Red-LAMP assay which detected no cross-amplification between Feline immune-deficiency virus and other feline viruses tested, indicating the high specificity (98.44%) of the novel FIV-LAMP primer.37 Additionally, the colorimetric assay confirmed that magnesium ions interact with the hydroxy naphthol blue dye, resulting in a detectable color change characteristic of the sky blue. Compared to RT-PCR, RT-LAMP exhibited superior sensitivity at lower viral titre making it a more effective method for early and rapid virus detection. The following research results are convergent with respect to the report of Gwande, who also demonstrated RT-LAMP higher sensitivity for TSV detection.17 Additionally, the RT-LAMP assay has been reported to exhibit greater sensitivity than quantitative PCR (qPCR).38 Supporting this, another study demonstrated that the positivity rate was substantially higher when using nested PCR and RT-LAMP (31.48%) compared to conventional PCR, which showed a much lower detection rate of only 1.85%.39 The validation of RT-LAMP through comparative RT-PCR assay further underscores its reliability. The amplification of 929 bp and 240 bp fragment using TSV CPF/CPR and F3/B3 primer, respectively confirmed the presence of TSV. Given its ability to detect low virus concentration and provide visual confirmation without electrophoresis, RT-LAMP present a practical, field-deployable alternative to RT-PCR. Similarly, a colorimetric RT-LAMP assay was developed for the detection of Feline Coronavirus, demonstrating superior sensitivity and rapidity compared to conventional PCR method.40 Overall, our study highlighted the effectiveness of isothermal amplification as a rapid, sensitive detection technique for TSV detection. Its advantages over RT-PCR, including ease of use, no need of thermal cycler and real-time visualization make it an invaluable asset for disease surveillance and early management in cotton and other susceptible crop. Future research should focus on integrating RT-LAMP with portable detection platforms to facilitate on-site virus diagnostic and improve disease management in agricultural ecosystem.
RT-LAMP demonstrates transformative potential for the rapid and reliable detection of TSV in cotton, offering tenfold higher sensitivity than RT-PCR with results achievable within an hour using minimal equipment. This is especially critical in field settings, where TSV symptoms are often confused with nutrient deficiencies leading to frequent misdiagnosis and delayed management. By enabling visual colorimetric detection through HNB dye without the need for electrophoresis, RT-LAMP facilitates early and accurate identification even in resource-limited environments. The implementation of portable LAMP-based diagnostic kits possibly integrated with smartphone-assisted image analysis and GPS-tagged results can further enhance its usability for on-site detection. Such advancements will empower farmers and agricultural workers to act rapidly, thereby reducing reliance on laboratory-based diagnostics. In practical terms, early detection enabled by RT-LAMP can help prevent widespread outbreaks potentially minimizing TSV-related cotton yield losses, while offering significant cost savings by reducing unnecessary pesticide use. For broader impact, RT-LAMP could be embedded within existing agricultural extension services and diagnostic networks to ensure timely disease alerts and farmer training, particularly in vulnerable cotton-growing regions. Overall, RT-LAMP stands out as a practical, scalable and impactful tool that addresses both technical and logistical challenges in TSV management paving the way for more resilient and sustainable cotton production.
ACKNOWLEDGMENTS
The authors are thankful to the Department of Plant Pathology and The Dean, School of Post Graduate Studies, Tamil Nadu Agricultural University, Coimbatore, for technical support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Sarangdhar AA, Pawar VR. Machine learning regression technique for cotton leaf disease detection and controlling using IoT. International Conference of Electronics, Communication and Aerospace Technology (ICECA). 2017;2:449-454.
Crossref - Nahar S. Cotton Production in India: An Economic Analysis. JournalNX. 2016;2(5):47-50.
- Internationl Cotton Advisory Committee(ICAC). Data Portal. 2021. https://icac.org/DataPortal/ProductionDetails?country=WLD#Production (Accessed January 3, 2022).
- Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273.
Crossref - Padmanabhan C, Gao S, Li R, Zhang S, Fei Z, Kai-SHU L. Complete genome sequence of an emerging genotype of tobacco streak virus in the United States. Genome Announc. 2014;2(6):10-1128.
Crossref - Neeleman L, Linthorst HJ, Bol J F. Efficient translation of alfamovirus RNAs requires the binding of coat protein dimers to the 3′ termini of the viral RNAs. J Gen Virol. 2004 85(1):231-240.
Crossref - Jagtap GP, Jadhav TH, Utpal. Occurrence, distribution and survey of tobacco streak virus (TSV) of cotton. J Crop Sci. 2012;1(1):16-19.
- Sharman M, Persley JE. Distribution in Australia and seed transmission of Tobacco streak virus in Parthenium hysterophorus. Plant Dis. 2009;93(7):708-712.
Crossref - Rao RDVJ, Reddy AS, Chander Rao S, Varaprasad KS. Tobacco streak ilarvirus as causal agent of sunflower necrosis disease in India. J Oilseeds Res. 2000;17:400-401.
- Reddy AS, Rao RDVJP, Thirumala-Devi K, et al. Occurrence of tobacco streak virus on peanut (Arachis hypogaea L.) in India. Plant Dis. 2002;86(2):173-178.
Crossref - Rageshwari S, Renukadevi P, Malathi VG, Nakkeeran S. Occurrence, biological and serological assay of Tobacco streak virus infecting cotton in Tamil Nadu. J Mycol Pl Pathol. 2016;46(2):159-168.
- Vinodkumar S, Nakkeran S, Malathi Vg, et al. Tobacco streak virus: an emerging threat to cotton cultivation in India. Phytoparasitica. 2017;45(23):729.
Crossref - Valarmathi P, Amutha Mari. Field evaluation of germplasm lines of extra-long staple cotton (Gossypium barbadense) for Tobacco Streak Virus (TSV) resistance based on symptoms expression. 2024.
Crossref - RAMESH SUSARLA. Andhra Pradesh: New disease affecting BT cotton creates panic among farmers in Kurnool. The Hindu. Accessed date, September 09, 2022. https://www.thehindu.com/news/national/andhra-pradesh/andhra-pradesh-new-disease-affecting-bt-cotton-creates-panic-among-farmers-in-kurnool/article65866173.ece
- Almeida AMR, Sakai J, Hanada K, et al. Biological and molecular characterisation of an isolate of Tobacco streak virus obtained from soybean in Brazil. Fitopatol. Bras 2005;30(4):366-373.
Crossref - Kumar NA, Narasu ML, Zehr UB, Ravi KS. Molecular characterization of tobacco streak virus causing soybean necrosis in India. Indian J Biotechnol. 2008;7(2):214-217
- Gawande SP, Raghavendra KP, Monga D, Nagrale DT, Kranthi S. Rapid detection of tobacco steak virus (TSV) in cotton (Gossopyium hirustum) based on reverse transcription loop medicated isothermal amplification (RT-LAMP). J Virol Methods. 2019;270:21-25.
Crossref - Rajamanickam S, Renukadevi P, Devappa V, Nakkeeran S. Identification and characterization of Tobacco streak virus, as a new causative agent of necrosis disease of Anthurium. Indian J Biotechnol. 2021;20(2):194-200.
- Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):e63.
Crossref - Parida M, Sannarangaiah S, Dash PK, Rao PVL, Morita K. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol. 2008;18(6):407-421.
Crossref - Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3(5):877-882.
Crossref - Wang D, Yu J, Wang Y, et al. Development of a real-time loop-mediated isothermal amplification (LAMP) assay and visual LAMP assay for detection of African swine fever virus (ASFV). J Virol Methods. 2020;276:113775.
Crossref - Zhao LM, Li G, Gao Y, Zhu YR, Liu J, Zhu X. Reverse transcription loop-mediated isothermal amplification assay for detecting tomato chlorosis virus. J Virol Methods. 2015;213:93-97.
Crossref - Almasi MA, Manesh ME, Jafary H, Dehabadi SMH. Visual detection of Potato Leafroll virus by loop-mediated isothermal amplification of DNA with the GeneFinder™ dye. J Virol Methods. 2013;192(1-2):51-54.
Crossref - Fukuta S, Kato S, Yoshida K, et al. Detection of tomato yellow leaf curl virus by loop-mediated isothermal amplification reaction. J Virol Methods. 2003;112(1-2):35-40.
Crossref - Bhat AI, Siljo A, Deeshma KP. Rapid detection of Piper yellow mottle virus and Cucumber mosaic virus infecting black pepper (Piper nigrum) by loop-mediated isothermal amplification (LAMP). J Virol Methods. 2013;193(1):190-196.
Crossref - Li JY, Wei QW, Liu Y, et al. One-step reverse transcription loop-mediated isothermal amplification for the rapid detection of cucumber green mottle mosaic virus. J Virol Methods. 2013;193(2):583-588.
Crossref - Johnson AA, Dasgupta I, Gopal DVRS. Development of loop-mediated isothermal amplification and SYBR green real-time PCR methods for the detection of Citrus yellow mosaic badnavirus in citrus species. J Virol Methods. 2014;203:9-14.
Crossref - Warghane A, Misra P, Bhose S, et al. Development of a simple and rapid reverse transcription-loop mediated isothermal amplification (RT-LAMP) assay for sensitive detection of Citrus tristeza virus. J Virol Methods. 2017;250:6-10.
Crossref - Hull R. Mechanical inoculation of plant viruses. Curr Protoc Microbiol. 2009;13(1).
Crossref - Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156-159.
Crossref - Rajamanickam S, Karthikeyan G. Sequence diversity analysis of Tobacco streak virus infecting okra (Abelmoschus esculentus L.) in India. J Pure Appl Microbiol. 2014;8(6):4759-4767.
- Lavakumar P, Prasad Rao RD, Reddy AS, Madhavi KJ, Anitha K, Wallyar F. Emergence and spread of tobacco streak virus menace in India and control strategies. Indian J Plant Prot. 2008;36(1):1-8.
- Bhat AI, Jain RK, Ramiah M. Detection of Tobacco streak virus from sunflower and other crops by reverse transcription polymerase chain reaction. Indian Phytopathol. 2002;55(2):216-218.
- Ladhalakshmi D, Ramiah M, Ganapathy T, et al. First report of the natural occurrence of tobacco streak virus on blackgram (Vigna mungo). Plant Pathol. 2006;55(4):569.
Crossref - Rajamanickam S, Ganesamurthy K, Karthikeyan, G. Molecular characterization and genetic diversity of tobacco streak virus infecting soybean (Glycine max L). Afr J Microbiol Res. 2016;10(21):759-767.
Crossref - Saejung W, Khumtong K, Rapichai W, et al. Detection of Feline Immunodeficiency Virus by Neutral Red-based Loop-Mediated Isothermal Amplification Assay. Veterinary World. 2024;17(1):72-81.
Crossref - Khamsingok P, Rapichai W, Rattanasrisomporn A, Rungsuriyawiboon O, Choowongkomon K, Rattanasrisomporn J. Comparison of PCR, Nested PCR, and RT-LAMP for Rapid Detection of Feline Calicivirus Infection in Clinical Samples. Animals. 2024;14(16):2432. Crossref
- Khumtong K, Rapichai W, Saejung W, et al. Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification with Xylenol Orange Targeting Nucleocapsid Gene for Detection of Feline Coronavirus Infection. Viruses. 2025;17(3):418.
Crossref - Rapichai W, Saejung W, Khumtong K, et al. Development of colorimetric reverse transcription loop-mediated isothermal amplification assay for detecting feline coronavirus. Animals. 2022;12(16):2075.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.