ISSN: 0973-7510
E-ISSN: 2581-690X
The Chromobacterium violaceum is a gram-negative facultative anaerobic bacterium that is known to cause human infections in lungs, liver, brain, spleen lymph nodes and urinary tract. It has Acyl Homoserine Lactone (AHL) regulated virulence features like violacein pigment production, swarming motility, biofilm formation and haemolysis. Bacterial pathogens form biofilms in natural as well as medical implants due to a complex signalling – “Quorum Sensing” (QS). QS builds an interaction among the cells, which increases the proliferation and mechanisms necessary for invasion into the host. Instead of using only bactericidal agents for infection control, suppression of QS by Quorum Quenching agents (QQ) can overcome limitations of currently used antimicrobial substances. In the present study biogenic silver nanoparticles (BSNPs) synthesized from selected five plant extracts were screened against Chromobacterium violaceum MCC 4212 for QQ potential. Biofilm inhibition of 91.8% and dispersal of 81.33% was found to be exhibited by BSNPsmade from extracts of Garcinia and Trachyspermum. Swarming nature was inhibited by 66% while there was complete inhibition of haemolysis by BSNPs. Therefore, the BSNPs synthesized were found potential to control the pathogenicity of C. violaceum 4212 as an antibiofilm agent.
Biogenic Silver Nanoparticles, Chromobacterium Violaceum, Quorum Sensing Inhibition, Violacein, Antibiofilm
Bacterial pathogens thrive in versatile habitats due to their strong cell signaling mechanisms i.e., Quorum Sensing (QS) behavior. QS enables bacteria to coordinate their virulence factors to establish a successful infection in host. Although antibiotics were initially used as powerful antimicrobial agents, resurgence of antibiotic resistance forced researchers to explore novel antimicrobials for therapy. Currently antimicrobial strategies started focusing on QS mechanism to weaken the pathogenic trait and make the pathogen more susceptible for therapy and avoid the chance for drug resistance.1
Gram positive bacteria communicate by Acyl Homoserine Lactone (AHL)-mediated QS systems with the Lux homologues LasI/LasR while Gram negative bacteria use Autoinducer peptide (AIP) for signaling. However, in some organisms like Pseudomonas multiple QS systems are employed to control the pathogenic trait. Thorough understanding of these QS circuits and their mechanism helps in identification of targets for disruption of QS mechanism. Quorum Sensing Inhibitors (QSI) or Quorum quenching (QQ) agents are the chemicals which could disrupt these QS pathways at their sub-MIC values.2
The development of therapeutical strategies based on QQ mechanism is mainly driven by the need for alternative or complementary approaches. Many synthetic and natural QQ molecules have been investigated in past decade as novel antimicrobials or as antivirulents. Natural QSIs were observed to be abundant in many medicinal plants, vegetables and edible fruits and extracted from all types of plant tissues: roots and rhizomes, flowers, bark, leaves, stems, seeds and fruits. Extracts of edible fruits such as Ananas comosus, Musa paradisiaca, Manilkara zapota, Ocimum sanctumhave been shown to inactivate AHL molecules produced by Chromobacterium violaceumand P. aeruginosa.3 In order to design potential antibiofilm agent against C. violaceum 4212 pathogen with both QSI and antimicrobial activity, natural plant extracts were used. In present study, after screening around 20 edible plant products for QQ activity, five commonly used aqueous edible extracts were identified. Most potential among all were extracts of Garcinia cambogia fruit rind, Terminalia chebula seed, Trachyspermum ammi leaves, Zingiber officinale rhizome and Agaricus bisporous fruit. All the extracts have been identified for antimicrobial potential.
Chromobacterium violaceum is an opportunistic pathogen in which QS circuits control virulence traits like biofilm formation, production of cyanide, exoprotease and chitinase enzymes and violacein pigment.C. violaceum has been reported to cause infections in lungs, liver, spleen, brain, and lymph nodes.4 Further reports were seen in literature indicating its role in pneumonia, urinary tract infections, meningitis, and endocarditis and reported to be resistant to penicillin, ampicillin, and cephalosporins. As a combinatorial approach, antibiofilm agent against Chromobacterium violaceum MCC 4212 was developed with biogenic silver nanoparticles (BSNPs)with QQ potential.
Microbial Cultures
Chromobacterium violaceum 12472 is a standard bioindicator organism used globally for QSI activity screening was purchased from MCC Pune. Chromobacterium violaceum MCC 4212 was used as model pathogen to evaluate QQ activity was also obtained from NCL Pune. Bacterial strains were cultured in Nutrient broth at 32°C.
Preparation of aqueous medicinal extracts
Dried Garcinia cambogia (Malabar tamarind) fruits rind, Zingiber officinale (Ginger), Trachyspermum ammi (Ajwain), Terminalia chebula (Myrobalan) and Agaricus bisporous (Button mushroom) were sun dried to prepare the extract. 1g/ml concentration was prepared by dissolving the dried powder in sterile Milli Q Water at a temperature of 100°C for 15 minutes in a water bath. The cooled extract was filtered using Whatman No.1 filter paper and stored at 4°C in screw cap vials5.
Biogenic Silver Nanoparticles (BSNPs) synthesis
The BSNPs were synthesized by preparing a reaction mixture in 150 ml Erlenmeyer flasks using 100ml of 1mM AgNO3 (prepared in Milli Q Water) and 1 ml of the crude extract (Garcinia cambogia, Zingiber officinale, Trachyspermum ammi, Terminalia chebula and Agaricus bisporus). The aqueous solution was incubated at RT and monitored for synthesis of BSNPs by bioreduction which is indicated by colour change.6 The BSNPs were purified by centrifugation at 10,000 rpm for 15min at 4°C. They were suspended in MilliQ water and stored at 4°C.
QSI Bioindicator Assay
Bioindicator assay was performed by well diffusion assay on Nutrient Agar medium plates. The logarithmic phase culture of Chromobacterium violaceum 12472 (Bioindicator strain) at approximately 2.5x 106 CFU/ml was inoculated aseptically by spread plate method. The plates were incubated for 10 min at Room Temperature (RT) for adsorption of the inoculum onto the surface of the agar medium. Agar wells were made using cylindrical tubes of 1.5mm diameter. The wells were dispensed with 50µl medicinal extract of concentrations 100µg/ml, 200 µg/ml, 300 µg/ml, 400 µg/ml and 500 µg/ml. The Nutrient Agar plates were incubated at 32°C for 48hrs in upright position for halo zones of depigmentation.7
Characterization of BSNPs
UV- Visible characterization of biogenic silver nanoparticles (BSNPs) was carried out by scanning in the wavelength range between 350 nm to 450 nm for characterization based on the specific absorption peak in the aqueous solution. Zeta potential and Nanoparticle size analysis of the biofabricated silver nanoparticles were characterized in Nanoparticle Size Analyzer. (Model No.202008181725004.nsz). FTIR analysis was carried out by recording Spectrum in the range of 4000-500cm-1 using a SHIMADZU Fourier Transform Infrared Spectrophotometer. The scanning data was obtained after 10 scans.
MIC and MBC determination
The MIC of individual BSNPs were determined by Microtiter Plate based assay. Overnight grown Chromobacterium violaceum 12472 culture was diluted in sterile nutrient broth to obtain cell density equivalent to 0.5 McFarland corresponding to 5×108 CFU/ml and used as inoculum. Biogenic silver nanoparticles (BSNPs) with 1.5 µg/ml, 3µg/ml, 6µg/ml, 12 µg/ml and 24 µg/ml concentrations were subjected for MIC testing. To 50 µl of broth, aliquots of BSNPs with the concentrations mentioned above were added from the stock solution making up the total volume of 100 µl. 10µl of culture was added finally to all wells in the microtiter plate in triplicates. After 24 hrs. of incubation, resazurin was added to determine the viability of the cells. Viability was indicated by the color change of resazurin from blue to pink coloured resorufin (7 -Hydroxy -3H- phenoxazin -3-one)and helped in determining MIC values. The MIC concentration and the next higher concentration containing test solutions were plated on the solid media plates to detect the bacteriostatic as well as bactericidal concentrations based on the presence and absence of bacterial colonies.8
Quantification of violacein production
Quantification of violacein pigment was carried out by Choo et.al method.9 For this purpose, triplicates of 250ml Conical flasks with 20ml of sterile nutrient broth in each with 1mg/ml, 2mg/ml, 4mg/ml, 6mg/ml, 8mg/ml, 10mg/ml of extracts respectively per flak were taken. These flasks were inoculated with 50 µl overnight grown. C.violaceum 4212 culture and incubated at 32°C for 48hrs on a shaker at 100 rpm. Violacein was extracted in 2ml of DMSO from cell pellets after centrifugation of 2ml culture medium at 15000g for 10min. Cell bound violacein pigment released into DMSO was collected after centrifugation at 15000g for 10 min and absorbance was measured at 585 nm using DMSO as blank. Percentage inhibition of violacein is calculated by the formula:
Inhibition = [A585 nm (control)- A585 nm (test)/ A585nm (control)]x100
Biofilm Inhibition Assay
Biofilm inhibition assay was carried out in flat bottomed 96-wells microtiter plates. To evaluate the antibiofilm activity of plant extracts and BSNPs were added to the overnight grown C. violacieum culture 4212 suspension in microtiter plates at sub inhibitory concentrations and incubated at 32°C for 48 hours in triplicates for biofilm formation. Microtiter plates were decanted and stained with 50 μl of 0.1% crystal violet for 5 min after 48 hrs. of incubation. Microtiter plates were rinsed twice with 200 μl of sterile water, and destained with 200 μl of 70% acetic acid. Biofilm inhibition was quantified by measuring optical density of the suspension at 630 nm using a microplate reader.10 The biofilm inhibition was calculated by the formula-
Percentage biofilm inhibition= 100- [(A630nm of test / A630nm control) x 100]
Biofilm dispersal Assay
C. violaceum 4212 culture was grown in nutrient brothin 96 well microtiter plates in triplicates for 48 hrs. at 32°C for biofilm formation. After the incubation period, BSNPs at sub inhibitory concentrations were added in the microtiter plates for evaluation of biofilm dispersal activity by incubating the plates further for 24 hrs. Microtiter plates were decanted and stained with 50 μl of 0.1% crystal violet for 5 min after 48 hrs. of incubation. Microtiter plates were rinsed twice with 200 μl of sterile water, and destained with 200 μl of 70% acetic acid. Biofilm dispersal was quantified by measuring optical density of the suspension at 630 nm using a microplate reader10. The biofilm dispersal was calculated by the formula-
Percentage biofilm dispersal = 100- [(A630nm of test / A630nm control) x 100]
Swarming Assay
Swarming motility is mediated by flagella across the semi solid medium. Sterilized Swarming agar media with different sub inhibitory concentrations of either extracts or BSNPs were poured into sterile petri dishes and allowed to absorb for 3 to 4 hrs. at 37°C. Then 5μl of culture was placed in the centre of the plate and incubated at 32°C. After incubation the diameter of the swarming was measured.11
Haemolytic Assay
The C. violaceum 4212 was treated with subinhibitory concentration of BSNPs and inoculated into a blood agar medium enriched with 5% sheep erythrocytes. The triplicate set plates were incubated at 32°C for 48hrs along with appropriate controls. After incubation the plates were observed for zones of complete haemolysis and screened for β haemolysis.
AHL Extraction
Overnight grown culture samples after treatment with BSNPs were centrifuged at 15,000g for 10min at 4°C to obtain cell free supernatants. These supernatants were filtered through a 0.2µm filter. AHL extraction was done by addition of equal volumes of acidified ethyl acetate and followed by constant shaking for 5 min. The extracts were concentrated in a Rotavapor at 40°C and dried. It was resuspended in ethyl acetate for further analysis.12
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
Culture supernatants of BSNPs treated and untreated or control were analysed after extracting AHL by a Shimadzu GCMSQP-2010 plus detector gas chromatograph. It was equipped with a RTx-5MS (30.0 m × 0.32 mm ID., 0.5 μm thickness) cross bond diphenyl dimethyl polysiloxane-fused silica capillary column. Helium, ≥99.99%, was used as a carrier gas with a constant flow rate of 0.8 mL/min. 1μL of the sample was injected (split ratio of 100:1) into GC− MS using an AOC5000 auto-injector.13
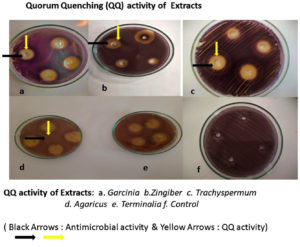
Screening for QQ activity
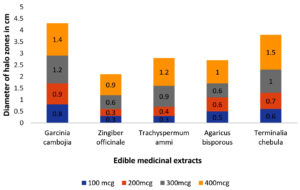
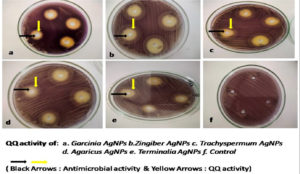
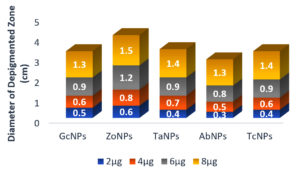
Initially, several plant extracts were screened for QQ activity in the lab. Concentrations ranging from 100µg/ml to 400µg/ml of each medicinal were loaded into agar wells on Nutrient agar plates seeded with bioindicator strain and incubated for overnight at 32°C. The halo zones due to loss of pigment were measured and graphically represented. (Figure 1 and Figure 2). At 100µg/ml of extract concentration, Terminalia chebula and Garcinia cambogia showed maximum activity with halo zone of 1.5cm and 1.4cm respectively. Zones for Trachyspermum ammi and Agaricus bisporous were recorded as 1.2cm. The least activity was found with Zingiber officinale as 0.9cm. For all the extracts with increasing concentrations there was increase in the zone’s diameter. Among all the extracts, Garcinia at low concentrations (100µg) showed 0.8 halo zone as QQ activity. The order for QQ activity of extracts was observed as Terminalia chebula>Garcinia cambogia>Trachyspermum ammi >Agaricus bisporous > Zingiber officinale.
Synthesis of Biogenic Silver Nanoparticles (BSNPs)
The synthesis of silver nanoparticle is achieved by reduction of Ag+ ions in the 1mM AgNO3 due to the biologically active compounds present in the aqueous medicinal extracts. Formation of the nano entities of the silver has been indicated by gradual development of dark brown to black suspension visualized that was originally colourless. GcNPs is indicated by black colour, TcNPs- Orange brown, TaNPs- Dark brown, AbNPs and ZoNPs in light brown colour as shown in (Figure 3). All the extracts used in the present study could lead to transformation of silver to nano silver in time ranging from 1 to5 Hrs. The time taken for synthesis of BSNPs mentioned in (Table 1).
Table (1):
Time of synthesis for BSNPs.
S. No |
Biofabricated AgNPs |
Time of Synthesis (Hrs.) |
|---|---|---|
1 |
GcNPs |
2 |
2 |
TcNPs |
4 |
3 |
TaNPs |
4 |
4 |
AbNPs |
3 |
5 |
ZoNPs |
1 |
Characterization of BSNPs
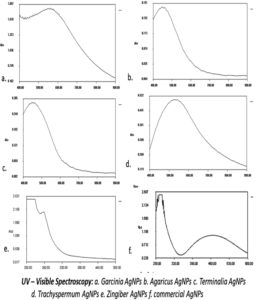
Spectral analysis of the biogenic nanoparticles suspended in aqueous suspension has been shown in the Figure 4. When scanned in a range from 400 to 900nm, the highest absorption for Garcinia cambogia (582nm) and least with Agaricus bisporous (497nm)was observed. The absorption maxima of BSNPs are shown in the (Table 2). The absorption peaks in the range of 400 -600nm confirmed the presence of silver nanoparticles.
Table (2):
Physical properties of BSNPs.
Fabricated Extract |
Nanoparticle abbreviation |
Absorption Maxima |
Zeta Potential |
Electrophoretic Mobility |
|---|---|---|---|---|
Garcinia cambogia |
GcNPs |
582nm |
-24.4mV |
-0.000189cm2/Vs |
Zingiber officinale |
ZoNPs |
562nm |
-28.6mV |
-0.000221 cm2/Vs |
Trachyspermum ammi |
TaNPs |
528nm |
-31.2mV |
-0.000241 cm2/Vs |
Terminalia chebula |
TcNPs |
495nm |
-32.9mV |
-0.000255 cm2/Vs |
Agaricus bisporous |
AbNPs |
497nm |
-30.1mV |
-0.000233 cm2/Vs |
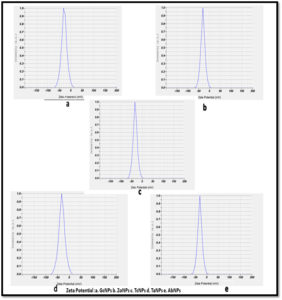
Zeta potential and electrophoretic mobility of the nanoparticles has been determined by Nanoparticle analyser and it was found that all the biogenically synthesized silver nanoparticles show negative surface charges. Table 2 and Figure 5. The values obtained were ranging from -24.4 to -32.9mV. ZP values lower than -28mV indicates agglomeration of nanoparticles. ZP values lower than -30mV are considerably much stable in suspension.
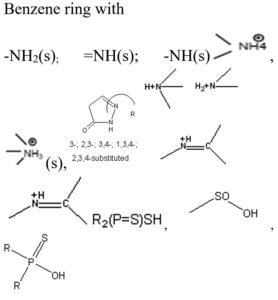
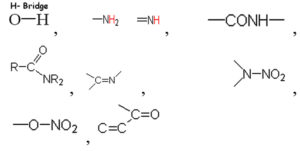
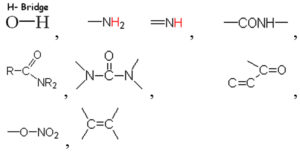
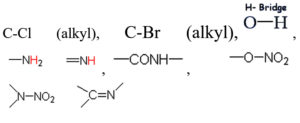
Based on the vibrational frequencies of the chemical bonds present around the nanoparticles, different functional groups could be predicted by Fourier Transform infra-red Spectroscopy (FTIR) analysis. This analysis aims to predict the functional groups of phytochemicals which were involved the formation of AgNPs. Using online spectroscopic tools [http://www.science-and-fun.de/tools/] the functional groups interacting on the surface charges of nanoparticles are listed in (Table 3). Among the five biogenic nanoparticles, GcNPs IR spectrum revealed interactions of unique functional groups like phosphinic acids (medium interactions), sulphonic acids (weak interactions) at 2359.02cm-1 and 2343.59cm-1. Overall, from the results obtained surface interactions of biogenic nanoparticles with primary amines, thiol groups, alkenes, unsaturated ketones, alcohol groups were observed as prominent peaks.
Table (3):
Functional groups identified as capping agents on BSNPs by FTIR analysis.
Nanoparticle |
Wavelengths |
Interacting functional group |
|---|---|---|
GcNPs |
3452.70cm-1,2359cm-1, 2343.59cm-1, 2200- 3000cm-1(m; O-H & N-H Stretching Vibration), 1450-1670cm-1, 2200-2360cm-1 |
|
ZoNPs |
3444.8cm-1,3437.26cm-1, 1629.90cm-1 |
-CONH-(m), -C=C-C=O(s), -N-NO2(s), -NH2 (m), |
TaNPs |
3600-3200cm-1,3500-3300cm-1, 3470- 3400cm-1,1637cm-1, 1640-1620cm-1, 1285-1270cm-1, 1630-1550cm-1, 1300-1250cm-1 |
|
TcNPs |
3452.70cm –1, 1650-1560cm-1 |
|
AbNPs |
646.17cm-1,3450.77cm-1, 3439cm-1, 1629.90cm-1 |
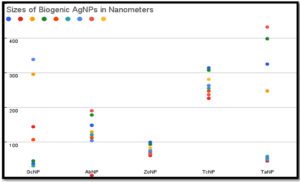
Size determination by Nanoparticle analyzer has shown synthesis of variable size silver nanoparticles.Size range for GcNPs 31.6nm- 338.6nm; ZoNPs 61.6nm – 99.7nm; TaNPs 45.6nm- 431.8nm, TcNPs 226.4nm- 314.3nm were detected (Table 4). Colour of the AgNPs synthesized had light brown to dark brown colour which is due to agglomeration. During the process of synthesis colour variation from light yellow, orange to darker shades was observed, indicating formation of smaller sized nanoparticles initially. In course of time nanoparticles agglomerated and resulted in particles greater than 100nm. Highest size range was featured in the TaNPs and GcNPs. Least sized nanoparticle (4.3nm) was observed in AbNPs. Very less variations in sizes of ZoNPs and TcNPs could be identified 31.6nm, 45.7nm and 37.6nm were the smaller sized particles as shown in the (Figure 6).
Table (4):
Size of BSNPs.
QSI extract used for biogenic |
BSNPs |
Size of Nanoparticles analyzed in Nanoparticle Analyzer |
|---|---|---|
Garcinia cambogia |
GcNPs |
31.6nm,144.3nm, 295.8nm, 45.7nm, 107.1nm, 144.3nm, 37.6nm, 205.2nm, 338.6nm |
Agaricus bisporous |
AbNPs |
4.3nm, 129.3nm, 178.3nm,112.4nm, 120.9nm, 104.2nm, 148.6nm, 190.5nm |
Zingiber officinale |
ZoNPs |
61.6nm, 75.7nm, 93.6nm, 69.0nm, 72.4nm, 75.7nm, 65.8nm, 82.9nm, 99.7nm |
Terminalia chebula |
TcNPs |
226.4nm, 263.4nm, 307.3nm, 247.5nm, 255.3nm, 263.4nm, 236.8nm, 281.0nm, 314.3nm |
Trachyspermum ammi |
TaNPs |
45.6nm, 247.6nm, 398.2nm, 53.7nm, 58.7nm, 49.6nm, 325.1nm, 431.8nm |
QQ assay
Plate based assay was performed, in order to determine the ability of BSNPs as QQ agents. Halo zones around the agar wells were observed for all the BSNPs which indicated their QQ potential. The diameters of the zones were measured and compared as shown in the (Figure 7) and was found to have zones > 1.0cm at highest concentration, for all the BSNPs. Highest diameter of halo zones was observed for ZoNPs, followed by TaNPs, TcNPs, GcNPs and AbNPs. From this assay it was also evident that greater activity was achieved for BSNPs when compared to extracts from which the BSNPs were synthesized. (Figure 8).
Determination of MIC for BSNPs
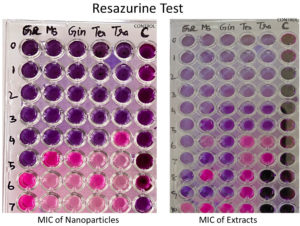
In order to formulate potent antibiofilm agent, the efficacy of BSNPs against the pathogenic C. violaceum 4212 was determined as Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentration (MBC) by resazurin assay. A comparative analysis of MIC & MBC of medicinal extracts and the BSNPs was carried out in order to determine the difference in their activity potential. Minimum Inhibitory Concentration (MIC) is the lowest concentration of the extracts and BSNPs which showed inhibition of growth. The resazurin assay has been performed in a 96 well microtiter plate as shown in Figure 9. The resazurin dye acts as an indicator of cell growth in the study. The live cells have oxidoreductases that reduces resazurin salt to resorufin which is indicated by the color change from blue to pink/colorless. The biocidal activity of extracts and BSNPs were analyzed by plating a sample of inoculum that showed absence of growth on Nutrient Agar. The concentrations that inhibited the growth on subculturing was determined as MBC.
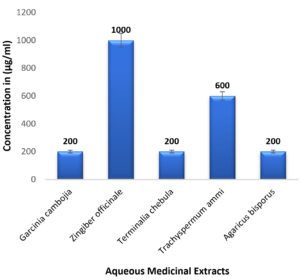
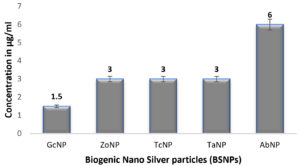
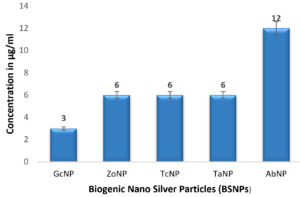
In case of medicinal extracts, concentrations ranging from 200µg/ml – 1200 µg/ml was observed as the MIC & MBC concentrations as shown in Figure 10 & Figure 11. Interestingly there was a manifold decrease,1.5 µg/ml to 12 µg/ml in the concentrations when BSNPs were employed. As per the MIC concentrations, GcNPs showed inhibition at lowest concentrations (1.5µg/ml) there by indicating as the most potent antimicrobial agent. ZoNPs, TcNPs and TaNPs showed inhibition at MIC of 3µg/ml with medium effect and least by AbNPs at MIC 6µg/ml. The MIC and MBC of BSNPs are shown in the Figure 12 and Figure 13.
The MIC and MBC of the biofilm forming bacteria can be considered as the – Minimum Biofilm Inhibitory Concentration (MBIC) and Minimum Biofilm Eradication Concentration (MBEC).
Quantitative assay of violacein pigment inhibition
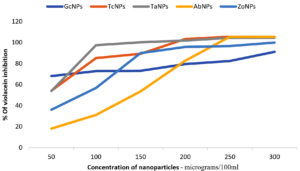
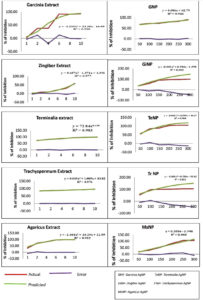
The QQ activity of BSNPs was quantified by measuring the decrease in violacein production. Violacein pigment is a water-soluble compound, whose percentage in reduction upon treatment is quantifiable by colorimetric assay. Flask-based assay was performed to evaluate the impact of concentrations of BSNPs ranging 50µg – 300µg/100ml on C. violaceum MCC 4212 pathogen. A comparative analysis of the QSI effect of medicinal extract and its corresponding BSNPs was performed in order to establish the efficacy. The percentage of reduction in pigment was calculated after extracting the cell bound violacein in DMSO. Results from (Figure14) indicated a decrease in violacein production with increased concentrations of biogenic silver nanoparticles. At a concentration of 50µg/100ml maximum percentage reduction was observed for GcNPs with 68.06% while other nanoparticles had lesser inhibition. At a concentration of 100 µg/100ml TaNPs showed 97.44% which was maximum and at 150 µg/100ml all the biogenic nanoparticles have achieved 100% inhibition as shown in (Figure 15). Experimental data reveals that ZoNPs even at higher concentrations show a significant inhibition of violacein pigment. ZoNPs were more effective when compared to ginger extract.
A statistical analysis of Linear Regression Model was performed to generate difference with predicted values and evaluate the significant error. From the R2 value obtained a comparison was done to determine the efficacy of BSNPs with corresponding medicinal extract that were used for fabrication. From the graphs small differences were identified between the observed and predicted values. This indicated that the predicted values were neither too high nor low from the experimental data. The R2 values obtained showed a strong relationship since there was a perfect fit between observed and the fitted values as shown in (Figure 16). R2 values depicted in the (Table 5) shows there is a perfect fit within the data. In comparative analysis ZoNPs, GcNPs, TcNPs and TaNPs were found to be more effective than the extracts. However, in case of AbNPs, the corresponding extract showed enhanced QQ activity.
Table (5):
Comparison of Linear regression coefficient (R2) values of Extract and BSNPs.
| Edible Medicinal Extracts | BSNPs | ||
|---|---|---|---|
| Zingiber officinale | R2 = 0.977 | ZoNPs | R2 = 0.969 |
| Garcinia cambogia | R2 = 0.906 | GcNPs | R2 = 0.944 |
| Trachyspermum ammi | R2 = 0.976 | TaNPs | R2 = 0.852 |
| Terminalia chebula | R2 = 0.983 | TcNPs | R2 = 0.960 |
| Agaricus bisporous | R2 = 0.912 | AbNPs | R2 = 0.960 |
As part of the analysis, it was also noted that TaNPs and TcNPs at concentrations 100 µg/100ml showed highest inhibition, 96% and 85% respectively which was observed to be consistently increasing at higher concentrations. Since this was a QS (AHL) regulated phenomena, detection of AHL molecules was performed in the treated samples.
Detection of AHL- signals
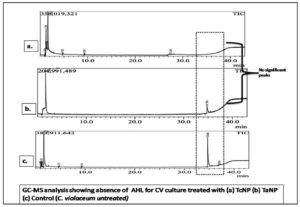
In Chromobacterium violaceum the production of violacein pigment is promoted by the positive regulation of the N– Acyl homoserine lactone CviR QS system. The purple colour violacein pigment is in response to signal molecule N– Hexanoyl homoserine lactone (C6HSL). In the present study, since the significant violacein inhibition was noted, it was relevant to screen the impact on C6HSL molecules after treatment with BSNPs i.e., TcNPs and TaNPs.
GC-MS analysis of cell free supernatant after AHL extraction showed no significant peaks when compared to the control (Figure 17). Number of multiple peaks in the data were observed which could be degraded AHL molecules. These results substantiated the hypothesis that BSNPs TcNPs and TaNPs have QQ potential. Hence further experiments were designed to evaluate their impact on virulence factors.
Antibiofilm activity
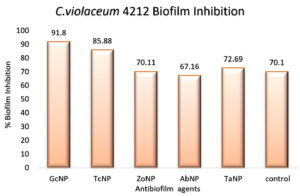
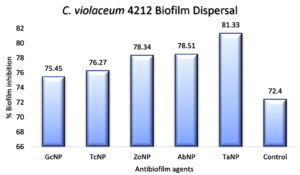
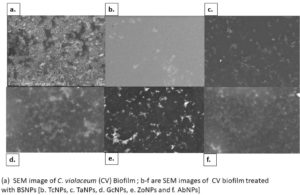
An in vitro biofilm assay was carried out in microtiter plate for all BSNPs. Specific MBC (Minimum Bactericidal Concentrations) was employed in order to evaluate the antibiofilm efficacy. This assay was performed by determining the biofilm inhibition and biofilm dispersal activity. BSNPs could inhibit the establishment of Chromobacterium violaceum MCC 4212 biofilm when added along with the inoculum. This biofilm inhibition was evaluated as 91.80% for GcNPs which was maximum among all the five BSNPs, as shown in (Figure 18). The TcNPs and TaNPs showed 85.55% and 72.69 % respectively while the least activity has been recorded for AbNPs (67.16%). Biofilm dispersal was studied by adding BSNPs to preformed biofilm in microtiter plates. Figure 19 shows the dispersal activity of BSNPs. Maximum biofilm dispersal activity was shown by TaNPs while AbNPs, ZoNPs and TcNPs it was 78.51%, 78.34%, 76.27% respectively. Comparison of biofilm inhibition and dispersal activity indicates more inhibition effect for GcNPs & TcNPs while the rest of the BSNPs show enhanced dispersal. The evidence of biofilm eradication was also observed in Scanning Electron Microscope (SEM) images, (Figure 20) which were taken after treatment with BSNPs. Observations clearly indicated absence of the biofilm matrix and reduction in the cell density when compared to the control.
Effect on Swarming Motility
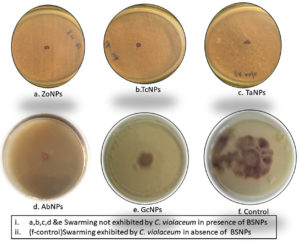
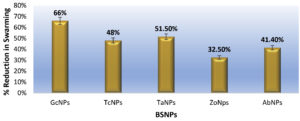
Effect on the swarming motility was evaluated, since it has been established that the inhibition of AHL signals, occurs very significantly after treatment with BSNPs. Swarming motility is a QS dependent virulence factor in Chromobacterium violaceum which is relevant in the pathogenesis and spreading of biofilm. It was observed that C. violaceum MCC4212 showed inhibition of swarming motility in the Swarming Agar assay at a sub-MIC concentration (1µg/ml). Absence spreading nature of the C. violaceum MCC4212 as seen in Figure 21 is more evident when compared to the control plates. The reduction in swarming was observed as GcNPs- 66%, TcNPs- 48%, TaNPs- 51.50%, ZoNPs-32.50% and AbNPs- 41.40%. The decrease in swarming nature has been depicted in the Figure 22.
Haemolytic Assay
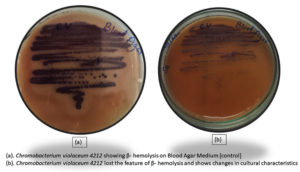
Haemolytic activity is a virulence trait, which was assessed by growing C. violaceum 4212 on 5% sheep blood agar plates. The bacteria that show hemolysis are identified by clear zones of hemolysis around the colonies due to production of hemolysins. The different types of hemolysis are alpha-hemolysis (partial hemolysis indicated by greenish discoloration), beta-hemolysis (complete hemolysis showing clear zones) and gamma-hemolysis (absence of hemolysis). Chromobacterium violaceum 4212 shows beta- hemolysis with clear zones of clearance.
In order to evaluate the effect of BSNPs on this pathogenic trait, actively growing culture of C. violaceum was incubated with sub inhibitory concentration of BSNPs. The treated cultures along with controls (untreated) when inoculated in the blood agar medium, incubated for 48hrs at 320C, was observed to show absence of hemolysis. This indicates inhibition of hemolytic activity by BSNPsas shown in the Figure 23.
Edible plant extracts of medicinal significance contain diverse compounds with multiple modes of action which enable them to overcome the development of resistance. Present study aims to explore novel anti-biofilm agents with medicinal plant extracts depicting quorum quenching (QQ) activity14-17 for which several plant extracts were screened, and five potentially active QQ agents were selected.18
Aqueous extracts of dried fruit rind of Garcinia cambogia (Malabar Tamarind), rhizome of Zingiber officinale (Ginger), dried nut like fruits of Terminalia chebula (Myrobalan), seeds of Trachyspermum ammi (Ajwain)and dried fruiting body of Agaricus bisporus (Button Mushrooms)were identified with potential QQ activity in the plate-based screening wherein they depicted halo zones greater than 0.9cm. In the present study, the potential of Garcinia aqueous extract with QQ activity was observed which has not been reported earlier.
The QQ activity of all five aqueous extracts were proportional to the concentration tested which was observed as halo zones or loss of violacein pigment production. Terminalia chebula aqueous extract showed the maximum halo zones of 1.5cm while Zingiber officinale had the least i.e., 0.9cm. The variation in the QQ activity among the extracts is due to the diversity of the aqueous soluble bioactive compounds. The use of aqueous extract has been revealed to be potent and hence it was further explored for synthesis of biogenic silver nanoparticles (BSNPs).
Among the various approaches to eradicate pathogenic biofilm, many studies have combined the antibiotics with QQ agents to enhance their bactericidal effect. Study reported by Brackman G et al., stated the enhanced activity of tobramycin when combined with baicalin hydrate which was a potential QSI agent.19,20 Hymes SR et al., have reported the control of Gardnerella vaginalis biofilm effectively when DNase was combined with metronidazole antibiotic. A similar study on clindamycin and metronidazole when combined with subtilisin or lauramide arginine ethyl ester showed effective antibacterial activity.21 Hence in order to enhance the antimicrobial spectrum and synergistic activity with multiple modes of action, combinatorial approach plays an important role. In order to improve the efficacy and reduce the toxicity of AgNPs, a study was undertaken to synthesize biogenic nanoparticles. Bio fabrication with QQ agents as surface capping agents on silver nanoparticles would be more compatible with biological systems.
The bioreduction of silver to form silver nanoparticles has been mediated by adding the selected medicinal extracts. The synthesis was initiated by addition of 1% of aqueous extract into 1mM filter sterilised silver nitrate solution at room temperature. The formation of silver nanoparticles (AgNPs)for each extract had a characteristic colour, like silver nanoparticles synthesised by Garcinia cambogia (GcNPs)- black, Terminalia chebula (TcNPs)- Orange brown, Trachyspermum ammi (TaNPs)- Dark brown, Zingiber officinale (ZoNPs) and Agaricus bisporus (AbNPs) -light brown. The colour transformation into light yellow to brown, in their nano forms is because of the interaction of phytochemical components. The fabrication of biologically active plant derived compounds form a coating on the surface making them more stable in the biological environment.
Silver nanoparticles synthesized show colour variations based on their size and rate of agglomeration. Greater cohesive forces were observed to increase in surface tension which causes the clustering of AgNPs in the colloidal suspension resulting in dark colours as reported by Badi’Ah HI et al.22 In the present study, biogenic silver nano synthesis involves use of crude extract, which has resulted in a mixture of AgNPs, with variable proportions and properties. Study of the physicochemical parameters of biogenic silver nanoparticles (BSNPs) were carried out to specifically to understand their mode of action when used as an antibiofilm agent.
Formation of AgNPs was indicated by colour development which was due to the reductive ability of edible medicinal plant extract. Further characterization was carried out by observing their UV- Visible spectral properties, which is based on the surface plasmon resonance of the particles. The suspension of AgNPs were scanned through a wavelength range of 400 nm- 900nm, and the maximum absorption of each were recorded as, GcNPs-582 nm, ZoNPs- 562 nm, TaNPs- 528 nm, TcNPs – 495 nm, AbNPs- 497 nm. Broader peaks of absorption range between 400nm – 600nm for all BSNPs, indicating the presence of silver nanoparticles >100 nm.
Nanoparticle analyser was used to measure zeta potential, electrophoretic mobility and size of nanoparticles synthesized in the aqueous nano colloidal suspension. All the BSNPs synthesized were observed to have negative values ( -24 to -32.9), which shows greater stability in aqueous solutions. Similar observations were stated by Patil MP et al. and Raja S et al. in their studies using silver nanoparticles23.
Erdogan O et al. have reported that the zeta potential between -30mV and+30mV has greater stability in dispersion medium. This property is of significance if the AgNPs are to be applied in biological systems, because the conditions in them are aqueous. Since the BSNPs synthesized from edible medicinal extracts also had zeta potential as stated by Erdogan O et al. they could be explored as antimicrobial and antibiofilm agents.24
To understand the interaction of various functional groups involved in the formation of nano silver after addition of medicinal extracts, FTIR characterization was carried out. The presence of a specific functional group was observed in the form of peaks indicating different stretches of bonds. As reported by Jyoti K et al. presence of O-H bonds indicates the alcohols while the same when conjugated with benzene rings indicates phenolic compounds in the bioreduction process. Some of the functional groups identified in study were C-C stretching as non-conjugated; C-N, -N and N-H for presence of proteins; -S(Sulphur) containing thiol groups; -NO2 as nitro groups; C-Br as alkyl groups. These results indicate the presence of biological active components as surface capping agents.25
BSNPs synthesized were analysed for size range in Nanoparticle analyser as depicted in Table 2-As mentioned in the studies of Mohanta YK et al, nanoparticles of size less than 100 nm are known to have a bactericidal effect due enhanced penetration into the bacterial cells.26 These different sized nanoparticle formulations have greater potential for penetration into the biofilm matrix that have varied sized pores. The biofilm matrix is a complex polymeric aggregation of nucleic acids, proteins, polysaccharides, lipids and lipoproteins that is often referred to as “matrixome” (Karygianni L et al.) that can be differentially targeted by a mixture of multiple sizes of BSNPs.27 Biogenically synthesized nanoparticles have proven to be more active due to their surface-active compounds. Electrostatic, van der waals, hydrophobic, hydrogen bonding, 𝝅-𝝅 interactions and electrostatic repulsions are observed to be the major interactions between the nanoparticles and the biomolecules making up the biofilm.
In order to evaluate the quorum quenching ability of BSNPs, Chromobacterium violaceum 4212 was selected as a model organism. This is a soil isolate which is a saprophyte and also an opportunistic pathogen in humans.28 A better understanding of AHL based regulating factors of biofilm formation in C. violaceum can be substantial evidence for the role of BSNPs as QQ agents.
During the preliminary studies the MIC and MBC concentrations of BSNPs were determined in microtiter plates using resazurin assay. These results reveal that MIC & MBC concentrations for BSNPs were found to be in 1.5 µg-12µg. These results prove the efficacy of BSNPs as antimicrobial agents. Based on the MIC values, GcNPs with 1.5µg was found to be more potent than others.
A plate-based assay was performed to evaluate the ability of the quorum quenching nature based on the presence of halo zones of lack of pigmentation.29 The detection of AHL production can be directly correlated to the production of violacein pigment production as well as other virulence factors regulated by these molecules. All the BSNPs showed significant quorum quenching activity in the current study with a zone greater than 1.0cm at concentrations of 10µg/ml. Therefore, all the five types of BSNPs have been further evaluated for quantitative evaluation of violacein pigment inhibition to determine potential agents as anti-biofilm components.
Studies have reported that CviR interacts with QQ agents and shows QS inhibition in a dose dependent manner. Even in the current analysis of violacein quantification using C. violaceum 4212, it was found that BSNPs at very low concentrations (0.05 µg/ml-0.30µg/ml) could significantly show impact on violacein production. 97.44% of violacein inhibition was recorded with TaNPs at 0.1µg/ml and 100% inhibition for all BSNPs with concentrations from 0.15µg- 30 µg/ml.
A statistical linear regression analysis was performed to understand the significance of the data. The regression coefficient (R2)value closer to 0.9 indicates significant impact on the violacein inhibition. At a sub inhibitory concentration of 0.1µg/ml, 96% inhibition for TaNPs and 85% inhibition for TcNPs, were observed. Results indicate that BSNPs possess potent quenching activity when compared with their corresponding crude extracts at lower concentrations.
The regulation of the QS mechanism in C. violaceum is reportedly influenced by signal molecule Acyl Homoserine Lactone (AHL). Investigations were made to detect AHL signal molecules by GC-MS analysis. The treated culture supernatants after extraction of AHL in ethyl acetate, were analysed for specific AHL peaks. Interestingly when compared with the control, TaNPs and TcNPs treated samples have not shown any significant AHL specific peaks. This could be because of the QQ of TaNPs and TcNPs .
Antibiofilm efficacy of BSNPs were evaluated by using in vitro microtiter assay. GcNPs could cause 91.80% biofilm inhibition, which was highest among all the BSNPs used in the study. TaNPs depicted high dispersal activity of 81.33% on C. violaceum 4212. Interestingly GcNPs & TcNPs exhibited more of Biofilm inhibition activity than the dispersal. Inhibition of C. violaceum 4212 biofilm was confirmed through SEM analysis wherein observations clearly indicated absence of the biofilm matrix and reduction in the cell density in comparison with control.
One of the essential traits for biofilm formation is the surface-active swarming motility of bacterial cells. C.violaceum depicts this type of motility because of its single polar flagellum. As Swarming motility is regulated by QS mechanism, this feature was investigated by treating the C. violaceum culture in a plate-based assay. When compared with control, there was about 66% reduction in the swarming behavior at sub inhibitory concentrations i.e. 0.5µg/ml and 1µg/ml. Venkatramanan M et al. have found 72.8% reduction in swarming motility of C. violaceum12472 while evaluating antibiofilm activity of Passiflora edulis extract. Interestingly reduction in swarming motility of C.violaceum 4212 using BSNPs was not reported so far.30
Another virulence trait in bacterial pathogens is production of hemolysins which cause lysis of RBC resulting in enhanced pathogenicity. This trait is identified by growing bacteria on blood agar enriched with 5% sheep erythrocytes. C. violaceum 4212 has been identified to exhibit b- hemolytic activity when inoculated in 5% sheep erythrocytes enriched blood agar medium. Brumbach KC et al. have reported the presence of Serratia– like hemolysins in pathogenic strains of C. violaceum.31-33 In the present study, experiments were performed to study the impact of BSNPs on the hemolysis activity of C. violaceum 4212, by treating with BSNPs at sub-inhibitory concentration i.e 0.5µg/ml and 1µg/ml. After incubation at 37°C, b- Hemolysis was not observed in treated samples when compared with the control, indicating drastic inhibition of this pathogenic trait.
With all these experiments, the current study could establish that BSNPs possess, QQ effect, anti-virulent properties against C. violaceum 4212 and could be used as a potential application in control of pathogens by reducing their virulence expression.34
The present study focuses on establishment of use of BSNPs as QQ agents. Since QS signals are essential for virulence expression of pathogenic species, sub inhibitory concentrations of BSNPs were influential in controlling the growth and establishment of C. violaceum 4212 once its QS mediated factors were inhibited. BSNPs have been found to suppress the development of pathogens rather than eradication. Exposure of BSNPs in smaller concentrations has fewer challenges in terms of toxicity less probability of development of drug resistance. These BSNPs were also evaluated on a clinical isolate of P.aeruginosa.
ACKNOWLEDGMENTS
The authors would like to thank the Management of Bhavan’s Vivekananda College of Science Humanities and Commerce for the support and encouragement to carry out the research work in the college. Authors are also thankful to the College of Technology, Osmania University for extending their support and infrastructure for specimen analysis.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both the authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Miller MB, Bassler BL. Quorum sensing in bacteria. Ann Rev Microbiol. 2001;55(1):165-199.
Crossref - Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2(11):a012427.
Crossref - Ahmad A, Viljoen AM, Chenia HY. The impact of plant volatiles on bacterial quorum sensing. Lett Appl Microbiol. 2015; 60(1):8-19.
Crossref - McClean KH, Winson MK, Fish L, et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143(12):3703-3711.
Crossref - Bacha K, Tariku Y, Gebreyesus F, et al. Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: Implication for development of potent antimicrobial agents. BMC Microbiol. 2016;16(1):139.
Crossref - Krithiga N, Rajalakshmi A, Jayachitra A. Green synthesis of silver nanoparticles using leaf extracts of Clitoriaternatea and Solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. Journal of Nanoscience. 2015;2015:928204.
Crossref - Poli JP, Guinoiseau E, de Rocca Serra D, et al. Anti-Quorum Sensing Activity of 12 Essential Oils on Chromobacterium violaceum and Specific Action of cis-cis-p-Menthenolide from Corsican Mentha suaveolens ssp. Insularis. Molecules. 2018;23(9):2125.
Crossref - Loo CY, Rohanizadeh R, Young PM, et al. Combination of silver nanoparticles and curcumin nanoparticles for enhanced anti-biofilm activities. J Agric Food Chem. 2016;64(12):2513-2522.
Crossref - Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42(6):637-641.
- O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Ann Rev Microbiol. 2000;54(1):49-79.
Crossref - Champalal L, Kumar US, Krishnan N, Vaseeharan B, Mariappanadar V, Raman P. Modulation of quorum sensing-controlled virulence factors in Chromobacterium violaceum by selective amino acids. FEMS Microbiol Lett. 2018;365(23):fny252.
Crossref - Ravn L, Christensen AB, Molin S, Givskov M, Gram L. Methods for detecting acylated homoserine lactones produced by Gram-negative bacteria and their application in studies of AHL-production kinetics. J Microbiol Methods. 2001;44(3):239-251.
Crossref - Li T, Wang D, Ren L, et al. Involvement of exogenous N-acyl-homoserine lactones in spoilage potential of Pseudomonas fluorescens isolated from refrigerated turbot. Front Microbiol. 2019;10:2716.
Crossref - Santo BLSDE, Santana LF, Kato Jr. WH, et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules (Basel, Switzerland). 2020;25(19):4513.
Crossref - Mao QQ, Xu XY, Cao SY, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8(6):185.
Crossref - Zarshenas MM, Moein M, Samani SM, Petramfar P. An overview on ajwain (Trachyspermum ammi) pharmacological effects; modern and traditional. Journal of Natural Remedies. 2014; 14(1):98-105.
Crossref - Muhammad S, Khan BA, Akhtar N, et al. The morphology, extractions, chemical constituents and uses of Terminalia chebula: A review. J Med Plants Res. 2012; 6(33):4772-4775.
Crossref - Usman M, Murtaza G, Ditta A. Nutritional, medicinal, and cosmetic value of bioactive compounds in button mushroom (Agaricus bisporus): a review. Applied Sciences. 2021;11(13):5943.
Crossref - Ravichandran V, Zhong L, Wang H, Yu G, Zhang Y, Li A. Virtual screening and biomolecular interactions of CviR-based quorum sensing inhibitors against Chromobacterium violaceum. Front Cell Infect Microbiol. 2018;8:292.
Crossref - Brackman G, Cos P, Maes L, Nelis HJ, Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilm to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011;55(6):2655-2661.
Crossref - Chhatre A, Solasa P, Sakle S, Thaokar R, Mehra A. Color and surface plasmon effects in nanoparticle systems: Case of silver nanoparticles prepared by microemulsion route. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2012;404:83-92.
Crossref - Badi’Ah HI, Seedeh F, Supriyanto G, Zaidan AH. Synthesis of silver nanoparticles and the development in analysis method. InIOP Conference Series: Earth and Environmental Science. 2019;217(1):012005.
Crossref - Raja S, Ramesh V, Thivaharan V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab J Chem 2017;10(2):253-261.
Crossref - Erdogan O, Abbak M, Demirbolat GM, et al. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PloS One. 2019;14(6):e0216496.
Crossref - Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci. 201;9(3):217-227.
Crossref - Mohanta YK, Biswas K, Jena SK, Hashem A, Abdallah EF, Mohanta TK. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front Microbiol. 2020;11:1143.
Crossref - Karygianni L, Ren Z, Koo H, Thurnheer T. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 2020;28(8):668-681.
Crossref - Sharma SK, Dhyani R, Ahmad E, et al. Characterization and low-cost preservation of Chromobacterium violaceum strain TRFM-24 isolated from Tripura state, India. J Genet Eng Biotechnol. 2021;19(1):142.
Crossref - Arunkumar M, Suhashini K, Mahesh N, Ravikumar R. Quorum quenching and antibacterial activity of silver nanoparticles synthesized from Sargassum polyphyllum. Bangladesh J Pharmacol. 2014;9(1):54-59.
Crossref - Venkatramanan M, Ganesh PS, Senthil R, et al. Inhibition of quorum sensing and biofilm formation in Chromobacterium violaceum by fruit extracts of Passiflora edulis. ACS Omega. 2020;5(40):25605-25616.
Crossref - Brumbach KC, Eason BD, Anderson LK. The Serratia-type hemolysin of Chromobacterium violaceum. FEMS Microbiol Lett. 2007;267(2):243-250.
Crossref - Zhuang X, Zhang A, Chu W. Anti-quorum sensing activity of Forsythia suspense extract against Chromobacterium violaceum by targeting CviR receptor. Int Microbiol. 2020;23(2):215-24.
Crossref - Vasavi HS, Arun AB, Rekha PD. Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol Immunol. 2014;58(5):286-293.
Crossref - Kothari V, Sharma S, Padia D. Recent research advances on Chromobacterium violaceum. Asian Pac J Trop Med. 2017;10(8):744-752.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.