ISSN: 0973-7510
E-ISSN: 2581-690X
A thermophilic esterase isolated from Rhodococcus sp. LKE-021. This enzyme was purified with purification fold 60 from the crude extracts of enzyme and recovery of enzyme obtained approximately 21%. The specific activity of the LKE-021 esteraseis 795.1 U/mg. SDS-PAGE analysis determined the molecular weight of LKE-21 esteraseis around 32,000Da/32KDa. The enzyme activity of LKE-021 esterase exhibited over a wide range of temperature i.e. 30° to 80°C and the enzyme remained stable when incubated on 60° for 2h. This indicates that the isolated LKE-021 esterase is thermostable. The isolated enzyme exhibits activity on various pH ranges from 2.0 to 12.0 and the highest activity observed on 11.0 pH.The LKE-021 esterase was active after proteinase K treatment and shows over 75 % specific activity i.e. 50 U/µg Proteinase K.
Rhodococcus, isolation & purification,characterization, Polyacrylamide Gel, Extremophiles
Extremophiles enzymes are the most promising for industrial applications. Thermophilic microorganisms producing a valuable thermostable enzyme stable in solvent and detergent, giving this enzyme considerable potential application in many industries1,2. According to Enzyme commission classification of enzyme lipases and esterases,are come in the hydrolases class and catalyze synthesis as well as hydrolysis of ester bonds. Ester compounds containing short-chain carboxylic acids and soluble in water hydrolyze by esterases (EC 3.1.1.1) whereas acyl-glycerides with long-chain catalyzes by lipases (EC 3.1.1.3) however the enzyme’s 3D structures share the α/β hydrolase fold3,4.
Esterases are most abundant in the living organism and isolated from microorganisms, plants, as well asanimals5,6. The hydrolysis of fat and fatty acid esterssynthes is is catalyzed by Esterases7,8. Microbial esterases have enantioselectivity and regiospecificity as desired characteristics exhibiting considerable industrial potential9. The emergent importance of esterases in numerous aspects mainly in quantitation, targeted synthesis, production, and purification and therefore used in various fields and scientists produce enzymes from microorganisms, plants, and animals10. Esterases are exploited in the dairy industry, the beverage industry for the production of fruit juices, wine, alcohol,and beer. Esterases are employed in catalysts of trans-esterification to transform fats and oils of low-value into more valuable products. For instance, esterases isolated from Lactobacillus casei CL96 are exploited for milk fathydrolysis for flavor enhancement in cheese and its productsmanufacturing11,12.
Thermophilic microbes are important for the production of thermostable esterase. Esterase exhibits a tolerance against toxic compounds at a high level and resistance to denaturation leads to their extremophilic characteristics like salt tolerance, high temperature, and resistance to protease13. Esterases have an extensive range of applications in combination with protease and amylase14. The present research focuses on the esterase separation and characterization from Rhodococcus sp. LKE-021.
Separation of esterase
Rhodococcus sp. LKE-021 was inoculated in shake flasks method containing modified nutrient broth (pH 7.0) having5.0g/l NaCl; 5.0g/l peptone; 3.0g/l yeast extract, and 10.0g/l glucose with constant shaking at 135 rpm at 60°. After 24 hrs extracellular enzyme was recovered by centrifugation for 5 min at 10×103relative centrifugal force in liquid fraction15.
Purification of Rhodococcus sp. LKE-021 esterase was done in four steps. The broth solution containing crude enzyme was cooled and slowly add the crystal of ammonium sulfate with constant stirring until 30% saturation achieve. At 4°C the solution was left overnight and centrifugation was done at 12×103 relative centrifugal force for 15 min and 4° temperature maintained during centrifugation. To recover a one by the tenth volume of the crude sample, 20Mm Tris-Cl buffer dissolved in solution. Now the solution centrifuge 12×103 relative centrifugal force at 4°C for 15 min discarded the pallet. Estimation of the enzyme was done in the precipitated protein solution. Tris-Cl buffer 20mM, pH 8.0used for dialyzed protein solution to remove the excess salt. The protein solution containing concentrated enzyme loaded onpre-equilibrated Diethylaminoethyl cellulose- c column of size 2.2cm x 20cmwith 20Mm 2-Amino-2-(hydroxymethyl)propane-1,3-diol-HCl buffer (PH 8.0)(Himedia, India). The 2-Amino-2-(hydroxymethyl)propane-1,3-diol-HCl buffer passed in the column until bound protein was eluted by Sodium chloride (NaCl) gradient and ʎ280 nm of effluent become zero. Each fraction of eluted volume i.e. 1mL collected and fraction having high specific activity was lyophilized. Lyophilized sample dissolved in 2-Amino-2-(hydroxymethyl)propane-1,3-diol-HCl buffer of 20mM, PH 8.0 pre-equilibrated carboxymethylcellulose (Sigma) column of size 2.2cm x 25cm used to running the sample. The columneluted with 2-Amino-2-(hydroxymethyl)propane-1,3-diol-HCl buffer until zero absorbance at l280 nm observed. Now bound protein eluted by NaCl of 0.05M in PH 8.0 of 2-Amino-2-(hydroxymethyl)propane-1,3-diol-HCl buffer and the bioactive fraction collected. It passed to Sephadex-G100 column of size 2.25cm x 35cm (Fluka Chemical) and the 1mL fraction at 5mL/h of flow rate was collected and examined purification by SDS-PAGE. The pure enzyme fraction with bioactivity collected & lyophilized for further characterization16.
Molecular mass determination and enzyme activity staining
SDS-PAGE performed according to Laemmli17 by using 7.5% polyacrylamide. For the molecular weight determination of the purified enzyme, 14-97 kDamarkers were used. β-mercaptoethanolomitted in the buffer. Silver staining used to visualized the protein bands and washed by 2-Amino-2-(hydroxymethyl)propane-1,3-diol-HCl buffer of 7.5 pH for 30 min. Lastly, the gel was incubated in 2mM Fast Red TR (Sigma- Aldrich) in sodium sulfate buffer of 100 mM, pH 7.5. The activity of esterase estimated by the presence of a band of deep purple colored18.
pH and temperature effect on the activity of the enzyme
pH effect on activity of esterase examinedat 5.0 – 12.0 pH ranges by several buffer solutions such as 50 mM, pH 5.0–6.0sodium acetate buffer, 50 mM, pH 6.0–8.0 potassium phosphate buffer, 50 mM; pH 7.0–9.02-Amino-2-(hydroxymethyl) propane-1,3-diol-HCl buffer, 50 mM, pH 8.0–11.0 glycine–NaOH buffer, and 50 mM, pH 12.0 Na2HPO4NaOH buffer at 70°C. The activity of enzyme estimated by using P-nitrophenyl acetate (pNPA). Estimation of pH stability of lyophilized enzymes was done by incubation at 70°C for 2 hours and dissolved in buffers of various pH. Reaction pH adjusted with similar buffer mentioned above and examined residual activity. The temperature effects estimated byusing pNPA at 20°C to 120°C at pH 11. Thermostability estimated by incubation of enzyme at 60°C–90°C at pH 11 for 2.5hours16.
Substrate specificity of the refined enzyme
Substrate specificity estimated by measuring enzyme activity on substrate-like various fatty acid esters different pNP esters, triglycerides, and naphthyl esters16.
p-Nitrophenyl esters
Various pNP ester used with the standard assay method. The corresponding ester was substituted to pNP acetate19.
a-Naphthyl esters
The enzyme (250µL), a-Naphthyl acetate (50 µL) and glycine NaOH buffer (200 µL) was mixed and incubated with Fast Red TR (50 µL 10mM). degree of substrate hydrolysis was measured in terms of enzyme activity at ʎ560. Enzyme activity in one unit,defined as the quantity of enzyme used to discharge 1 µM naphthol in one hour20.
β-Naphthyl esters
β-Naphthyl acetate (50 µL), glycine NaOH buffer (200 µL) and enzyme (250 µL) mixed and incubated for 30 minutes and pour on to the ice to stop the reaction. The estimation of esterolytic activity observed at l320 nm. One unit enzyme activity determined as the amount of enzyme sufficiently liberating 1 µM β-naphthol in 1 hour21.
Fatty acid esters
Titration method used for the determination of enzymatic hydrolysis of fatty acid. Fatty acid ester (400 µL of 10mM), glycine NaOH buffer (300 µL) and enzyme (300 µL) was mixed and incubated for 30mins and reaction terminating by adding acetone, ethanol, and 10 µL of phenolphthalein (1%). The mixture was titrated against NaOH (0.05M)till thered color achieved. Lineweaver–Burk plotted for Michaelis–Menten constant (Km) and rate of reaction (Vmax) estimation22. A unit activity is defined as the quantity of enzyme consume to liberate fatty acid (1 µM) in 1 hour.
Metal ions effect, denaturing chemicals,and inhibitors
Enzyme and Glycine NaOH buffer (50mM) incubated at 70° for 1 hour with various metal ions (Ba2+, Cu2+, Ca2+, Fe3+, Co2+, K+, Hg2+, Mn2,Mg2+, and Zn2+), inhibiting agent (PMSF, Iodoacetic acid and EDTA), oxidizers (hydrogen peroxide),reducing sugar (β-mercaptoethanol) and 1% and 2% surface-active agent (SDS, Tween-80, TritonX-100, and Tween-20). pNP acetate utilized for the estimation of enzyme activity16.
NaCl effect on the enzyme activity
Enzyme activity due to the salinity effect estimated by using different NaCl concentrations (0 to 10M). Enzyme incubatedat 70°C for 3 hrs with glycine (50 mM) and NaOH buffer with various concentrations of NaCl16.
Organic solvents effect on the enzyme activity
Purified enzyme stability estimated against various organic solvents. The enzyme, glycine (50 mM), and NaOH buffer mixed with various organic solvent. The mixture incubated with moderate constant shaking at 70°C for 10 days. The related activity of the sample estimated at different days of intervals16.
Enzyme stability against protease
Stability against proteinase K (Sigma-Aldrich) assayed by refined enzyme incubated with various concentrations of proteinase at 70°C for 30 mins. The pNP substrate as acetate used to measure the activity of the enzyme16.
Separation of esterase
The separation of the esterase from the isolate Rhodococcus sp. LKE-021 achieved 60 fold purification of the crude enzyme (Table 1). Purified LKE-021 esterase attained higher recovery (20.7%) and specific activity (795.1 U mg−1 protein) in comparison with esterase recovery 14.4% isolated from Thermobifida fusca23.
Table (1):
Separation of lke-021 esterase.
Purification step |
Total Activity (U) |
Protein (mg) |
Specific Activity (U/mg) |
Purification fold |
Recovery (%) |
|---|---|---|---|---|---|
Crude extraction |
15720 |
1180 |
13.3 |
1.0 |
100 |
0-30%(NH4)2SO4 fraction |
10890 |
94.7 |
114.9 |
8.64 |
69.2 |
DEAE ion-exchange |
6870 |
28.34 |
242.4 |
18.22 |
43.7 |
CM- ion-exchange |
4650 |
8.8 |
528.4 |
39.7 |
29.5 |
Sephadex gel-filtration |
3260 |
4.1 |
795.1 |
59.7 |
20.7 |
Electrophoresis and zymography
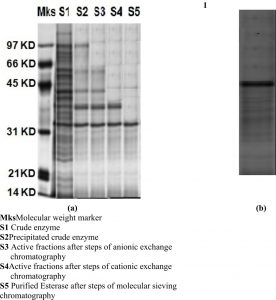
Under the reduced conditions a band observed in SDS-PAGE signifying the esterase was homogeneous. The molecular mass of the LKE-021 Esterase discovered by SDS-PAGE is approximately 32,000Da (Fig. 1a). Zymogram of activity also shown one clear zone of esterolytic activity that confirmed the purification of LKE-021 Esterase at homogeneity level (Fig. 1b).
(a) SDS-PAGE of the purified LKE-021 Esterase (b) Activity staining of LKE-021 purified Esterase
Mks: Molecular weight marker
S1: Crude enzyme
S2: Precipitated crude enzyme
S3: Active fractions after steps of anionic exchange chromatography
S4: Active fractions after steps of cationic exchange chromatography
S5: Purified Esterase after steps of molecular sieving chromatography
Fig. 1. SDS-PAGE Gel of the purified LKE-021 Esterase
pHeffect on the purified enzyme activity
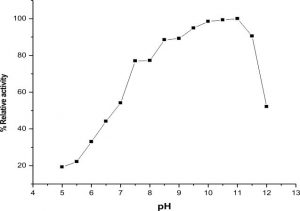
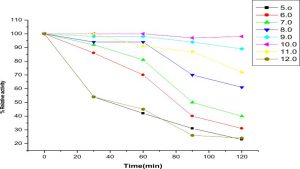
The highest activity of LKE-021 esterase shows on pH 11. The LKE-021 esterase shows a broad range activity on pH 5–12 (Fig. 2). Fig. 3 illustrates the relative residual activity of LKE-021 esterase at various pH values after 30, 60, 90 mins. This enzyme is stable in 5–11pH for a long as well as short duration. LKE-021esterase exhibited alkaliphilic nature. Alkaliphilic nature has been previously observed in enzymes extracted from extreme environmental condition16. Various pH and high temperatures require chemical methods recognized therefore enzymes muststabilize wide ranges of harsh conditions and there is anextensive interest in enzymes and their derivative isolated from extremophiles and without any pretreatment they are stable16,24.
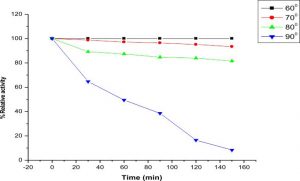
Thermostability and optimum temperature of the purified enzyme
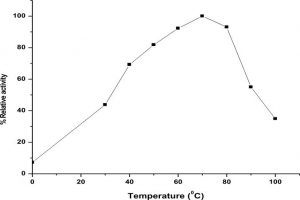
The LKE-021 esterase exhibits decent enzyme activity over a wide range of temperature 30–90°, the optimum activity being assayed at 70 – 80° (Fig. 4).The profile of the thermal stability of LKE-021 esterase examined as residual activity (Fig. 5). The enzyme activated by maintaining at 30-70° and nearly retained its optimum activity at 70° for 2 hours of incubation. Though, the activity of the enzyme was lost at 90° at 70° for 2 hours of incubation. LKE-028 esterase exhibited a half-life (t1/2) of 60 mins at 90°and at 80°C no significant reduction was detected. These results confer the work of Kumar et al., deliver a confirmation that LKE-021 esterase possesses exclusive properties for largescale production and application at extreme pH and temperature.
Metal ions effect on the enzyme activity
All metal ions examined and observed that it inhibit the refinedLKE-021 to some level, the LKE-021 esterase being inactivated to some extent by HgCl2, CoCl2 ions. Metal ions Ba+, Zn+, and Fe+improve the enzyme activity. The result of numerous metal ions on the activity of LKE-021 esterase is present in Table 2. It is familiar that metal ions are essential in maintaining protein integrity and stability by amino acid binding in specific sites of enzymes25. In the presence of Zn2+ and Ba2+LKE-021 esterase activity was improved up to 143.6 and 123.9%. These observations reflect similarities that describe Bachkatova and Severina26.
Table (2):
Metal ions effect on the enzyme activity.
Metal ions |
Specific activity(U/mg protein) |
% Relative activity |
|---|---|---|
Control |
795.1 |
100 |
CaCl2 |
856.3 |
107.7 |
HgCl2 |
73.94 |
9.3 |
FeCl3 |
909.59 |
114.4 |
KCl |
430.94 |
54.2 |
CuSO4 |
509.65 |
64.1 |
CoCl2 |
196.38 |
24.7 |
MgCl2 |
821.33 |
103.3 |
FeSO4 |
834.85 |
105.0 |
ZnSO4 |
1141.76 |
143.6 |
BaCl2 |
985.12 |
123.9 |
Inhibitors affect enzyme activity
The inhibitor’s effect on enzyme activity shown in Table 3. The serine inhibitor (PMSF) completely inhibited the LKE-021 Esterase indicating that the Esterase comes in the hydrolase family of serine. Enhancement of the activity of Esterase observed in the company of surfactants like Tween 80, Tween 20, and triton (1% to 2%). In each case, the enzyme subjected to 1 hour of pre-incubation. LKE-021 Esterase is stable in both reducing and oxidizing conditions and the activity of esterase retained in the presence of b-mercaptoethanol and H2O2.
Table (3):
Effect of adifferent inhibitor of LKE-021.
Inhibitors |
Specific activity(U/mg protein) |
%Relative activity |
|---|---|---|
EDTA |
704.29 |
88.58 |
Idoacetic acid |
610.64 |
76.8 |
SDS |
798.52 |
100.43 |
β-mercaptoethanol |
527.31 |
66.32 |
H202 |
625.58 |
78.68 |
PMSF |
40.63 |
5.11 |
Controle |
795.1 |
100% |
Triton 1% |
834.86 |
105 |
Triton 2% |
804.64 |
101.2 |
Tween 20 1% |
850.76 |
107.0 |
Tween 20 2% |
834.86 |
105.0 |
Tween 80 1% |
874.61 |
110.0 |
Tween 80 2% |
859.50 |
108.7 |
Salinity effect on the stability and enzyme activity
The purified LKE-021 Esterase shows great stability in NaCl about 100% activities when incubated with up to 10 M NaCl. The enzyme activity observed about two times when mixed with 1M NaCl and slightly decreases up to 3 M NaCl (Table 4). LKE-021 activity enhanced with 1 M NaCl and up to 10 M NaCl retained 100% activity and exhibited more halotolerant tha nearlier reported esterases18,20.
Table (4):
Effect of salinity on LKE-021 esterase.
Molarity of NaCl |
Specific activity(U/mg) |
Relative activity (%) |
|---|---|---|
Control |
795.1 |
100 |
1 M |
1941.63 |
244.2 |
2 M |
1798.51 |
226.2 |
3 M |
1272.16 |
160.5 |
4 M |
1209.34 |
152.1 |
5 M |
865.86 |
108.9 |
10 M |
823.7 |
103.6 |
Substrate specific activity of LKE-021
The activity of the LKE-021examined the effects of various substrates shown in Table5. The LKE-021 esterase was highly active on 4-Nitrophenyl butyrate and Ethyl laurate. On increasing the length of fatty acid and side-chain reduced activity of esterase. a-naphthyl acetateis a superior substrate than b-naphthyl acetate. The esterase exhibits low activity against fatty acid striglycerides. Substrate specificity of purified LKE-021 esterase estimated by pNPs ubstrate and straight-chain fatty acidsethyl esters. Esterase exhibits specificity of substrate on a set of substrates, Extreme activity of esterase was observed against pNP esters compared to ethyl esters.
Table (5):
Effect of different substrate on LKE-021 esterase.
Substrate |
Specific activity(U/mg) |
Relative activity (%) |
|---|---|---|
4-Nitrophenyl acetate |
795.1 |
100 |
2-Napthyl acetate |
454.00 |
57.1 |
4-Nitrophenyl palmitate |
181.28 |
22.8 |
Ethyl palmitate |
325.1 |
40.9 |
Ethyl linoleate |
223.34 |
28.09 |
4-Nitrophenyl caprylate |
414.24 |
52.1 |
Ethyl laurate |
681.40 |
85.7 |
4-Nitrophenyl butyrate |
751.36 |
94.5 |
Ethyl oleate |
52.47 |
6.6 |
Ethyl caprylate |
192.4 |
24.2 |
Ethyl lactate |
79.51 |
10.0 |
Ethyl decanoate |
249.66 |
31.4 |
Ethyl butyrate |
484.21 |
60.9 |
Ethyl isovalerate |
71.87 |
9.04 |
Ethyl formate |
446.06 |
56.1 |
Ethyl octanoate |
95.41 |
12 |
Ethyl heranate |
0 |
0 |
Triactin |
196.38 |
24.7 |
Tributyrin |
116.87 |
14.7 |
Tripalmitin |
41.34 |
5.2 |
Trilaurin |
0 |
0 |
Isopropyl-myristata |
0 |
0 |
Organic solvents Effect on the stability and activity of the LKE-021
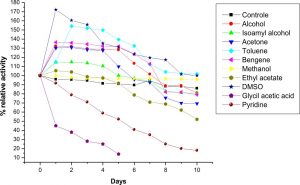
The LKE-021 Esterase exhibits stability in Toluene, DMSO, Methanol, benzene, butanol, Isoamyl alcohol,and acetone. The enzyme was retaining decent activity even after 10 days in the Benzene, Isoamyl alcohol and DMSO as compared to the other solvent and it is highly stable in Benzene and DMSO and retaining good residual activity incubation with these organic solvents for 10 days at 37°C (Fig. 6). LKE-021 Esterase is stable as well as the activity enhanced in organic solvents. 1.1–1.7 fold specific activity of LKE-021 Esterase dramatically increased over 10 days in Benzene and DMSO as compared to the aqueous medium. Therefore these properties could be beneficial in numerous enzymatic processes in the industry. The esterases exploited in trans-esterification and biocatalytic esterification has <1% water hence utilization of these esterases could be useful. The thermal stability of esterase could enhance by the use of these organic solvents27. LKE-021 esterase illustrated great stability in severalorganic solvents and maintained 100% relative activities subsequently 10 days in DMSO and toluene. The remarkable stability of LKE-21 esterase activity in organic solvents revealed by Karpushova18. Elend28reported Metagenome derived esterase which exhibits the highest activity after incubation of 1 hour with 30% and 15% DMSO, while LKE-028 esterase maintained 100% relative activity subsequently 10 days of incubation and same observation revealed by Sana20 in BSE01 esterase isolated from Bacillus sp.
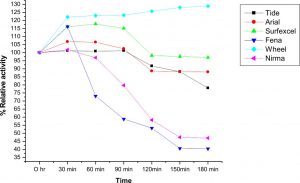
Compatibility of LKE-021 esterase with market available detergents and wash performance
The compatibility and stability of LKE-021 Esterase with most of the commercial laundry detergents like Wheel®, Ariel®, Tide®, Surf excel®, Nirma®, and Fena® as shown in the Fig. 7. It has shown good compatibility with Wheel and Surf excel detergents and more than 70% residual activity after 3 hrs. except the Nirma® and Fena® ®, detergent in all checked solid detergents. The stability and compatibility study of LKE-021 with most of the commercial laundry detergents at 37°C temperature was observed After 3 hr. in the following order: Wheel (128%) > Surf excel (97%) > Ariel (89%) > Tide (89%) %) >Nirma (48%) >Fena (41 %).
Stability of LKE-021 esterase against protease
The LKE-021 esterase was observed active after proteinase K treatment and shows over 75 % specific activity i.e. 50 U/µg Proteinase K (Table 6).
Table (6):
Stability of esterase LKE-021 with proteinase -K.
Prot-k (U/µg) |
Specific activity (U/mg) |
Relative activity (%) |
|---|---|---|
— |
795.1 |
100 |
5 |
786.35 |
98.9 |
10 |
689.89 |
87.9 |
20 |
652.78 |
82.1 |
50 |
601.10 |
75.6 |
100 |
194.00 |
24.4 |
LKE-021 esterasespecifies the extremophilic halophilic enzyme due to high salt tolerance. Thermostable, organic solvent,and salinity tolerant esterases might be exploited industrial applications such as effluent treatments, food industry for the production of inter-esterification substances, and non-natural hydrolysis of the substrate and alteration of molecules by nonaqueous biocatalysis. LKE-021 esterase shows potential uses in the non-aqueous biocatalyst due to stability and activity with protease, alkaline pH,organic solvents, and high temperatures.
ACKNOWLEDGMENTS
The authors are sincerely thankful to Mr. Sunil Sharma, Chancellor, and Dr. Sudhanshu, Chief Mentor of Suresh Gyan Vihar University, Jaipur, for providing a platform for this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
L S – Extraction and Purification of LKE-021 esterase. GS – Electrophoresis and molecular mass determination of LKE-021 esterase. AS – Carried out LKE-021 esterase activity. GA – Effect of metal ions, inhibitors, and denaturing chemicals on LKE-021 esterase activity. LK – Discussion writing. MIA – Formatting of paper as per Journal Guideline.SM – Grammatically checked and improved.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript and/or the Supplementary Files.
- Lasa I, Berenguer J. Thermophilic enzymes and their biotechnological potential. Microbiologia. 1993;9(2):77-89.
- Haki GD, Rakshit SK. Developments in industrially important thermostable enzymes: a review. Bioresour Technol. 2003;89(1):17-34.
Crossref - Nardin M, Dijkstra BW. a/b Hydrolase fold enzymes: the family keeps growing. COSB. 1999;9(6):732-737.
Crossref - Levisson M, van der Oost J, Kengen SW. Carboxylic ester hydrolases from hyperthermophiles. Extremophiles. 2009;13(4):567.
Crossref - Thomas TC, Szekacs A, Hammock BD, Wilson BW, McNamee MG. Affinity chromatography of neuropathy target esterase. Chem Biol. 1993;87(1-3):347-360.
Crossref - Shahjahan RM, Karim A, Begum RA, Alam MS, Begum A. Tissue specific esterase isozyme banding pattern in Nile tilapia (Oreochromisniloticus). Univ j zoolRajshahiUniv, 2008; 27: 1-5.
Crossref - Jaeger KE, Dijkstra BW, Reetz MT. Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu Rev Microbiol, 1999; 53(1): 315-351.
Crossref - Lopes DB, Fraga LP, Fleuri LF,Macedo GA. Lipase and esterase: to what extent can this classification be applied accurately. J Food SciTechnol,2011;31(3):603-613.
Crossref - Jaeger KE, Ransac S, Dijkstra BW, Colson C, van Heuvel M,Misset O. Bacterial lipases. FEMS Microbiol Rev, 1994; 15(1):29-63.
Crossref - Panda T,Gowrishankar BS. Production and applications of esterases. Appl Microbiol Biotechnol, 2005;67(2):160-169.
Crossref - Choi YJ, Lee B. Culture conditions for the production of esterase from Lactobacillus casei CL96. Bioproc Biosyst Eng,2012;4(1):59-63.
- Torres S, Martinez MA, Pandey A, Castro GR. An organic-solvent-tolerant esterase from thermophilic Bacillus licheniformis S-86. BioresourTechnol, 2009;100:896–902.
Crossref - Rigoldi F, Donini S, Redaelli A, Parisini E,Gautieri A. Engineering of thermostable enzymes for industrial applications. APB, 2018;2(1):011501.
Crossref - Raveendran S, Parameswaran B, BeeviUmmalyma S, Abraham A, Kuruvilla Mathew A, Madhavan, A, et al. Applications of microbial enzymes in food industry. Food Technol Biotech, 2018;56(1):16-30.
Crossref - Singh L, Sharma G, Awasthi G, Kumar L, Ali MI,Moin S. Screening, Isolation and Identification of Thermophilic Esterase Enzyme Isolated from Rhodococcus sp: LKE-021, J Pure Appl Microbiol, 2019;13(3):1855-1861.
Crossref - Kumar L, Singh B, Adhikari DK, Mukherjee J, Ghosh D. A thermoalkaliphilic halotolerant esterase from Rhodococcussp. LKE-028 (MTCC 5562): enzyme purification and characterization. Process Biochem, 2012;47(6):983-991.
Crossref - Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophageT4. Nature (London), 1970;227:680–685.
Crossref - Karpushova A, Brummer F, Barth S, Lange S, Schmid RD. Cloning, recombinantexpression and biochemical characterisation of novel esterases from Bacillus sp.associated with the marine sponge Aplysina aerophoba. Appl Microbiol Biotechnol, 2005;67: 59-69.
Crossref - Zhang J, Liu J, Zhou J, Ren Y, Dai X, Xiang H. Thermostable esterase from thermoanaerobacter tengcongensis: high-level expression, purification and characterization. Biotechnol Lett, 2003; 25: 1463–1467.
Crossref - Sana B, Ghosh D, Saha M, Mukherjee J. Purification and characterization of an extremely dimethylsulfoxide tolerant esterase from a salt-tolerant Bacillus species isolated from the marine environment of the Sundarbans. Process Biochem, 2007; 42:1571–1578.
Crossref - Hou WC, Chen HJ, Chang CF, Lin YH. Purification and characterization of fattyacid esterase from Yam (Dioscorea batatas Decne) tuber. Bot Bull Acad Sinica, 1999;40:305–310.
- Suzuki T, Nakayama T, Kurihara T, Nishino T, Esaki N. A cold-active esterase with a substrate preference for vinyl esters from a psychrotroph, Acinetobacter sp. strain no. 6: gene cloning, purification, and characterization. J Mol Catal B Enzyme, 2002;16: 255–263.
Crossref - Yang CH and Liu WH. Purification and properties of an acetylxylan esterase from Thermobifida fusca. Enzyme MicrobTechnol, 2008;42(2):181-186.
Crossref - Morana A, Di Prizito N, Aurilia V, Rossi M, Cannio R. A carboxylesterase from the hyperthermophilic archaeon Sulfolobussol fataricus: cloning of the gene,characterization of the protein. Gene, 2002;283: 107–115.
Crossref - Colak A, Pipik D, Saglam N, Guner S, Canakci S, Belduz AO. Characterization of athermoalkalophilic esterase from a novel thermophilic bacterium, Anoxybacillus genensis G2. Bioresour Technol, 2005;96:625–631.
Crossref - Bachkatova NA,Severina LO. Isolation and characterization of intracellularlipase from S. marcescens 345. Prikl Biokhim Mikrobiol, 1980;16:315–326.
- Heitmann P. In: Ruttoff H, editor. Industrial enzyme. Hamburg: Behr’s Verlag., 1994, p. 913.
- Elend C, Schmeisser C, Leggewie C, Babiak P, Carballeira JD, Steele HL, et al. Isolation and biochemical characterization of two novel metagenome derived esterases. Appl Environ Microbiol, 2006;72:3637–3645.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.