ISSN: 0973-7510
E-ISSN: 2581-690X
The current scientific studies have shown that extensive quantities of synthetic pigments are used worldwide in diverse industries. Synthetic pigments have shown enormous toxicity issues compared to natural colorants and dyes in current industrial usage. Diverse microbial communities, including fungi, bacteria, archaea, and yeast are current biopigment producers. However, the aforementioned biopigments are expensive, least efficient, and less eco-friendly to attain industrial sustainability. Thus, algae-based bio pigments are one of the best natural resources to meet today’s challenges. Algal pigments increase the product’s marketability and carry multiple therapeutic properties, including antioxidant, anti-inflammatory, and neuroprotective actions. These multidimensional qualities of algal pigments have piqued the interest of the food, pharmaceutical, cosmetic, and nutraceutical industries resulting in most potential implementation. Thus, a paradigm shift requires identifying potential algal communities having a higher biopigment-producing ability for future manufacturing and commercialization as a sustainable way forward. Hence, the current study has been designed for effective isolation and screening of algal isolates (i.e. DS1, DS2, DS3, DS4, DS5) from a different region of sundarban water resources concerning the investigation of algal pigments (i.e. chlorophyll-a, chlorophyll-b and carotenoid). In a nutshell, the current study shows that DS2 isolate produces a significant quantity of carotenoid (9.729 mg/g DCW), chlorophyll a (7.872 mg/g DCW), and chlorophyll b (7.176 mg/g DCW) amongst all isolates. Hence, the present study reveals that DS2 algal isolates might be a potential predecessor of biopigment production, having pivotal applications in food, pharmaceutical, cosmetic, and nutraceutical industries in the near future.
Algal Pigment, Isolation, Screening, Extraction, Quantification, Marketability
Algae are the unicellular eukaryotic and prokaryotic photosynthetic biotic entities with a category of the polyphyletic group and carry a wide range of potent characteristics as well. They are considered one of the planet’s oldest living species, usually dated 3465 million years. Algae have attracted interest because of their high production, which is related to their quick growth rate and excellent photosynthetic efficiency. Algae store an abundance of vital macro and micro-nutrients. However, projected increase in global population shows around 10 billion people by 2050, which would considerably increase macro and micro-nutrient demand; algae may become a new food and feed resource in the future.1 Recently, it has been anticipated that by 2030, the world’s population will have grown to nearly 9 billion people, with 12 percent of those individuals facing food scarcity. As a result, food crops are unlikely to be sufficient to meet the human population’s dietary needs. In this circumstance, there is a critical need to investigate new sustainable food sources, including algae.2 The algal consortia live in several settings, including freshwater, marine water, and the surface of damp rocks. They grow quickly, live in difficult situations, and survive environmental stresses such as heat, cold, anaerobiosis, salt, photo-oxidation, osmotic pressure, and UV radiation. Microalgae have an incredible biodiversity, with between 200,000 and 800,000 species in many different genera, of which roughly 50,000 have been reported and around 15,000 inert substances derived from the biomass of algae.3 Additionally, seaweeds are multicellular, macroscopic sea macroalgae. Based on their pigmentation, algae are split into three families: brown seaweeds (Phaeophyceae, around 1800 species), green seaweeds (Chlorophyceae, about 1500 species), and red seaweeds (about 1500 species) (Rhodophyceae, about 6500 species). Seaweed is currently harvested and cultivated in large quantities for food, phycocolloids extraction, fertilizers, fuel generation, medicine, and biomolecule recovery. While aquaculture produced over 1 million tonnes of seaweed in 2013, with green (2000t), red (193,000t), and brown (760,000t) seaweeds accounting for the majority of the harvest, overall yield lies beyond 23 million tonnes, distributed as follows: Green seaweeds account for 15,000 tonnes, brown seaweeds for 8 million tonnes, and red seaweeds for 15 million tonnes.4,5 Moreover, Thailand’s north-eastern wide availability is about a third of the country’s 16.9 million hectares, including 9.25 million hectares of agricultural land. Saline soil covers approximately 2.8 million hectares. North-eastern Thailand accounts for 17% of the country’s total land area. The development of algal businesses in saline-alkali environments is an economic growth model for this region.6 Furthermore, algae play a vital part in nature’s biological systems, contributing to the manufacture of various foods, medicines, cosmetics, and various crucial natural colors.
Algal pigment types and compositions differ depending on the group and species. Chlorophyll b is found mainly in green algae, whereas phycoerythrin and phycocyanin are found in red algae, and chlorophyll a, b, and fucoxanthin are found in brown algae. In the cellular metabolism of algae, each pigment ingredient serves a distinct purpose. Chlorophyll, for example, aids photosynthesis by absorbing and utilizing light, whereas carotenoids protect the photosynthetic equipment from photo-damage. Three algal pigments exist, including chlorophylls and carotenoids (water-insoluble), and phycobilins are water-soluble.7 Algal colorants are also highly demanded in the textile, polymers, paint, pulp, and printing industries. Natural color is becoming more popular in the culinary, pharmaceutical, cosmetics, printing, textile, and dye industries. It has bio-pharmacological, antioxidant, antimicrobial, and immuno-regulatory properties. There are several usages for these pigments in numerous sectors. It has the potential as a food colorant, antibacterial agent, and bio-indicator.8,9
Artificial colors (colorants) are being phased out of the food business due to changing market demand and legislation. Product coloration is critical because manufacturers strive to provide homogeneous products, while consumers desire appealing products. Solar energy is gathered by pigments, which are used in photosynthesis. They must execute three tasks: appropriate light intake, efficient excitation energy transfer to reaction centers (RCs) with limited risk, and efficient energy breakdown with minor damage to the photosynthetic apparatus. Algae’s color diversity has aided its adaptation to light conditions of varying quality and intensity.4 Many different algae groups produce various types of biopigment over the decades (i.e. Alaria crassifolia, Alaria esculenta , Analipus japonicas, Cladosiphon okamuranus, Cystoseira hakodatensis, Desmarestia viridis, Dictyopteris australis, Dictyota dichotoma, Ecklonia kurome, Fucus distichus, Fucus serratus, Himanthalia elongata , Hizikia fusiformis, Ishigeo kamurae, Iyengaria stellate, Kjellmaniella crassifolia, Laminaria japonica Laminaria digitata, Laminaria digitata, Laminaria saccharina, Leathesia difformis , Lobophora variegata , Melanosiphon intestinalis, Myagropsis myagroides, Padina australis , Padina gymnospora , Padina minor, Petalonia binghamiae , Saccharina japonica, Saccharina sculpera , Sargassum binderi, Sargassum confusum, Sargassum crassifolium, Sargassum duplicatum , Sargassum fulvellum, Sargassum fusiforme, Sargassum horneri, Sargassum linearifolium, Sargassum muticum, Sargassum plagiophyllum , Sargassum polycystum, Sargassum siliquastrum , Sargassum thunbergii, Scytosiphon lomentaria, Silvetia babingtonii, Spatoglossum asperum, Sphaerotrichia divaricata, Stoechospermum marginatum, Turbinaria ornate, Turbinaria turbinate, Feldmannia mitchelliae, Fucus vesiculosus, Padina tetrastromatica , Eisenia bicyclis, Undaria pinnatifida).10
Chlorophylls are one of the essential bioactive molecules produced in large quantities by algae. Green algae have a lot of chlorophyll a and chlorophyll b, two separate forms of chlorophyll. They have antioxidant, wound-healing, and antimutagenic effects and are utilized as a natural food coloring additive. Natural coloring agents E141 and E140, derivatives of chlorophyll and chlorophyll combined with copper are authorized by current European law (Regulation EC No 1333/2008) and revisions. Chlorophyll-a and chlorophyll-b are advertised as “E140i” in European markets after extraction methods from plant foods. Chlorophyllins formed through saponification of chlorophylls with intact color, on the other hand, are labelled “E140ii.”Copper chlorophyllins (CFR Section 73.125) are recognized as a natural green colorant under Title 21 CFR 73’s regulation of natural food colorants. The Food Safety and Standards Agency (2011) has released the Food Products Standards and Food Additives Regulations and Standards Authority of India, which has certified natural chlorophyll a and chlorophyll b as food additives.11 Each core and supplementary photosynthetic pigment has been widely realized for its commercial potential. Chlorophyll’s green tint has long been utilized as a natural colorant, and chlorophyll and chlorophyllin have been shown to work as antioxidants and anti-inflammatory agents in the prevention of chronic diseases and malignancies.12 As a result, numerous businesses, such as food, medicines, and cosmetics, heavily emphasize commercial chlorophyll production. Commercially, chlorophylls are primarily made from stinging nettle, spinach, alfalfa, or corn. It’s worth noting that microalgae’s chlorophyll content (about 7%) is far higher than that of other commercial sources of pigments like Alfalfa sp.(0.2 percent dry weight of biomass) and Spirulina sp. (0.76 percent dry weight of biomass).11
Carotenoids are a group of terpenoids pigments produced by photosynthetic organisms that range in color from yellow to orange–red. They are called antioxidants due to their ability to deactivate and bind free radicals, particularly singlet oxygen quenching. Carotenoids are divided into two groups: those made up of hydrocarbon structures, known as carotenes, and those made up of oxygen-atom-containing structures, known as xanthophylls. Xanthophylls, such as antheraxanthin, neoxanthin, zeaxanthin, violaxanthin, loroxanthin, canthaxanthin, lutein, and astaxanthin, can be synthesized by green microalgae, just like plants.13 According to the estimates, the CA market will reach USD 2.0 billion in 2026, with a 4.2 percent annual growth rate.14 Carotenoids are in high demand: the world market for carotenoids was valued at $1.24 billion in 2016 and is expected to reach $1.53 billion by 2021, representing a 3.78 percent annual compound growth rate from 2016 to 2021. Since they are created to resist oxidation and isomerization, synthetic carotenoids have been employed in commercial products up to this point because they are more stable than natural ones.15 Furthermore, one of the most significant value-added sources for nutraceuticals and cosmetics is considered to be carotenoids due to their diverse properties, including anti-aging action. During astaxanthin, lutein is the most commercially vital carotenoid from microalgae; in the upcoming years, it is anticipated that the carotenoid industry will grow rapidly.16 Although over 1100 naturally occurring sources of carotenoids include nearly 700 different organisms, canthaxanthin, astaxanthin, lycopene, carotene, and lutein are commercially important because they have antimicrobial, antioxidant, anti-inflammatory, and antitumor properties in addition to serving as a vitamin A precursor.17 Fucoxanthin, violaxanthin, neoxanthin, α-carotene, β-carotene, and lutein, which are termed pro-vitamin A, can be produced in significant numbers by Spirulina sp., Chlorella sp., Dunaliella sp., and Haematococcus sp.18
A large photoautotrophic group of unicellular to multicellular marine and freshwater organisms, the phylum Rhodophyta, popularly known as red algae, contains about 7000 species split into seven families. The phycobiliproteins (PBPs) i.e. phycoerythrin and phycocyanin, provides red color to these by obscuring chlorophyll and other photosynthetic pigments. PBPs are proteinic pigments made up of proteinic subunits, energy-transferring tetrapyrrolic open-chain chromophores (phycobilins), and other non-protein components. The four fluorescent pigments that are the strongest are B-phycoerythrin (B-PE), R-phycoerythrin (R-PE), C-phycocyanin (C-PC), and allophycocyanin (APC). B-PE was initially derived from the red algae order Bangiales.19 These contain anti-inflammatory, antioxidant, hepatoprotective, and free radical scavenging characteristics and can be easily extracted and utilized safely in cosmetics and food coloring. Phycocyanin is a protein primarily used in food as a colorant in chewing gums, candies, dairy products, soft drinks, and cosmetics such as lipstick and eyeliners, as well as in the pharmaceutical and cosmetic industries as a dye.7
Rather than the main three types of pigments, diverse groups of bio pigments can also be derived from algal species, and those are commercially demandable as well Non-pro-vitamin-A carotenoid (i.e. fucoxanthin) is a yellowish brown pigment that accounts for around 70% of the carotenoids found in brown algae. Antioxidant, hepatoprotective, anticancer, antimalarial, anti-obese, UV protective, anti-diabetic and anti-inflammatory activities have all been found for fucoxanthin.20 Several brown algae taxa, including Undaria sp., Sargassum sp., Laminaria sp., Eisenia sp., Alaria sp., Cystoseira sp., and Hijikia sp., have been examined for this chemical. These characteristics make the fucoxanthin pigment suitable for usage in various sectors, including the food, cosmetics, and pharmaceutical industries. Additionally, by 2022, the fucoxanthin market is predicted to have grown to $120 million.10 Microalgae, plants, yeast, bacteria, and a variety of seafood, including salmon, trout, red sea bream, shrimp, lobster, and fish eggs, can all create natural astaxanthin industrially used as an antioxidant. Among these, Haematococcus pluvialis, a microalga, is thought to be the highest source of natural astaxanthin (3–5% of its dry biomass). Synthetic astaxanthin currently leads the global market for this pigment, with a market value of over US$200 million and 130 metric tonnes of product produced each year.21,22 The second-most commercially valuable carotenoids are lutein, with a global market value of $233 million in 2010 and a projected market value of $309 million by 2018.23 Based on the aforementioned economic feasibility and multifarious prospects of algae-based pigments, the current experimental goal has been set forward to isolate, screen, extract and quantify algal pigments (e.g. chlorophyll a, chlorophyll b, carotenoids) in five algal isolates from mangrove water resources.
Collection of algal sample
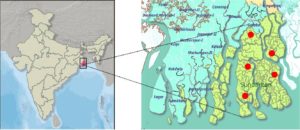
Water samples from the Sundarban islands’ mangrove ecology had been collected as part of an experimental investigation. Five water samples from the river stream Gosaba (Latitude: 22.1652°N, Longitude: 88.8079°E, pH: 6.8, Water Temperature: 28°C), Gazikhali (Latitude:22.4379°N, Longitude:88.8397°E, pH: 6.5, Water Temperature: 26°C), Pakhiraloy (Latitude:22.1419°N, Longitude:88.8335°E, pH: 6.8, Water Temperature: 25°C), Basanti (Latitude:22.112095°N, Longitude:88.401405°E, pH: 6.5, Water Temperature: 30°C), Bidyamandir (Latitude:25.05748°N, Longitude:88.28261°E, pH: 6.8, Water Temperature: 20°C) had been collected and filtration processed using whatman filter paper (Grade 113, Sigma-Aldrich) to remove plant leaves and other debris. Samples had been stored in a cold box for transportation to the research laboratory for further analysis (Figure 1).
Isolation and screening of pure algal stock
After the sample collection, filtered 2 ml water samples were inoculated in 8 ml sterile Bold basal medium (BBM)24 algal growth media (pH 7.2) and cultured for 7 days at 16 W/m² light intensity in shaking mode (50 rpm). Green color had been appeared in the culture tubes after 7 days of incubation. Then, on a BBM agar plate, these cultures from two distinct places were subjected to pure culture development (pH 7.2). A 1ml sample was placed uniformly across the surface of a BBM agar medium plate. For about 7 to 10 days, the algae were allowed to growon inoculated plates in a temperature-controlled incubator (20-25°C, approximately). Using a sterile technique, grown algal cultures were streaked onto additional sets of nutrient medium plates before being put back in the incubator for isolation. This streaking technique was applied until an axenic algal culture was isolated. An axenic algae isolate DS1, DS2, DS3, DS4, and DS5 were then preserved in 15 percent glycerol stock and stored at -20°C for future use. The pure isolates DS1, DS2, DS3, DS4, and DS5 were sub-cultured regularly to maintain optimal growth. For morphological characterization, light microscopic (1000x magnification in Magnus MLXi Plus, Magcam DC3) and optical density-biomass analysis (UV-Vis spectrophotometer from SHIMADZU, Model No: UV-1800) had been done.
Growth rate and pigment production profile of algal isolates
Following the isolation and screening procedures, the algal growth medium’s growth parameters and pigment profile were assessed in the 0-15 days time period using a spectrophotometer (Model 1280; Shimadzu) and measuring the absorbance at 663, 645, 470 nm to determine their exponential phase, which we could be used in our ongoing studies.25,8
Extraction and quantification procedure of isolated algal pigments
Experimental cultures’ biomass had been sedimented in a 1ml homogeneous solution and centrifuge (10000 rpm for 10 min at 4°C). Following centrifugation, the pellet biomass was taken and resuspended in a known volume of different extraction solvent mixtures (ethanol/methanol, and acetone) in a 2:1 ratio (v/v); after that, sonication was done for 20 minutes at 30Hz on an ice pack. The cultures were then kept at room temperature for 2hrs with regular manual shaking. After centrifuging the culture, the supernatant was transferred to a fresh tube, and 500 µl of the solvent combination was used to extract it. Thin Layer Chromatography (TLC) was used to identify the pigments extracted. Green and yellow stains on the silica gel plate were used to determine the different pigments. The following formula was used to generate (retardation factor) Rf values.
Rf value = Distance travelled by the solute/ Distance travelled by the solvent
After that, a spectrophotometer (Model 1280; Shimadzu) was used to test the supernatant’s absorbance at 663 (for chl-a), 645 (for chl-b), 470 (for car) nm for quantification of pigments. Using the formulae in the table, the amount of chlorophyll-a, chlorophyll-b, and carotenoid in the biomass had been determined.26,27
Pathogenicity test of highest pigment-producing isolate DS2
Hemolysin assays on sheep blood agar plates and broth media were used to examine the pathogenicity of the chosen isolates. The blood agar base was aseptically poured into a sterile petriplate with a 5 percent defibrinated sheep blood agar base medium. Algal cultures cultivated for 7-10 days were centrifuged for 5 minutes at 10000 rpm. The supernatant was thrown away. The cell palate was properly mixed in phosphate buffer saline (PBS) (pH 5.7). After that, the sheep blood agar base media plate was inoculated and incubated for 48 hours at 30°C. Similar to that, the same volume of positive control hemolysis buffer (ammonium chloride 8.02g, EDTA 0.37g, sodium bicarbonate 0.84g) was inoculated.28,29
As opposed to that, 2 mL of sheep blood and 8 mL of PBS pH 5.7 were thoroughly combined for the broth media experiment. After the four times washing process for 5 minutes of centrifugation at 6000 rpm, the supernatant was decanted. Lastly, 1.5 mL of PBS pH 5.7 and 0.5 mL of RBC blood were combined to create the control solution. Create two positive controls by adding 0.5 ml of blood solution to 1.5 ml of distilled water (DW) for one and 0.5 ml of blood solution to 1.5 ml of hemolysis buffer solution for the other 100X. Subsequently, cultivated cultures of the unknown sample were centrifuged for 5 minutes at 10000 rpm. The extra fluid was discarded. The cell palate was washed using PBS (pH 5.7). The sample’s pathogenicity was examined by adding 0.5 ml of blood solution to 1.5 ml of this solution. The four tested vials were then placed on a sheep blood agar plate and incubated for one hour at 30°C. After incubation, centrifugation was performed in each Eppendorf tube for 8 minutes at 8000 rpm. Afterwards, aseptically supernatants were separated, and finally, absorbances were measured at 541 nm.30-32
Isolation, screening, and identification of five algal isolates
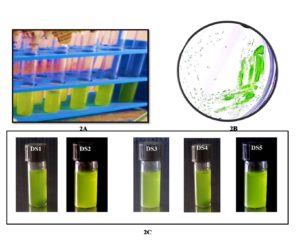
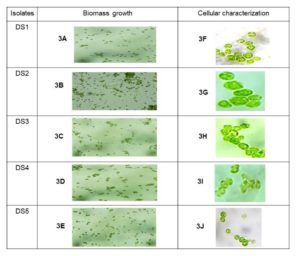
The proper procedure was followed to cultivate all the collected samples in the laboratory. The material was then processed to isolate a single cell. More than ten species were discovered in five samples successfully separated from the sampling water. Finally, the following five isolated species were bulk grown to extract and quantify natural pigments (Figure 2). Five samples were created after the isolation procedure, each of which was deemed a separate isolate. However, as algae can contain photosynthetic pigments in their cellular biomass, five mangrove algal isolates DS1, DS2, DS3, DS4, and DS5 have been successfully visualized under bright field microscopy. The microscopic examination had been revealed five different isolates were very diverse morphologically and as per biomass growth (Figure 3). As a result, the DS2 isolate was showed the biomass concentration microscopically observation rather than other isolates. Thus, DS2 algal isolate from the mangrove habitat had considered for detailed emphasize in this current study.
Figure 2. Algal culture growth in BBM medium [ 2A. algal culture cultivation in BBM medium. 2B. Grown algal cultures were streaked on BBM agar plate and green color algal colony has been observed. 2C. Pure isolates are used for further experiments].
Figure 3. Microscopic observation of algal isolate. [3A to 3E are the biomass growth of each isolates. 3F to 3J are the cellular morphology under 1000X microscopic view].
Growth and pigment content profile analysis of algal isolates
Growth pattern and pigment production profile were shown in 0-15 days experiment in 5 algal isolates (Table 1) and (Figure 4). All the isolates showed more or less similar growth patterns and pigment production rates during their lag and exponential phases. However, in the death phase, DS3 and DS5 reached 12-15 days, whereas DS1, DS2, and DS4 reached days 13-15. Significant differences were found among all the isolates in the growth and pigment production rate.
Table (1):
Growth kinetics of five algal isolates cultured in BBM Media.
| Algal isolates | Growth pattern (days 0-15) | ||||
|---|---|---|---|---|---|
| Lag Phase | Exponential Phase | Stationary phase | Death Phase | Growth rate (µ day⁻¹ ) | |
| DS1 | 2 | 6-9 | 10-12 | 13-15 | 0.45 |
| DS2 | 2 | 6-9 | 10-12 | 13-15 | 0.48 |
| DS3 | 2 | 6-9 | 10 | 12-15 | 0.33 |
| DS4 | 2 | 6-9 | 10-12 | 13-15 | 0.38 |
| DS5 | 2 | 6-9 | 10 | 12-15 | 0.4 |
Figure 4. Biomass Growth and Pigment production profile 0-15 days [throughout the lag phase to death phase the algal biomass growth rate and pigment rate was examined, where the exponential phase for all isolates shows after 9 days culture cultivation period].
Qualitative analysis on pigment identification of five algal isolates
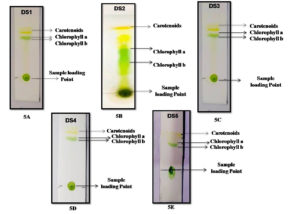
In the BBM medium, further algal isolates were maintained. The five isolates were taken for pigment identification throughout the TLC technique, and their Rf values were determined for more accuracy for the presence of pigments in algal biomass. 1 ml of the algal sample was centrifuged at 10000 rpm for 10 minutes at 4°C. After collecting the biomass solvent (Acetone: ethanol) 1:1 v/v mixed properly with biomass and further progress for TLC. As a result, the different pigment bands were shown in silica plates. According to thin-layer chromatography, two to three pigment fractions were recovered from the algal isolates. Chlorophyll a, b, and carotenoid pigments are the most abundant in these five isolates. DS2 was the most successfully identified pigment (chlorophyll a, chlorophyll b, and carotenoid) isolate, with Rf values of 0.77, 0.68, and 0.95, respectively. The Rf values were matched to data from the literature.24 Also, the Rf values of the chlorophyll and carotenoid pigments have different values for different isolates. During the qualitative analysis, the clearest pigments layer and highest Rf values were shown in DS2 isolates among the other four isolates DS2, DS3, DS4, and DS5 (Figure 5) (Table 2).
Table (2):
Retention factor (Rf) values of collected isolates.
| Isolates no. | Isolates name | Pigments name | Rf values |
|---|---|---|---|
| 1 | DS1 | Chlorophyll a | 0.54 |
| Chlorophyll b | 0.67 | ||
| Carotenoid | 0.88 | ||
| 2 | DS2 | Chlorophyll a | 0.77 |
| Chlorophyll b | 0.68 | ||
| Carotenoid | 0.95 | ||
| 3 | DS3 | Chlorophyll a | 0.66 |
| Chlorophyll b | 0.62 | ||
| Carotenoid | 0.73 | ||
| 4 | DS4 | Chlorophyll a | 0.71 |
| Chlorophyll b | 0.53 | ||
| Carotenoid | 0.77 | ||
| 5 | DS5 | Chlorophyll a | 0.6 |
| Chlorophyll b | 0.47 | ||
| Carotenoid | 0.75 |
Figure 5. Thin layer chromatography profile using algal pigment extracts. [5A. to 5E figure has been shows different pigment bands with their sample loading point, where the DS2 isolate shows the most prominent pigment bands on silica plate after the extraction procedure].
Quantitative experiment on pigment-producing five algal isolates
After the extraction procedure, the five isolates were prepared for pigment quantification. As an extraction solvent acetone: ethanol, acetone: methanol mixtures (1:1 v/v) were used. The chlorophylls and carotenoid were quantified by different equations33,34,5:
Chlorophyll a (mg/gm tissue): [12.7(A663) – 2.69 (A645)]*V/1000*W (Equation 1)
Chlorophyll b (mg/gm tissue): [22.9(A645) – 4.68 (A663)]*V/1000*W (Equation 2)
Where A = Absorbance of specific wavelength; V = Final volume of Chlorophyll extract in 80% Acetone: methanol/ ethanol; W = Fresh weight of Tissue extract.
C(x + c) = (1000A470 – 1.82Ca – 85.02Cb) / 198 (Equation 3)
Where, A = Absorbance at respective wavelength, Ca= Chlorophyll a, Cb= Chlorophyll b
Extensive quantitative tests had also been demonstrated that the pigment productivities for DS1, DS2, DS3, DS4, and DS5 which were depicted in the Table 3. During the 0-15 day cultivation phase, DS2 had been comparatively developed with higher pigment content than the other four isolates.
Table (3):
Algal isolates have been identified as a pigment source (Chlorophyll a, Chlorophyll b, Carotenoid) was extracted using traditional extraction techniques, and detection methods and quantification values were used to determine its presence.
| Algal isolates | Extraction Solvent | Pigment Extraction Conditions | Pigment Detection Techniques | Obtained Pigments yield (mg/g DCW) |
|---|---|---|---|---|
| DS1 | A:E | Room temperature for 2 hours | Spectrophotometry | Chl a : 2.484 Chl b : 1.786 Car : 4.226 |
| DS2 | A:M | Room temperature for 2 hours | Spectrophotometry | Chl a : 7.872 Chl b : 7.176 Car : 9.729 |
| DS3 | A:E | Room temperature for 2 hours | Spectrophotometry | Chl a : 3.804 Chl b : 3.459 Car : 4.182 |
| DS4 | A:M | Room temperature for 2 hours | Spectrophotometry | Chl a :3.049 Chl b : 2.546 |
| A.E | Car : 3.080 | |||
| DS5 | A.E | Room temperature for 2 hours | Spectrophotometry | Chl a : 3.804 Chl b : 3.507 Car : 4.691 |
The pathogenicity test activities of DS2 algal isolate
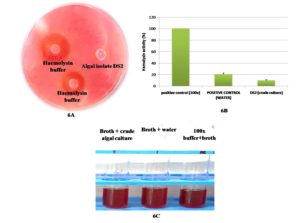
To assess pathogens for biosafety, the Blood Agar test was performed. Compared to the positive control, none of the chosen DS2 isolates developed blood lysis or halo zones on the blood agar medium. Beta-hemolysis was seen as a positive control (clear zones). The unknown sample’s test result also revealed gamma-hemolysis (no clear zones around colonies) (Figure 6A). The 100X buffer absorbance in a liquid medium was given the value of 100% hemolysis, and the formula was used to calculate the other absorbances’ percentage of hemolysis. Homolysing activity was evaluated at 20% (v/v) and 9% (v/v) in water and crude culture, respectively (Figure 6B and 6C). In this test, algal hemolysin activity was lower than water hemolysin activity. As experimental results, it could be concluded that this isolate (DS2) was competent for further scale-up studies and could be profitable for commercialization in various pharmaceutical, nutraceutical, and cosmeceuticals aspects.
Figure 6. Hemolysin assay of DS2 isolates for pathogenicity check. [6A. sheep blood agar plate, where hemolysis buffer (Hb) 25x and 100x shows a hallow zone and algal isolate DS2 shows no zone here. 6B. comparative study of hemolysin activity in sheep blood (n=3) with water. 6C. Hemolysin activity in 100x hemolysis buffer hemolysin activity in sheep blood with water, hemolysin activity on crude algal culture in sheep blood].
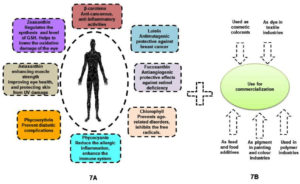
Figure 7A. Huge amount of therapeutic applications in pharmaceutical industries as well as health benefits. Figure 7B. Multi dimensional properties of algal pigment towards industrialization
The current study had shown that mangrove algal isolates DS1, DS2, DS3, DS4, and DS5 had broad spectrum of pigment productivity and noteworthy salient features. As a microbial cellular factory, the DS2 algae isolate had higher productivity towards bio-pigment production. Furthermore, no accurate dataset on commercially relevant indigenous algae in the sundarban mangrove were available. As a result, this research was aimed to identify potent mangrove algal isolates having higher natural pigments productivities towards economic sustainability. However, preservation of these natural isolates in axenic state in-situ condition would be quiet ascertains. Though Food and Agriculture Organization (FAO) had recommended the ideal physico chemical process parameter would be ranges for temperature (16-27°C), salinity (12-40 ppt), and pH (7-9), respectively. Moreover, different algal isolates could be able to tolerate temperature variations up to 15°C below their optimum, where growth might be hampered; whereas only a few degrees above optimum (temperature) could accelerate the growth minimization in natural algal isolates. In this context, halotolerant algal isolates were usually more stress tolerant than sweet water species. During the current experimentation, however, all process parameters/ independent variables were within the range.35,36
Consequently, algae based pigments or natural colorants could be claimed as better choice (i.e. higher biocompatibility and lower toxicity) in comparison to all synthetic colorants. Besides, broad spectrums of algal assemblages could also be important black horse regarding many potential commercialization in global market (Figures 7A and 7B). However, skilled labor and indigenous adaptive techniques might be required to establish algal biomass based industries in large scale. Due to their environmental adaptability, indigenous algal species could be the best choice. The potential outcome of current research work would be able to aid in the understanding of the growth behavior and pigment profiles of five significant algal isolates in the sundarban marine ecosystem, where the experimental research had showed that DS2 was the best isolate for a higher pigment production rate and least pathogenicity/ toxicity rather than another four isolates (DS2, DS3, DS4, and DS5). Hence, proper molecular characterization37,38 and multi process parameter optimization using response surface methodology (RSM).39-43 in natural algal regimes could also ameliorate biopigment biogenesis via deep understanding on metabolic cascades and its regulatory networks. Molecular approaches could be included as advancement of reverse engineering tools such as genetic transformation, gene targeting, various promoters, gene expression sequence, selection markers, and molecular biology techniques like clustered regularly interspaced short palindromic repeats (CRISPR), transcription activator-like effectors (TALEs) and zinc-finger nucleases (ZFN)44 as follow up avenues. Up- and down-regulation of transcription and translation genes, in addition to the knock-out and knock-in of desired genes, might also be the main components of genetic and metabolic engineering45; the algal strain may subsequently also put through a variety of mutagenesis processes designed to increase the strain’s productiveness for biopigments46 those are utilized to attain the maximum optimization of algal pigment yield (Figure 8). In addition, lower pathogenecity or lower toxicity of natural algal isolate could also open up new prospect in food and feed sectors to establish viable sustainable business to the global arena.47
ACKNOWLEDGMENTS
The authors would like to thank JIS University Kolkata and JIS Group Educational Initiatives for their encouragement to carry out this research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Grossmann L, Ebert S, Hinrichs J, Weiss J. Effect of precipitation, lyophilization, and organic solvent extraction on preparation of protein-rich powders from the microalgae Chlorella protothecoides. Algal Res. 2018;29:266-276.
Crossref - Chiellini C, Serra V, Gammuto L, Ciurli A, Longo V, Gabriele M. Evaluation of Nutraceutical Properties of Eleven Microalgal Strains Isolated from Different Freshwater Aquatic Environments: Perspectives for Their Application as Nutraceuticals. Foods. 2022;11(5):654.
Crossref - Patras D, Moraru CV, Socaciu C. Screening of bioactive compounds synthesized by microalgae: a progress overview on extraction and chemical analysis. Studia Universitatis Babes-BolyaiChemia. 2018;63(1):21-35.
Crossref - Dumay J, Morancais M. Proteins and pigments. Seaweed in Health and Disease Prevention. 2016;275-318.
Crossref - Ghosh D, Ghorai P, Debnath S, Indrama T, Kondi V, Tiwari ON. Algal biofertilizer towards green sustainable agriculture. New and Future Developments in Microbial Biotechnology and Bioengineering. 2022;27-45.
Crossref - Wu Z, Duangmanee P, Zhao P, Juntawong N, Ma C. The effects of light, temperature, and nutrition on growth and pigment accumulation of three Dunaliella salina strains isolated from saline soil. Jundishapur J Microbiol. 2016;9(1):E26732.
Crossref - Islam Z, Khatoon H, Rahman MR, Hasan J, Hasan SJ. Screening of natural pigments from indigenous marine microalgae isolated from different coastal aquafarms of Bangladesh. Bangladesh J Vet Anim Sci. 2021;9(2):61-72.
- Dave MR, Shetty R. Screening And Extraction Of Microbial Pigment From Organism Isolated From Marine Water. Int J Sci Res (IJSR). 2018;7(8):60-66.
Crossref - Tiwari ON, Ghosh D, Debnath S, Sahu M, Vanitha K. Multifaceted Utilization of Microalgal Biomass Towards Industrial Applications. InBiotechnology for Waste Biomass Utilization. 2022;pp. 79-127. Apple Academic Press.
Crossref - Lourenco-Lopes C, Garcia-Oliveira P, Carpena M, et al. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods. 2020;9(8):1113.
Crossref - Sarkar S, Manna MS, Bhowmick TK, Gayen K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem. 2020;96:58-72.
Crossref - Kim U, Cho DH, Heo J, et al. Two-stage cultivation strategy for the improvement of pigment productivity from high-density heterotrophic algal cultures. Bioresource Technol. 2020;302:122840.
Crossref - Haoujar I, Cacciola F, Abrini J, et al. The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules. 2019;24(22):4037.
Crossref - Pereira AG, Otero P, Echave J, et al. Xanthophylls from the sea: algae as source of bioactive carotenoids. Mar. Drugs. 2021;19(4):188.
Crossref - Imbimbo P, Bueno M, D’Elia L, Pollio A, Ibanez E, Olivieri G, Monti DM. Green compressed fluid technologies to extract antioxidants and lipids from Galdieria phlegrea in a Biorefinery Approach. ACS Sustainable Chem Eng. 2020;8(7):2939-2947.
Crossref - Borowiak D, Krzywonos M. Bioenergy, biofuels, lipids and pigments—Research trends in the use of microalgae grown in photobioreactors. Energies. 2022;15(15):5357.
Crossref - Rajput A, Singh DP, Khattar JS, Swatch GK, Singh Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1. 40. under optimized culture conditions. J Basic Microbiol. 2021;62(9):1156-1166.
Crossref - Wang J, Hu X, Chen J, Wang T, Huang X, Chen G. The Extraction of β-Carotene from Microalgae for Testing Their Health Benefits. Foods. 2022;11(4):502.
Crossref - Saluri M, Kaldmae M, Tuvikene R. Extraction and quantification of phycobiliproteins from the red alga Furcellaria lumbricalis. Algal Res. 2019;37:115-23.
Crossref - KoduvayurHabeebullah SF, Surendraraj A, Jacobsen C. Isolation of Fucoxanthin from Brown Algae and Its Antioxidant Activity: In Vitro and 5% Fish Oil-In-Water Emulsion. J Am Oil Chem Soc. 2018;95(7):835-843.
Crossref - Fabryova T, Tumova L, da Silva DC, et al. Isolation of astaxanthin monoesters from the microalgae Haematococcus pluvialis by high performance countercurrent chromatography (HPCCC) combined with high performance liquid chromatography (HPLC). Algal Res. 2020;49:101947.
Crossref - Hong ME, Choi HI, Kwak HS, et al. Rapid selection of astaxanthin-hyperproducing Haematococcus mutant via azide-based colorimetric assay combined with oil-based astaxanthin extraction. Bioresour Technol. 2018;267:175-81.
Crossref - Asker D, Awad TS. Isolation and characterization of a novel lutein-producing marine microalga using high throughput screening. Food Res Int. 2019;116:660-667.
Crossref - Toyub MA, Miah MI, Habib MA, Rahman MM. Growth performance and nutritional value of Scenedesmus obliquus cultured in different concentrations of sweetmeat factory waste media. Bangladesh J Anim Sci. 2008;37(1):86-93.
Crossref - Elumalai S, Saravanan GK, Kanna GR, Sangeetha T, Singh DR. Biochemical and pigment analysis on phycoremediatedmicroalgal biomass. Gold. Res. Thoughts. 2014;3(7).
- Henriques M, Silva A, Rocha J. Extraction and quantification of pigments from a marine microalga: a simple and reproducible method. Communicating Current Research and Educational Topics and Trends in Applied Microbiology Formatex. 2007;2:586-93.
- Sathya S. Separation of algal pigments by thin layer chromatography (TLC) and high performance liquid chromatography (HPLC). World J Pharm Res. 2017;6:1275-84.
Crossref - Huang B, Liang Y, Pan H, Et Al. Hemolytic And Cytotoxic Activity From Cultures Of Aureococcus Anophagefferens-A Causative Species Of Brown Tides In The North-Western Bohai Sea. China Chemosp. 2020;247:125819.
Crossref - Wauford N. Hemolysis Assay V1. 2016.
Crossref - Tseng SY, Liu PY, Lee YH, et al. The pathogenicity of Shewanella algae and ability to tolerate a wide range of temperatures and salinities. Can J Infect Dis Med Microbiol. 2018;6976897.
Crossref - Wu ZY, Ho SP, Cheng JF, et al. Whole-genome characterization of Shewanella algae strain SYT3 isolated from seawater reveals insight into hemolysis. Future Microbiol. 2018;13(16):1709-1717.
Crossref - Kizhakkekalam VK, Chakraborty K. Pharmacological properties of marine macroalgae-associated heterotrophic bacteria. Arch Microbiol. 2019;201(4):505-518.
Crossref - Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350-382.
Crossref - Lichtenthaler HK, Buschmann C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem. 2001;1(1):F4-3.
Crossref - Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev. 2010;14(1):217-222.
Crossref - Lavens P, Sorgeloos P. Manual on the production and use of live food for aquaculture. Food and Agriculture Organization. 1996;361.
- Ozturk BY, Asikkutlu B, Akkoz C, Atici T. Molecular and morphological characterization of several cyanobacteria and Chlorophyta species isolated from lakes in Turkey. Turkish J Fish Aquat Sci. 2019;19(8):635-43.
Crossref - Hadi SI, Santana H, Brunale PP, et al. DNA barcoding green microalgae isolated from neotropical inland waters. PloS One. 2016;11(2):e0149284.
Crossref - Eze CN, Ogbonna IO, Aoyagi H, Ogbonna JC. Comparison of growth, protein and carotenoid contents of some freshwater microalgae and the effects of urea and cultivation in a photobioreactor with reflective broth circulation guide on Desmodesmus subspicatus LC172266. Braz J Chem Eng. 2022;39(1):23-33.
Crossref - Abdulsamad JK, Varghese SA, Thajudeen J. Cost effective cultivation and biomass production of green microalga Desmodesmus subspicatus MB. 23 in NPK fertilizer medium. J Microbiol Biotechnol Food Sci. 2021;599-604.
Crossref - Aburai N, Tamura S, Abe K. Enhancement of carotenogenesis regulated by phosphorylation signaling in the aerial microalga Coelastrella sp. KGU-Y002. Phytochem Lett. 2020;40:121-125.
Crossref - Dimitrova P, Marinova G, Pilarski P. Preliminary studies on the growth and biochemical composition of a promising carotenoid producing strain Coelastrella sp. Nat Math Sci. 2016;6(3):139-149.
- Venkatachalam M, Shum-Cheong-Sing A, Dufosse L, Fouillaud M. Statistical optimization of the physico-chemical parameters for pigment production in submerged fermentation of Talaromyces albobiverticillius 30548. Microorganisms. 2020;8(5):711.
Crossref - Jagadevan S, Banerjee A, Banerjee C, et al. Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol Biofuels. 2018;11(1):1-21.
Crossref - Saini DK, Chakdar H, Pabbi S, Shukla P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit Rev Food Sci Nutr. 2020;60(3):391-405.
Crossref - Fu W, Chaiboonchoe A, Khraiwesh B, et al. Algal cell factories: approaches, applications, and potentials. Mar. Drugs. 2016;14(12):225.
Crossref - De K, Debnath S, Ghosh D. Elevating algal biomass generation toward sustainable utilization for high value added biomolecules generations. J Appl Biol Biotechnol. 2022;11(1):16-27.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.