ISSN: 0973-7510
E-ISSN: 2581-690X
Medicinal herbs that are in use for centuries to treat infections and other illnesses. Prunella vulgaris L. is traditionally used for its therapeutic attributes for the alleviation of various infectious diseases. The objective of this study on Prunella vulgaris was to reveal relevant pharmaceutical information to understand its beneficial medicinal uses for human beings. The methanolic and petroleum ether extracts after removal of the solvent under reduced pressure from the Prunella vulgaris plants were prepared and these extracts were analyzed in vitro for their activity against B. subtilis, E. coli, S. aureus and S. typhi (with ATCC numbers 6051, 25922, 23235 and 14028 respectively). Likewise, in vivo studies were conducted using the E. coli-induced peritonitis in laboratory rat models where the rats were given allopathic antibiotic ofloxacin and the results were compared with those rats who received plant extracts under controlled conditions. The results were analyzed for the efficacy of the plant extracts was compared with ofloxacin; the methanol extracts exhibited equally if not better results in clearing the pathogen from the system of animals. The petroleum ether extracts exhibited the least antimicrobial activity in comparison to those extracted in methanol isolates. In conclusion, the herbal extract demonstrated significant antibacterial activity both under in-vitro and in-vivo studies which is a major outcome of the study. During this study, all standards and norms were followed as per government animal authority.
Prunella vulgaris, methanolic extracts, pathogenic microorganisms
Plants are an incredible source of therapeutic agents since time immemorial and their medicinal efficacies are still being investigated globally. Secondary metabolites help plants and microbes in resisting the onslaught of abiotic and biotic stresses by the changing environmental and growth conditions and it are under these constraints that the organisms start synthesizing secondary metabolites that act as adaptive molecules. As many as two and a half million such secondary metabolites are reported in the literature and the great majority of them are known to possess bioactivity. These bioactive compounds play important roles in imparting defense mechanisms against pest attacks also. Their most important bioactivity is that these compounds can be used as potent herbal drugs and have been isolated and evaluated for their efficacy. Not many of them are commercialized yet as a purified molecule to be used as medicine; however, their crude extracts and powder formulation are prescribed in the Indian Ayurveda and Unani systems of medicine. Humanity has long realized their potential as therapeutic agents and the ancient people almost completely relied upon them as medication for various ailments. Micro-organisms are well-known examples of biosynthesizing secondary metabolites. All antibiotics are obtained from the metabolic machinery of microorganisms and all of them are classified as secondary metabolites. But, in addition to microbes, plant extracts are also known to exhibit antibacterial activity.
Members of the family Lamiaceae (Lavender/Mint family) are a rich source of essential oils, and these are known to possess beneficial therapeutic agents. One of its members, Prunella vulgaris L. has also been endowed with a treasure of compounds that are exploited as antimicrobial drugs. Therefore, it has been used as a disinfectant for past many decades. Out of these bioactive molecules, few have been tested and sold in the market as crude pharma compounds and used in home-grown enterprises1. Researchers got inclined towards remedial plants in the wake of watching increasingly symptoms of engineered drugs contrasted with their advantages2. Prunella vulgaris plants are one to two feet high, that grow wild in and around in Chitrakut region of Madhya Pradesh, India, and also in most other regions of India. In the present study, we have collected its natural populations and attempted to evaluate its antimicrobial activity.
J. vulgaris, owing to its numerous curative properties and primarily because of its flavonoids and phenolic compounds is also known as self-heal3. In the present investigation, the total phenol and flavonoid content of P. vulgaris was assessed. In the wake of widespread resistance to antibiotics, the complexity in peritonitis caused by E. coli limits the peritoneal function that affects patients adversely4. With its almost negligible side effects and broad-spectrumanti-microbial activity, the P. vulgaris extracts were also tested against peritonitis in Albino Wistar rats. This examination was also intended to decide the reason behind the seriousness of peritonitis brought about by E. coli. In this study all guidelines were followed for animal use and the experiments were performed with the permission from Institutional Animal Ethical Committee (IAEC) of the PBRI, Bhopal.
Structurally, antibiotics fall in the group of secondary metabolites and they come from either bacteria or fungi. Because of their over-usage, wrong prescription and incessant mutation among antibiotic-yielding bacteria, most of the pathogenic microbes have developed resistance. Hence, the focus of the scientists has shifted towards searching for new antibiotics. Plants have a huge reservoir of secondary metabolites. Since P. vulgaris is rich in essential and aromatic oils, therefore, to evaluate its antimicrobial efficacy, these experiments were conducted.
Selection and collection of plant material
An exhaustive literature survey is required in the plant selection for such studies, especially for its specific biological efficacy. In this study, Prunella vulgaris L. and its parts were selected based on an ethno botanical survey. Natural populations of the plant were collected from the wild and the herbarium of the plant species was prepared and deposited to the Department of Botany, Safia College of Science, Bhopal [Madhya Pradesh, India], for identification and authentication. Plants were authenticated by the Head of the Botany department. The herbarium specimen had authentication voucher numbers 140/Bot/Safia/19 and 139/Bot/Safia/19 for Prunella vulgaris is preserved.
It is not without reason that P. vulgaris is regarded as self-heal, it synthesizes secondary metabolites that have tremendous potentialities to be exploited to alleviate various ailments in human beings.
Consequently, the entire investigation was divided into two sections. The in vitro investigation included the antimicrobial actions of the extracts against standard bacterial strains obtained from American Type Culture Collection (ATCC), USA. The in vivo studies were based on three parameters i.e., E. coli-induced peritonitis, neutrophils function test, and the survival studies. Furthermore, the phenolic and flavonoids content of the extracts was assessed using standard laboratory protocols.
Processing and preparation of plant extract
Solvents
To prepare extracts of the shade-dried leaves of the P. vulgaris, with analytical grade methanol 99.8 % purchased from Sigma-Aldrich; and petroleum ether was obtained from Merck was used.
Maceration
The shade-dried plant material was broken down into very small pieces in such a way that minimum damage was done to the cells to preserve the essential oils in them. Maceration is a well-tested technique to reduce the plant material intended to be used in the isolation of active compounds. It involves putting the plant material and the organic solvent into contact with occasional shaking in an air-tight container overnight5.
Shade dried and macerated P. vulgaris (1 kg) powder was transferred to 250 ml conical flasks and methanol was added and kept for 72 hours at room temperature and was shaken at regular intervals. The resultant crude extract was filtered first using a muslin cloth and then after by Whatman filter paper (No. 1). After filtration, the solvent was removed under reduced pressure. The solvent-free dried extract was designated as PVME and was weighed and kept inside the air-tight desiccators for further use5.
Photochemical Analysis
Spectrophotometric Quantification of Whole Phenol Content
Procedure
Total amount of phenolics present in the extracts was estimated using Folin and Ciocalteu’s reagent. Then, Gallic acid was used to prepare the standard curve, and the total phenolics were expressed in terms of Gallic Acid Equivalent (GAE, mg/g). Gallic acid measuring 0.01 to 0.05 mg/ml was dissolved in methanol to prepare the standard curve. The plant extracts were diluted (10-1) using methanol and 0.5 ml of each of these samples were taken in a test tube, to which 2.5 ml of diluted (10-fold) Folin and Ciocalteu’s reagent and finally 7.5% sodium carbonate (2 ml) were added. These test tubes were then covered with para film and were incubated for 30 min. at ambient room temperature (15-25°C). After 30 min. the absorbance in each test tube was measured at 760 nm via spectrophotometer. All of these operations were performed in technical triplicates. The Folin and Ciocalteu’s reagent is reactive to polyphenols and upon reaction, it generates a blue color that was measured spectrophotometrically6.
A Gallic acid standard curve was used as a reference for the evaluation of unknown total phenolics content in the P. vulgaris extracts. Line of regression from gallic acid was used for estimation of unknown phenolic content in our sample7. From this curve of gallic acid, the regression line was estimated to be:
y = 0.002x + 0.063 and R2 = 0.995; (where y is the absorbance)
Spectrophotometric Quantification of Total Flavonoid Content
Methodology
Colorimetric assay described by Dewanto et al20 was employed to find out the total flavonoids present in the plant extracts. The diluted sample of extracts and the standard solution of quercet in were added into a 75μl of sodium nitrite solution, and it was mixed continuously for 6 min. To this mixture, 0.15 ml of aluminum chloride solution (100 g/L) was added; and after 5 min., 0.5 ml of sodium hydroxide was added to this mixture. Using distilled water; the volume was finally made up to 2.5 ml. and mixed thoroughly. Then, the absorbance of this mixture was measured at 510 nm. This mixture without the extract was taken as blank. The total content of flavonoid was expressed in terms of mg quercetin/g dry weight (mg/g DW), using Querctin’s calibration curve prepared earlier. This experiment was conducted in triplicates. The regression line drawn for quercetin was used to estimate flavonoid (unknown) content in the extracts. From quercetin’s standard curve, a regression line was found as: Y = 0.001x + 0.120 and r2 = 0.988.
Thus, by putting the value of the absorbance of the tested sample (Y = absorbance) the goodness of fit was calculated7.
Whole Flavonoid Contents (TFC)
In-Vivo Antibacterial Study
Peritonitis is an inflammation of the inner lining of our body. It is a silk-like membrane that encloses the internal organs also, and the inflammation of this membrane is caused by a bacterial or fungal infection. Infection or no infection, peritonitis causes systemic inflammation because it damages the peritoneal layer which often leads to mortality. In the present study, the peritonitis was induced by injecting E. coli (ATCC 25922) in the Albino Wistar rats and the antibacterial activity of the extracts was studied in vivo7.
Parameters included in E. coli-induced peritonitis in the in vivo antibacterial study
- E. coli-in the Albino Wistar rats.
- Neutrophils Function Test
- Survival Study
Characteristics of the animals used
Albino Wistar rats were employed in the study were procured from the Pinnacle Biomedical Research Institute (PBRI), Bhopal, India. All the animal experiments were approved and cleared by the Institutional Animal Ethical Committee (IAEC) of the PBRI, Bhopal bearing. The committee for Control and Supervision of Experiments on Animals (CPCSEA) registration no. – 1824/PO/ere/s/15/CPCSEA. The convention reference number is pbri/iaec/pn. Following were the features of the animals used in the study:
Strain – Albino Wistar rats
Age 13- 14 weeks
Sex – either sex
Bodyweight – 200±20 g
Route of administration- P.O
Groups for E. coli-induced peritonitis
The animals were divided into three groups. Details of the treatment are being given below:
Group-I: Animals acted as Control where Normal saline + E. coli were injected via I.P.
Group-II: Animals were given ofloxacin 10 mg/Kg body mass per os (oral administration). + E. coli was injected via i.p.
Group-III: Animals were administered with the plant extract at 100 mg/kg body mass p.o. + E. coli via i.p.
E. coli strain in this study was acquired from the American Type Culture Collection, USA, and maintained on supplement agar in our laboratory, and these cultures were used for all examinations. The E. coli (ATCC-25922) lifted from a single and well-isolated colony was cultured on supplement broth and incubated at 37°C for 16-18 hrs. to attain uniform developmental stage. The culture suspension was then spun at (200 rpm) for 10 min. at 4°C, and the pellet was re-suspended in Phosphate Buffered Saline (PBS), and Optical Density (OD) was taken at 660 nm, a cell density that concorded to approximately 108 CFU/ml8.
Neutrophiles function test
Neutrophils are the cells that are the type of phagocytes that assist in the innate immune system and together with monocytes are among the first to respond at the site of the injury or infection earliest responders to infection. Neutrophils help in disabling the invading microorganisms by digesting them and thus preventing infection9.
After 14 day’s treatment, the Differential Leukocyte Count (DLC) was determined (UB) from the blood taken from the retro-orbital plexus of each rat into heparin vials9. Once the counting was done, these blood samples along with 80 mg nylon fibers/ml (NFTB) were kept at 37°C for 15 min. After the incubation, the blood samples were analyzed again for differential and total leukocyte counts respectively to arrive at the neutrophil index. The neutrophil adhesion percentage was determined according to the formula given below:
Neutrophil adhesion % = NIu – NIt × 100/NIu.
Here, NIt is the neutrophil index of treated blood samples (NFTB) and NIu is the neutrophil index of untreated blood samples (UB)10.
Survival studies
Animals were orally given the extracts and the standard antibiotics on the zero-day; 1×108 viable E. coli was injected intraperitoneally (i.p.). In the first twenty hours, the survival of the rats was monitored on an hourly basis, and for the remaining period till 14 days, it was monitored daily basis11.
Bacterial studies
Blood samples of model rats used in the experiment were taken from their tail vein at the end of the experiment (the time when maximum mortality occurred) was used to determine the presence of E. coli in all groups12. The blood samples were serially diluted and spread on the nutrient agar in triplicate and the colonies were counted after 24 hrs of incubation at 37°C.12.
In-Vitro Antibacterial Study
To find out whether these plant extracts possess any antibacterial activity, different extracts were subjected to the agar well diffusion assay13. Following four bacterial strains obtained from American Type Culture Collection (ATCC) were used in the study. E. coli– ATCC- 25922, S. aureus– ATCC-23235, B. subtilis– ATCC 6051, S. typhi– ATCC-14028
Nutrient Agar Medium
Nutrient agar medium (HiMedia) was prepared by adding 28 g of dehydrated powder, in one liter of double-distilled water (DDW), and autoclaved; and the molten state of the medium was poured into 90mm diameter Petri plates (25-30 ml/plate).
Procedure
The methanolic extracts of the P. vulgaris were diluted and used at the concentration of 25, 50, 75, and 100 mg/ml. Petri plates containing nutrient agar media were prepared. Microbial suspension of the density of 1×108 CFU/ml of each of E. coli, S. aureus, B. subtilis, and S. typhi was used for spreading on the Petri plates containing the Nutrient agar media. Then, 4 wells of 5 mm depth and 6 mm diameter were made on the semi-solid agar in each Petri plate using a borer. Different concentrations of the plant extracts were prepared, and a positive control consisting of ofloxacin, 10 μg/ml, were dispensed (50 μl) in different wells. The inoculated plates were kept at room temperature for 1 hr. to allow the diffusion of the extract, and consequently, these plates were incubated for 24 hrs at 30°C. After incubation, the zones of inhibition around each well were measured with the help of Vernier caliper and the mean values of the diameter of zones of inhibition and the standard deviations were further calculated. All experiments were conducted in triplicates.
Statistical Analysis
All data was statistically analyzed through SPSS one-way ANOVA.
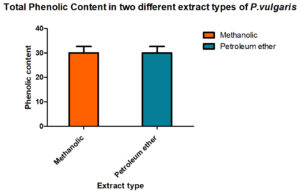
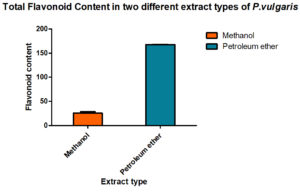
To assess its bioactivity, P. vulgaris methanol and petroleum ether extracts were prepared. All the standard tests were done to estimate the total content of phenolic, flavonoids compounds and the results are given in Fig. 1 & 2.
Fig. 2. Total flavonoid content (TFC) in petroleum ether and methanolic extract of P. vulgaris (PVME)
To find out the amount present, suitable standard curves were generated and quantities were derived out by comparing them with these curves. Interestingly, the methanol is better organic solvents as compared to petroleum ether while preparing the extracts of the P vulgaris, hence, when total phenolic content in P. vulgaris was compared in the two extracts, methanolic extracts showed a better yield of total phenolic content than found in the petroleum ether. Total flavonoids also showed the same trend.
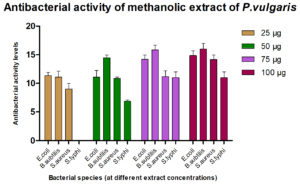
For assessing their antimicrobial efficacy, standard microbiological laboratory methods were employed, and standard growth media and reagents were used. The results of antibacterial action demonstrated by the plant extracts against the four following bacterial strains are given in Fig. 3.
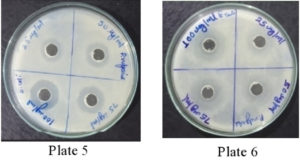
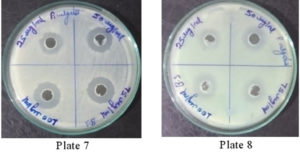
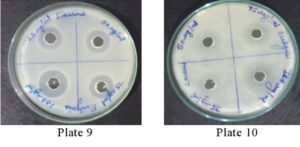
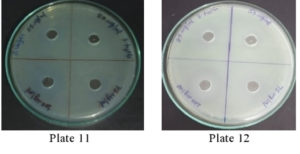
It was found that the methanolic extracts of P. vulgaris effectively restricted the growth of E. coli at conc. of 100 µg/ml Fig. 6 (Plate 5 & 6) while against B. subtilis the extracts were effective at a conc. of 50 µg/ml. Fig. 7 (Plates 7 & 8). Whereas the extracts against S. aureus were able to kill the bacteria at 100 µg/ml, Fig. 8 (Plates 9 & 10), showing lesser bioactivity compared to other mentioned. It was, however, interesting to note that the Salmonella typhi showed complete resistance towards methanolic extracts of P vulgaris Fig. 9 (Plates 11 & 12).
Fig. 7. Plate 7 & 8. Anti-Bacterial Activity of Methanolic Extract of P. vulgaris against B. subtilis
Fig. 8. Plate 9 & 10 Anti-Bacterial Activity of Methanolic Extract of P. vulgaris against S. aureus.
Fig. 9. Plate 11 & 12. Anti-Bacterial Activity of Methanolic Extract of P. vulgaris against S. typhi.
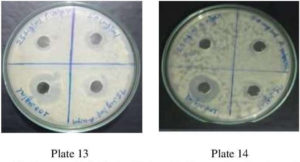
Fig. 11. Plate no. 13 & 14. Anti-Bacterial Activity ofMethanolic Extract P. vulgaris against A. niger.
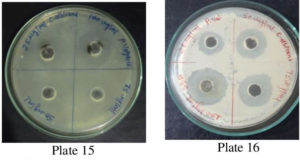
Fig. 12. Plate 15 &16. Anti-Bacterial Activity of Methanolic Extract P. vulgaris against C. albicans.
Anti-Bacterial Activity of Methanolic Extract of P. vulgaris
Standardantibacterial Agent (Ofloxacin10µ g/ml)
Name of the Organism 10 µg/ml
E. coli 22.25±0.500
B. subtilis 30.50±0.577
S. aureus 25.50±0.577
S. typhi 33.50±0.408
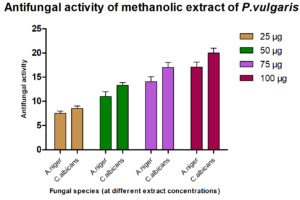
The antimicrobial ability of the methanolic extracts of P. vulgaris was also tested against the two fungal strains. The methanolic extract (ME) of P. vulgaris demonstrated significant clearing zones (anti-fungal activity) in the case of A. niger but the zones of inhibition were much more pronounced against C. albicans where the clearing started appearing at the 25 µg/ml but the maximum zone was observed at the conc. of 100 µg/ml of ME extract of P. vulgaris Fig.10-12 (Plates13-16).
Table (1):
Treatment groups for in vivo anti-bacterial study.
Group No. |
Treatment |
No of animals per group |
|---|---|---|
I |
Vehicle |
Six |
II |
Ofloxacin (10 mg/kg b/w) |
Six |
III |
PVME (100 mg/kg b/w) |
Six |
In vivo antibacterial study
Animal house condition
Animals in this experiment were selected randomly from the animal house and were segregated into various treatment groups randomly. The animals were maintained in a propylene-coated cage with sterile straw spread on the floor. The relative humidity of about 30.7 % at 22±2°C and 12hrs each of light and dark cycles were maintained in the animal house. These animals were fed using the standard pellets (Golden Feeds, New Delhi, India) and water.
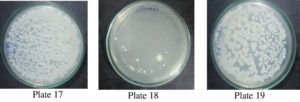
The bacterial clearance ability when compared with the allopathic ofloxacin antibiotic and P. vulgaris extract, it was revealed that even among Albino Wistar rats the standard ofloxacin is effective at 10 mg/kg body weight which is an expected result but the PVME also shows clearance activity though at higher concentration i.e., at 100 mg/kg BW Table 2 & Fig. 13 (Plates 17-19), confirmed significant in vitro results.
Table (2):
Bacterial clearance study.
Control |
Ofoxacin 10 mg/kg BW |
PV ME 100 mg/kg |
|---|---|---|
253.83±13.378 |
77.83± 6.177 |
111.83±4.262 |
In vivo Bacterial Clearance
E. coli-induced peritonitis, sampling of the tail blood at the end of the experiment (a time when maximum mortality occurs) was used to determine the presence of E. coli in all the groups by counting well separated bacterial colonies on the Petri plates. The blood samples thus collected were serially diluted and a measured amount was placed on Petri plates on the nutrient agar in triplicate and Petri plates were incubated at 37°C; thus produced colonies were then counted after 24 hrs. of incubation. The mortality recorded in the sample consisting of PVME at 100 mg/kg body weight was reduced to 60 % compared to 100 % mortality in control, suggesting that ME of P vulgaris showed a positive effect in reducing the mortality among E. coli induced peritonitis in laboratory-reared rats Fig. 13 (Plates 17, 18 and 19).
Table (3):
Neutrophils Functions (Phagocytic Index).
| Animals groups |
TLC (A) | Neutrophile % (B) | Neutrophile index (A×B) |
Neutrophile Adhesion percentage | |||
|---|---|---|---|---|---|---|---|
| UB | NFTB | UB | NFTB | UB | NFTB | ||
| Control | 7.90±0.441 | 7.31±0.545 | 23.59±1.212 | 21.50±1.460 | 186.72±17.965 | 156.92±13.645 | 15.69±6.107 |
| Standard | 8.59±0.514 | 6.686±0.503 | 26.99±0.648 | 24.64±0.799 | 231.93±14.986 | 168.80±11.068 | 26.80±8.713 |
| Sample PV ME 100 mg/kg | 8.13±0.547 | 7.45±0.589 | 26.16±1.272 | 21.67±0.387 | 212.47±11.375 | 161.37±12.256 | 23.98±5.551 |
UB = Untreated Blood; NFTB = Nylon Fiber Treated Blood.
Neutrophil Functions (Phagocytic Index) results are provided in Table 3. The neutrophil adhesion percentage was reduced in the sample of 100 mg/kg which was 5.55 as compared to the control and standard which showed 6.10 and 7.13% of neutrophile adhesion. Phagocytosis by neutrophils is taken as the main defense system against microbial infections in peritonitis. Phagocytic cells play a significant role in preventing sepsis by eliminating bacteria from the system. Therefore, effective clearing of bacteria is critical for host survival from infection. Here, the effect of ofloxacin at 10 mg/kg body weight was 6.177 while in the sample of PVME was 4.26. Statistically showing almost similar action but in the case of ofloxacin, expectedly, better results of clearance were observed at much lower concentration compared with the action shown by the MEPV.
Animals pre-handled with extracts and antibiotics on the 0 days, approximately 1×108 E. coli were injected intraperitoneally (i.p.), and Table 4 shows the results of survival rate after 14 days.
Table (4):
Survival Rate (14 days).
Groups |
Number of Deaths |
% of Mortality |
|---|---|---|
Control |
5 |
100 |
Standard drug |
1 |
20 |
Sample PV ME 100 mg/kg |
3 |
60 |
In this parameter, the mortality was observed for 14 days i.e., after the completion of the experiment mentioned in Table 4. A sampling of the tail blood was done at the end of the experiment (the time when maximum mortality occurred), and the appearance of well-separated bacterial colonies on the nutrient agar plates was counted to determine the presence of E. coli in all the groups.
In the Control group the number of dead rats was 5 and mortality was found to be 100%; the number of deaths in the group given the standard drug was only one and mortality was calculated to be 20%, but when the survival rate for the animal group treated with the sample polyvinyl methyl ether (PVME) 100 mg/kg was analyzed, the number of deaths was 3, and mortality was found to be 60%. These results of in vivo studies carried out with the Albino Wistar rats also further support the findings of the in vitro antimicrobial studies that the PVME is playing a role in eliminating pathogenic microbes from the host system and it could be inferred that the PVME possess abilities to restrict and contain the growth of microbes.
Although it has been amply demonstrated here that the PVME does possess anti-bacterial and anti-fungal traits and is capable not only to restrict the growth of both bacteria and fungi in vitro but also assists in eliminating the pathogens from the living systems as revealed by the in vivo anti-bacterial studies. It must be kept in mind that these results constitute preliminary studies and therefore these results must be subjected to further rigorous testing at a large scale and it is not surprising that it may lead to the discovery of some new antibacterial and antifungal molecules to be formulated by the pharmaceutical industry, and eventually the plant extracts may be added to the basket of antimicrobial compounds for the benefit of the human race.
The microbes are becoming resistant these days using many strategies. In many countries, antibiotics are not adequate, very expensive, and have side effects. Therefore, the present need is to find a solution to such a problem.
The present study attempts to investigate the antimicrobial activity of the Prunella vulgaris crude extracts because it synthesizes an enormous amount of secondary metabolites in the form of flavonoids, phenols, alkaloids, and tannins. P. Vulgaris is also rich in essential volatile oils that impart antioxidant and antimicrobial activity.
To assess their bioactivity, methanol and petroleum ether were selected as the extracting organic solvents. It is very surprising to note that the methanolic extract appeared as a better solvent among the two in terms of phenolic extraction. Total flavonoids also showed the same trend. All standard tests were performed to evaluate the presence and content of amino acids, carbohydrates, alkaloids, glycosides, saponins, triterpenoids, flavonoids, sterols, tannins, phenolic compounds, and oil and fats. To find out the amount present, suitable standard curves were generated and quantities were derived out by comparing them with these curves. . In one other study, chloroform extract had the highest phenolic content while flavonoid content was highest in the hydro alcoholic extract19.
The antimicrobial activity of the methanolic extracts was also tested against the two fungal strains. The ME of P. vulgaris demonstrated significant clearing zones in the case of A. niger but the zones of inhibition were much more pronounced against C. albicans where the clearing started appearing at the 25 µg/ml but the maximum zone was observed at the conc. of 100 µg/ml of ME extract of P. vulgaris Plate 23 26. Similarly, methanol extract had shown better inhibition than ethanol extract. But, A. niger had lower MIC than C.albicans18.
In general, out of the two types of extracts, ME was found to impart a higher protective effect as compared to petroleum ether that may be attributed to the higher amounts of phenolics compounds in ME.
Acute oral toxicity study of an extract of methanol of Prunella vulgaris was carried out according to OECD-423 guidelines in mice. Five mg/kg, 50 mg/kg, 300 mg/kg and 2000 mg/kg were used as dose range. The results of the methanolic extracts of P. vulgaris yielded identical reading suggesting that there was no lethality observed hence, showing no effect whatsoever.
Regarding E.coli induced peritonitis, sampling of the tail blood at the end of the experiment was used to determine E. coli bacteria in all groups. Samples were serially diluted and plated on Nutrient agar in triplicates and counted the Colony at 24 hrs after incubation at 30°C. The mortality recorded in the sample consisting of P. vulgaris ME at 100 mg/Kg weight was reduced to 60 % compared to 100 % mortality in control suggesting that ME of P vulgaris showed a positive effect in reducing the mortality of E. coli induced peritonitis in laboratory-reared rats. Our study is in line with Azaz et al. found that hydro alcoholic extract (250 mg/Kg) of P. vulgaris showed the best inhibition in growth of E. coli in rats, and C.albicans growth was reduced at a dose of 400 mg/kg19.
Clearance Test is given in Table 2, Neutrophils Functions (Phagocytic Index) results are provided in Table 3. The neutrophil adhesion percentage was reduced in the sample of 100 mg/kg which was 5.55 as compared to the control and standard which showed 6.10 and 7.13 percent of neutrophile adhesion. Phagocytosis by neutrophils is taken as the main defense system against microbial infections in peritonitis. Phagocytes play a crucial role in the prevention of sepsis by eliminating bacteria from the system. Therefore, effective clearing of bacteria is critical for host survival from infection.
P. vulgaris is widely distributed all across the world and grows wild; it constitutes a great repository of secondary metabolites with demonstrated therapeutic ability, and is available at relatively economical prices. In vitro results revealed that the methanolic extracts of Prunella vulgaris (MEPV) possess substantial antimicrobial activity both against bacteria and fungi. Under in vivo studies, it was confirmed that MEPV showed a positive effect in reducing the mortality of E. coli-induced peritonitis in laboratory-reared rats. Statistically showing almost similar action but in the case of ofloxacin, better results of clearance were observed at much lower concentration compared with the action shown by the MEPV. This investigation is unique in the sense that it evaluates the antimicrobial activity of the MEPV in both in vitro and in vivo and reports that the MEPV extracts restricted the growth of pathogenic bacteria to an acceptable extent not only in Petri plates but also in living animals, consequently, this report might constitute a pioneering study.
The results are although preliminary but support the existing scientific reports. The authors strongly recommend that since the plant extracts have the proven potential of antibiotic activity, an exhaustive study at a much greater scale may be carried out worldwide under the strict control of either reputed research laboratories or by the pharmaceutical industries to precisely evaluate and assess the extent of bioactivity contained in the crude extracts of at least some selected plants that may have demonstrated a track record of antimicrobial potential.
ACKNOWLEDGMENTS
The authors are grateful to Dr. M.P. Dobhal and Prof. Arif Ali for critical assessment / review and English editing of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This study was carried out in accordance with the recommendations of Institutional Animal Ethics Committee (IAEC) of Pinnacle Biomedical Research Institute (PBRI), Bhopal (Reg. No. 1824/PO/ERe/S/15/CPCSEA). The protocol was approved by the Institute Animal Care and Use Committee (NIH) under the protocol number PBRI/ IAEC/PN-18032.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Renisheya JJ, Malar T, Johnson M, Mary UM, Arthy A Antibacterial activities of ethanolic extracts of selected medicinal plants against human pathogens. Asian Pac J Trop Biomed. 2011;1(1):S76-S78.
Crossref - Imtiaz B, Fozia, Waheed A, et al. Antimicrobial activity of Malva neglecta and Nasturtium microphyllum. Int J Res Ayurveda Pharm. 2012;3(6):808-810.
Crossref - Lamaison JL, Petitjean-Freyet C, Carnat A. Medicinal Lamiaceae with antioxidant properties, a potential source of rosmarinic acid. Pharm Acta Helv. 1991;66(7):185-188
- Valdes-Sotomayor J, Cirugeda A, Bajo MA, et al. Increased severity of Escherichia coli peritonitis in peritoneal dialysis patients independent of changes in in vitro antimicrobial susceptibility testing. Perit Dial Int. 2003;23(5):450-455.
Crossref - Ingle KP, Deshmukh AG, Padole DA, Dudhare MS, Moharil MP, Khelurkar VC. Phytochemicals: Extraction methods, identification, and detection of bioactive compounds from plant extracts. J Pharmacogn Phytochem. 2017;6:32-36.
- Prior R, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53(10):4290-4302.
Crossref - Yeabyo S, Teka MZ, Gopalakrishnan VK, Kamalakararao K, Muthulingam M, Chaithanya KK. Immuno protective potential of Sida schimperiana chloroform root extract against E. coli 018: K1 induced peritonitis in albino Wistar rats. Res J Pharm Technol. 2021;14(4):2262-2269.
Crossref - Teixeira-da-Cunha MG, Gomes RN, Roehrs N, et al. Bacterial clearance is improved in septic mice by platelet-activating factor-acetylhydrolase (PAF-AH) administration. PloS One. 2013;8(9):e74567.
Crossref - Ghule BV, Yeole PG. In vitro and in vivo immunomodulatory activities of iridoids fraction from Barleriaprionitis Linn. J Ethnopharmacol. 2012;141(1):424-431.
Crossref - Patel P, Asdaq SM. Immunomodulatory activity of methanolic fruit extract of Aeglemarmelos in experimental animals. Saudi Pharmaceutical Journal. 2010;18(3):161-165.
Crossref - Cloutier S, Wahl K, Baker C, Newberry RC. The social buffering effect of playful handling on responses to repeated intraperitoneal injections in laboratory rats. J Am Assoc Lab Anim Sci. 2014;53(2):168-173.
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1(2):87-93.
Crossref - Daoud A, Malika D, Bakari S, et al. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arabian Journal of Chemistry. 2019;12(8):3075-3086.
Crossref - Maurya S, Singh D. Quantitative analysis of total phenolic content in Adhatodavasica Nees extracts. Int J Pharmtech Res. 2010;2(4):2403-2406.
- Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolke RH. Manual of Clinical Microbiology, 6th edition. ASM, Washington, DC. 1995.
- Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz J Microbiol. 2000;31(4):247-256.
Crossref - Khadagwanshi D, Trivedi S, Patel P, Jha M. Effect of Phaseolus vulgaris on E. coli induced peritonitis and bacteremia in mice. J Drug Delivery and Therapeutics. 2019;9(4-s):310-314.
Crossref - Mahboubi M, Mahboubi A, Kazempouur N. The antimicrobial activity of Prunella vulgaris extracts.Herba Polonica. 2015;61(1):31-38.
Crossref - NayaA, Khan MA, Sharma P, Mishra RM. Phytochemical screening, antioxidant and antimicrobial activities of Prunella vulgaris for oral thrush. Journal of Drug Delivery &Therapeutics. 2018;8(5-s): 251-258.
Crossref - Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry. 2002;50(10):3010–3014.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.