ISSN: 0973-7510
E-ISSN: 2581-690X
L-asparaginase is an extremely demanding biocatalyst that is employed to treat acute lymphoblastic leukemia (ALL) and lessen the development of acrylamide in fried food products. Here, an endophytic F. oxysporum MJS2, obtained from the stem tissues of M. jalapa was evaluated for the synthesis of L-asparaginase. The fermentation conditions were optimized through One Variable At a Time (OVAT) joined with a Central Composite Design (CCD) for the maximum production of enzymes. A 2.32-fold increase in the enzyme action was detected in the post-optimized condition (32.47 U mL-1) in a fermentation condition of pH 7, incubation temperature of 37 °C, and 120 hours of incubation time, glucose (5 gL-1), ammonium sulphate (7 gL-1), and NaCl were the best options for L-asparaginase synthesis by the endophyte. Crude enzyme was dialyzed and purified using Sephadex G-100 column chromatography with a molecular weight of 35 kDa determined through SDS-PAGE. MJS2-derived L-asparaginase acts optimum at a substrate concentration of 50 mM. Endophytic fungi MJS2 could be pharmaceutically exploited to produce L-asparaginase and open up new horizons in the biotechnological aspects of endophytes of common Indian Medicinal plants.
Mirabilis jalapa, Endophytic Fungi, Fusarium oxysporum, L-asparaginase
The enzyme L-asparaginase, denoted as L-asparagine aminohydrolase EC 3.5.1.1, is an essential material in the therapeutic process of pediatric acute lymphoblastic leukemia (ALL) and lymphosarcoma cancer, as it hydrolysis asparagine and glutamine into aspartic acid and ammonia.1 Interest in L-asparaginase has grown considerably since this enzyme was found to have anti-tumor activity.2 This enzyme has anti-leukemic activity in humans. It is mainly found in a diverse array of organisms, i.e., bacteria, fungi, plants, and in the serum of certain rodents but not revealed in humans. However, microbial sources are always preferred from an industrial perspective due to their economic feasibility, high consistency and stability, ease of changing the fermentation parameters, and optimization. Soil microbial pools have previously been screened extensively for L-asparaginase production; nowadays, the focus has been slowly shifted to a new type of microbial organisms, called endophytes. They are the hidden microbes that remain within inside the host plant and facilitate overall development of host under stressful situations. Also, they are popular because of their biotechnological potential, which can be exploited in the field of pharmaceuticals.
In therapeutics, L-asparaginase acts as an anti-proliferative agent. Malignant cells have a high requirement of L-asparagine in comparison to non-malignant ones for abnormal cell division and protein synthesis. The synthesis of L-asparagine depends on the availability of L-asparagine synthetase, which is lacking by the tumor cells. So, they depend on outside sources of L-asparagine.3 L-asparaginase dissociates L-asparagine to ammonia and aspartic acid in the patient’s blood and reduces the availability of this enzyme for cancer cells in ALL patients.4 Also, in food processing, this enzyme blocks the production of acrylamide, which is generated at high temperatures during frying or baking starchy food items. Thus, the enzyme is in high demand, and to date, only two native (Escherichia coli and Erwinia carotovora) and one pegylated E. coli L-asparaginase preparation are clinically approved and commercially available as a chemotherapeutic component of ALL and in the food processing industry.5 L-asparaginase has a global market value of 380 million USD in 2017, which is estimated to escalate to 420 million USD by 2025.6
Bacterial L-asparaginase is classified into two types: type I and type II. Type I L-asparaginases are found in the cytoplasm and aid in the catalysis of both L-asparagine and L-glutamine; type II L-asparaginases are found anaerobically inside the periplasmic region of the bacterial membranes and specifically linked to L-asparagine hydrolysis. Actually, as per protein databases of National Centre for Biotechnology Information (NCBI), L-asparaginase synthesizing genetic signals are majorly (95.5%) detected among the bacterial members (i.e., Kingdom Monera) whereas fungi, animal, plant, archaea, and virus accounts for 1.68%, 1.25%, 0.24%, 0.88%, <0.01% respectively.7 That’s why bacterial L-asparaginases have been studied vividly, and some are (Erwinia chrysanthemi, Erwinia carotovora, and Escherichia coli) being commercially used for therapeutic purposes, i.e., treatment of ALL.8 They come with several side effects, i.e., hypersensitivity, allergic responses, anaphylactic shocks, antigenicity, etc.9-11 So, potent options are of urgent need and fungi, be it soil or endophyte, are required for this purpose. There are some reports of fungi and endophytes as potent L-asparaginase producers. Different species of Aspergillus, Penicillium, Fusarium, Trichoderma, and Cladosporium isolated from various terrestrial and rhizospheric habitats are reported to produce L-asparaginase with cytotoxic and antioxidative abilities.12-15 These fungi-derived L-asparaginases are mainly used in the food industry for the removal/reduction of acrylamide formation, but are not used as a cytotoxic agent. Though these are considered in the Generally Recognised as Safe (GRAS) category, and some are commercialized as PreventASe, Acrylaway by DSM, and Novozymes, respectively, but issues related to stability during food processing, proteolytic action, allergic reaction, or toxicity in post-consumption conditions are vital drawbacks.16-18 That’s why potent alternatives are in high demand, and endophytic fungi are major candidates for this. As endophytes are a unique group of microorganisms that promote plant health in biotic/abiotic stress situations, and produce a variety of bioactive components due to their gene sharing phenomenon with their host, they are interesting candidates for the exploration of L-asparaginase production. Recently, endophytic fungi have caught attention for the production, optimization, characterization, and bioactivity assessment related to L-asparaginase, and hence, more research is needed to obtain novel enzymes with strong pharmaceutical utilities.19-21 Endophytic fungi Colletotrichum sydowii, Fusarium sporotrichioides, Fusarium solani, Cercospora canescens, Penicillium crustosum, Penicillium pancosmium, Phoma sp., Penicillium ubiquetum, Septoria sp., and Penicillium commune isolated from Prunus Africana, Periploca linearifolia are effective producers of L-asparaginase in optimized media compositions.22 Endophytes Phyllosticta catimbauensis URM 7672, Cladosporium sp., Plectosphaerella sp., Fusarium sp., Stemphylium sp., Septoria sp., Alternaria sp., Didymella sp., Phoma sp., Chaetosphaeronema sp., Sarocladium sp., Nemania sp., Epicoccum sp., Ulocladium sp., Chaetomium sp., and Cladosporium sp., from Mandevilla catimbauensis, Matricaria chamomilla, Matricaria parthenium, Anthemis triumfetii, Anthemis altissima, Achillea millefolium, Achillea filipendulina and Cichorium intybus, Tinospora cordifolia.23-26
Medicinal plants are known to harbour potent endophytic fungi, and here, the focus has been given to a common Indian plant of the Nyctaginaceae family-Mirabilis jalapa.27 Ethnomedicinally, it has numerous uses and is being used by the locals/tribals.28 It has a diverse phytochemical profile and serves a variety of pharmacological attributes.29 It is known for its strong antioxidative, cytotoxic, and antimicrobial activity.30-33 Methanolic seed extracts of this plant are known to exhibit cytotoxic action.33,34 Watthanachaiyingcharoen et al. reported anti-cancerous/apoptosis proteins from this plant, too.35 As this plant has such a well-known cytotoxic, antioxidative activity, we studied it for its endophytic fungal association. As endophytes share valuable genes with their hosts, some anticancer activity-related components may be shared between them, and the isolates may produce L-asparaginase, as a tool to tackle the growth of cancer cells. To the best of our knowledge, no such study related to this plant’s endophytes from this particular geography focusing on the L-asparaginase has been performed. Few reports on endophytic bacteria, and endophytic fungi from M. jalapa are available.36,37
In the present investigation, fungal endophytes from Mirabilis jalapa have been screened for their L-asparaginase activity. The potent isolate was identified as Fusarium oxysporum MJS2, and the enzyme action was optimized through Central Composite Design (CCD) analysis. The obtained parameters, such as pH 6.8, incubation temperature of 37 °C, fermentation time of 30 minutes, glucose (as a sugar source), and ammonium sulphate (as a protein source) were detected as the most optimum ones. Biochemical characterization of the enzyme reveals that a substrate concentration (asparagine monohydrate) of 50 mM is highly effective in maximum enzymatic action. Also, the enzyme was purified, and the approximate molecular weight was determined as 35 kDa. This outcome potentially indicates the pharmaceutical utility of fungal metabolites of endophytic origin, which opens up new biological sources for L-asparaginase production.
Isolation of fungal endophytes
Healthy plants of M. jalapa were collected from Gopegarh village of West Midnapore district to study the fungal endophytes. Explants (i.e., stem and leaf) were surface sterilized in clean water and then using 5% NaOCl and 75% ethanol for 1 minute. Sterilized plant parts were placed in 2% water agar media, and placed at 28 ± 2 °C for 3-4 days. Upon emergence of fungal hyphae from the explants they are placed to potato dextrose agar (PDA) media and cultured at 28 ± 2 °C for 2-4 weeks.
Evaluation of fungal endophytes for L-asparaginase action
Qualitative screening
Fungal isolates were inoculated on modified Czapek dox (MCD) media with an initial pH of 6.2 and mixed with 0.09% phenol red to screen their L-asparaginase action, adopting the standard methodologies of Gulati et al.38 The plates were cultured at 38 °C for 3-5 days in a BOD shaker.
Quantitative estimation
The isolates with potent enzymatic activity were further estimated from broth culture following the standard procedure. The isolates with the highest enzymatic activity were next quantitatively estimated. Out of the different endophytic fungi, MJS2 exhibited an enzyme production zone of 3 cm and 5.8 cm upon 3 and 5 days of incubation, whereas the other isolates, MJS1, MJS4, MJS9, and MJS11, had a production of 1.85-2.9 and 2.65-3.9 cm respectively, which is almost 25-50% less than MJS2. Also, MJS2 produced a larger zone (diameter) of L-asparaginase enzyme, even larger than the growth zone of MJS2, which signifies its ability to synthesise larger quantities of L-asparaginase. Hence, it was further studied. The isolate was first grown on 50 mL MCD broth for 5 days with an agitation of 120 rpm. 2 mL of the culture filtrate was collected in a centrifuge tube after 24 h consecutively and stored at -4 °C for up to 5 days. The raw protein was spined at 13000 rpm for 10 min at 4 °C after proper incubation. Then 100 µL of supernatant was taken in a 2 mL Eppendorf tube, in which 100 µL of Tris HCl at pH 7.2, 200 µL of 0.04 M L-asparagine and 100 µL of ddH2O were added. After an hour of incubation at 37 °C, 100 µL of 1.5 M Trichloroacetic Acid (TCA) was included to cease the enzymatic action. Then 100 µL of the mixture was added to the 750 µL sterile distilled water and 300 µL of Nessler reagents and incubated for 20 min at 28 ± 2 °C as described by Kumar et al.39 Post-incubation, the enzymatic action was evaluated by measuring the absorbance at 450 nm of wavelength.40
Identification of the potent isolate
The most potent enzyme producer (fungal isolate MJS2) was first studied through plate morphology, then microscopically, and finally, identification was confirmed through Internal Transcribed Spacer (ITS) sequencing. The obtained sequence was submitted to the National Centre for Biotechnology Information GenBank database, and a phylogenetic tree was constructed using MEGA 10 software after aligning sequences with more than 95% similarity from the BLAST search result.41
Optimization of fermentation conditions of MJS2 regarding L-asparaginase synthesis
The most potent enzyme producer, MJS2, was subjected to optimization regarding maximum synthesis of L-asparaginase using the OVAT method. It was inoculated on modified Czapek dox media added with different sugar sources (glucose, maltose, sucrose, mannitol, xylose, and starch) and nitrogen sources (ammonium sulphate, ammonium nitrate, and ammonium chloride). The medium temperature, pH, and fermentation period were optimized by calculating the enzymatic activity. After evaluating how the concentration of supplementary carbon source affected the enzyme activity, the impact of supplementary nitrogen source and its concentration were examined. The impact of the fermentation parameter on the substrate concentration was also determined by adding different substrate (L-asparagine) strengths, i.e., 5, 7.5, 10, 12.5, 15 g/L.
Further optimization was done by adopting Response Surface Methodology (RSM); specifically, Central Composite Design (CCD) using the results obtained from OVAT. The three most influencing parameters-concentration of glucose, ammonium sulphate, and L-asparagine were utilized. A set of 20 experiments was performed; eight factorial, six axial, and six centrals were conducted in a random sequence with 6 replicates at the center point to calculate the pure error of the model. The CCD results were placed to the second-order polynomial equation:
Y = a0 + a1 × A + a2 × B + a3 × C +a12 × A × B + a13.A × C + a23 × B × C + a11 × A2 + a22 × B2 + a33 × C2
Here, Y is denoted as the L-asparaginase activity (U mL-1); A, B, and C represent the coded value of glucose, ammonium sulphate, and L-asparagine concentration. a0 is a constant, a1, a2 and a3 are linear, a11, a22 and a33 are squared, a12, a13 & a23 are interaction parameters respectively. CCD plots are prepared for the clear elucidation of different interactions involved among the three fermentation parameters.
Biochemical characterization of L-asparaginase produced by MJS2
Impact of L-asparagine concentration
To determine the most appropriate substrate concentration; various strengths of asparagine monohydrate (10 to 100 mM) were used. They were mixed with the semi-purified L-asp and the reaction ran for 30 min at 37 °C temperature, and at a pH of 6.8.
Effect of temperature
The stability of the semi-purified enzyme was assessed at various temperatures (20-60 °C) through incubation, and the residual activity was evaluated using a spectrophotometer at 450 nm.
Effect of pH
Enzymatic activity was determined in different pH ranges (5-11) at a time interval of 30 min under optimum conditions and measured at 450 nm.
Influence of salts, chelating agents, and other additives
The L-asparaginase enzyme activity was checked in the presence of a 10 mM concentration of different metal ions, chelating agents, additives, and inhibitors. The 10 mM concentration of Fe2+, Na+, Mg2+, K+, Cu2+, Zn2+, Mn2+, Cd2+, Pb2+, Hg2+, Ag, Ba2+, Ca2+, Sn2+, urea, PEG, and EDTA were checked on L-asparaginase action by adding the same concentration on the purified enzyme and keeping it at 37 °C for 30 mins. The activity was calculated and recorded in comparison to the control (absence of any metal ion).
Partial purification of MJS2 produced L-asparaginase
Organism was inoculated in MCD broth for 5 days of incubation period under optimum conditions and the crude enzyme was filtered. The purification step is performed at 4 °C. Culture extract was filtrated at 6000 rpm for 15 min, collected the supernatant with extracellular enzyme that was treated with 80% saturated ammonium sulphate solution. Continuous overnight stirring was provided and the precipitated protein was dissolved in 50 mM Tris-HCl buffer (pH 7.5) to dialyze against the same buffer solution for a day to remove the salt.
Gel filtration chromatography
The dialyzed protein was run over a Sephadex G-100 column (2.5 × 60 cm) that had been pre-equilibrated with Tris-HCl buffer of 50 mM concentration at pH 7.5, to eliminate any excess ammonium sulphate. Then, elution of the protein was done in the same buffer and a fraction was collected and stored at 4 °C.
Electrophoretic separation of L-asparaginase
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to elucidate the structure of L-asp.
Statistical Information
Response Surface Quadratic Evaluation based optimisation of the L-asparaginase production has been optimised using Design Expert Equipment (Stat-Ease, Minneapolis, USA) freely available on the internet (trial version). The statistical differences between the data presented in the Figure 3 and 5 are calculated in Prism Graph Pad (version 8.4.3.).
Elucidation of the L-asparaginase-synthesising fungal endophyte
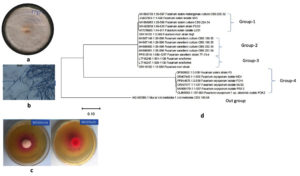
Screening for L-asparaginase production, i.e., both qualitative and quantitative was done using the isolated fungi. The formation of a pink zone is a clear indication of a positive response in the plate assay due to the presence of phenol red. Among all the isolates, MJS2 (Figure 1a, b) exhibited the highest zone diameter in the plate assay method (Figure 1c). In qualitative estimation, the asparaginase action of MJS2 was 13.97 U mL-1, which was identified as Fusarium oxysporum based on the ITS-based molecular method (Figure 1d). Endophytic F. oxysporum isolated from the medicinal plant Murraya koenigii is known to exhibit L-asparaginase action of 0.013 µM mL-1 min-1.42 Meanwhile, other Fusarium species such as F. solani, and F. proliferatum with asparaginase enzyme activity of 3.58 IU mL-1 and 16.75 IU mL-1 have also been reported from Withania somnifera and Cymbopogon citratus, respectively.43,44 Ashok et al. suggested that Trichosporon asahii IBBLA1, isolated from arctic soil, showed the L-asparaginase activity of 20.57 U mL-1 at an optimum condition of 30 °C and pH 7.45 Chakraborty and Shivakumar reported about the L-glutaminase and urease-free L-asparaginase producer, Ganoderma australe GPC191 which, showed enzyme action of 1.57 U mL-1.46 The study of de Andrade et al. reported about the glutaminase and urease free L-asparaginase enzyme activity of many microbes as Aspergillus, Diaporthe, Xylaria, Epicoccum, and Trametes, obtained from Antarctic mosses.47 It is seen that our presently studied isolate produces L-asparaginase higher than the contemporary ones. An isolate of Fusarium, i.e., F. equiseti, has produced an enzyme of 40.78 U/mL, which is somewhat near to our presently reported one (32.47 U/mL), but to the best of our knowledge, F. oxysporum has not been reported for such an activity earlier.
Figure 1. Plate (a) and microscopic (b) morphology of MJS2 along with its L-asparaginase producing ability (c) and phylogenetic position (d)
Fusarium oxysporum has been reported as an endophytic fungus by several researchers from various types of plants48 and is known to synthesise a variety of bioactive metabolites.49,50 Though they are potent plant pathogens but not all the strains are virulent, and F. oxysporum, isolated from Catharanthus roseus, is known to produce anticancer drugs vincristine and vinblastine.51 Different anticancer compounds i.e., oxysporidinone analogue, 3-hydroxyl-2-piperidinone derivative, -4,62 -anhydrooxysporidinone, (+)-fusarinolic acid, gibepyrone D, beauvercin, cerevisterol, fusaruside, and (2S,22 R,3R,32 E,4E,8E)-1-O-Dglucopyranosyl-2-N-(22-hydroxy-32-octadecenoyl)-3-hydroxy-9-methyl-4,8-sphingadienine, obtained from F. oxysporum, an isolate of Cinnamomum kanehirae, exhibited a cytotoxicity against PC-3, PANC-1, and A549 cell lines with an IC50 dosage of 10.4 ± 1.6-49.5 ± 3.8 µM.52 Kour et al. isolated podophyllotoxin (a precursor of anticancer medicine-etoposide, teniposide and etopophose phosphate) from F. oxysporum, an endophyte of Juniperus recurva.53 Not only in the pharmaceutical sector, but also F. oxysporum is effective in controlling weeds in wheat crop fields and stimulating wheat growth.54 So, in the present investigation, the F. oxysporum MJS2-derived L-asparaginase will not be a safety issue.
Precipitation of crude L-asparaginase using ammonium sulphate
The initial stage in the L-asparaginase purification was precipitating the crude enzyme with ammonium sulphate.55 It was noted that 80% of ammonium sulphate showed the highest recovery of the L-asparaginase enzyme. The highest enzymatic activity was 56.69 U mg-1 after 80% fractional precipitation following the gel filtration technique. There are several techniques related to the precipitation and purification of the enzyme L-asparaginase, of which ammonium sulphate precipitation is the most popular one and adopted by the majority of the researchers.56-58 Methanol-based precipitation is performed for L-asparaginase from Cladosporium species, which has a lower yield than the former.12
Characterization of MJS2-produced L-asparaginase
Evaluation of the molecular weight (MW)
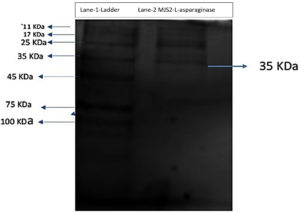
A pre-stained protein ladder (HIMedia Laboratories MW 11-245) was used to compare the MW of the purified L-asp. Figure 2 shows the SDS-PAGE of the purified L-asparaginase, showing one protein band, and the approximate molecular weight was 35 KDa. The results are comparable to those of purified Lactobacillus casei subsp. casei ATCC 393, which had a molecular weight of 35 kDa.59 The study of Yap et al. examined the extracellular L-asparaginase-producing activity of an endophytic fungus Colletotrichum gloeosporioides that consists of monomeric units of 25 kDa.60 An endophytic fungus Lasidiplodia theobromae, which can produce asparaginase with a molecular weight of 70 kDa, has been shown to have antileukemic activity.61 Jenila et al. identified L-asparaginase enzyme-producing endophytic species of Fusarium sp. with a molecular weight of 66 kDa.62 Arredondo-Nunez et al. reported that the MW of type-II L-asp from halotolerant Bacillus subtilis was 38 kDa in the SDS-PAGE study.63 Darwesh et al. reported that the recombinant L-asp isolated from Burkholderia pseudomallei had a MW of 33.6 kDa in SDS-PAGE.64 Alongside, researchers screened Trichoderma species obtained from soil and agricultural waste for their ability to produce antileukemic L-asparaginase, which revealed that the purified enzyme had molecular weights of 69.3 kDa and 72 kDa, respectively.65 All these studies unveiled the different ranges in molecular weight of L-asp from distinct microorganisms. Actually, L-asparaginase enzyme is a dimer of dimers, highlighting the anticancer activity related to four active sites found at the interface of two subunits.66 The molecular weight of L-asparaginase was found to differ based on the enzyme’s source.
Influence of different culture compounds on L-asparaginase action
Enzymatic change of L-asparagine monohydrate to the corresponding aspartate and ammonia are among the most interesting bio-transformations. Therefore, L-asparaginase production needs to be optimized using a quick and effective methodology. Reports state that enzyme production could be increased severalfold by a statistics-based experimental design. Culture conditions and concentration parameters during fermentation at the shake flask level have shown a gradual increase in enzyme activity. The statistical experimental design allows examining numerous variables at the same time, making it faster and more cost-effective.
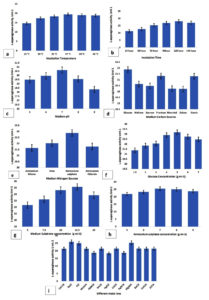
This present study showed that endophytic isolate MJS2 exhibits the highest enzyme production of 32.47 ± U mL-1 at a fermentation time of 120 h, 37 °C of fermentation temperature, and an initial medium pH of 7. The effect of various sugar and protein sources on L-asparaginase synthesis was evaluated (Table 1). The growth of the studied endophytic fungi MJS2 was assessed by the estimation of biomass and is represented in Table 1. The biomass development (i.e., growth of the fungus) is mostly associated with L-asparaginase activity of the isolate, and a higher production of L-asparaginase is also accompanied by increased biomass. 120 hours of fermentation time has a biomass output of 1.35 ± 0.04 g/L and enzymatic activity of 18.05 ± 0.03 U/mL and at 37 °C of incubation temperature 1.39 ± 0.01 g/L of biomass is recorded with a L-asparaginase action of 19.39 ± 0.04 U/mL. This same trend has been followed for other parameters also, i.e., at glucose concentration of 2 g/L (1.41 ± 0.09 g/L and 21.87 ± 0.16 U/mL), ammonium sulphate of 6 g/L (1.49 ± 0.06 g/L and 22.87 ± 0.03 U/mL). The increase in enzyme production is simultaneous with the biomass generation in all the cases.
Table (1):
Optimization of fermentation conditions regarding L-asparaginase production from MJS2
| Factors Evaluated | Values of the Factors | The concen. of the components (gL-1) | Biomass (g/L) | L-asparaginase activity (U mL-1) |
|---|---|---|---|---|
| Incubation Time (hours) | 24 | – | 0.9 ± 0.01 | 11.23 ± 0.02 |
| 48 | – | 1.12 ± 0.02 | 12.65 ± 0.05 | |
| 72 | – | 1.15 ± 0.03 | 15.31 ± 0.14 | |
| 96 | – | 1.28 ± 0.05 | 16.88 ± 0.13 | |
| 120 | – | 1.35 ± 0.04 | 18.05 ± 0.03 | |
| 144 | – | 1.31 ± 0.03 | 17.02 ± 0.08 | |
| Incubation temp. (°C) | 25 | – | 1.11 ± 0.02 | 14.63 ± 0.06 |
| 30 | – | 1.17 ± 0.01 | 17.32 ± 0.05 | |
| 35 | – | 1.19 ± 0.03 | 18.34 ± 0.06 | |
| 37 | – | 1.39 ± 0.01 | 19.39 ± 0.04 | |
| 39 | – | 1.30 ± 0.01 | 19.02 ± 0.07 | |
| 40 | – | 1.28 ± 0.04 | 18.81 ± 0.06 | |
| Medium pH | 5 | – | 1.20 ± 0.03 | 19.48 ± 0.05 |
| 6 | – | 1.22 ± 0.04 | 19.94 ± 0.10 | |
| 7 | – | 1.34 ± 0.05 | 20.63 ± 0.14 | |
| 8 | – | 1.30 ± 0.04 | 19.56 ± 0.12 | |
| 9 | – | 1.27 ± 0.03 | 18.32 ± 0.10 | |
| Medium Carbon Sources | Glucose | 2.0 | 1.41 ± 0.09 | 21.87 ± 0.16 |
| Maltose | 2.0 | 1.40 ± 0.04 | 20.64 ± 0.12 | |
| Sucrose | 2.0 | 1.38 ± 0.03 | 20.48 ± 0.07 | |
| Fructose | 2.0 | 1.37 ± 0.01 | 21.32 ± 0.10 | |
| Mannitol | 2.0 | 1.30 ± 0.02 | 20.25 ± 0.12 | |
| Xylose | 2.0 | 1.22 ± 0.04 | 20.22 ± 0.11 | |
| Starch | 2.0 | 1.28 ± 0.02 | 21.30 ± 0.13 | |
| Medium Nitrogen Sources | Ammonium Nitrate | 6.0 | 1.40 ± 0.03 | 21.63 ± 0.04 |
| Urea | 6.0 | 1.41 ± 0.04 | 22.02 ± 0.07 | |
| Ammonium sulphate | 6.0 | 1.49 ± 0.06 | 22.87 ± 0.03 | |
| Ammonium Chloride | 6.0 | 1.42 ± 0.04 | 21.75 ± 0.02 | |
| Glucose Concen. | Glucose | 1.5 | 1.47 ± 0.04 | 21.37 ± 0.06 |
| 2 | 1.50 ± 0.05 | 21.83 ± 0.07 | ||
| 3 | 1.50 ± 0.04 | 22.05 ± 0.12 | ||
| 4 | 1.49 ± 0.03 | 22.89 ± 0.02 | ||
| 5 | 1.55 ± 0.04 | 23.18 ± 0.02 | ||
| 6 | 1.54 ± 0.03 | 22.74 ± 0.03 | ||
| 7 | 1.53 ± 0.02 | 22.46 ± 0.08 | ||
| Medium Substrate Concen. | L-asparagine monohydrate | 5 | 1.55 ± 0.05 | 22.32 ± 0.08 |
| 7.5 | 1.57 ± 0.04 | 23.21 ± 0.03 | ||
| 10 | 1.58 ± 0.02 | 24.63 ± 0.05 | ||
| 12.5 | 1.62 ± 0.03 | 25.13 ± 0.02 | ||
| 15 | 1.58 ± 0.04 | 23.85 ± 0.02 | ||
| Ammonium Sulphate Concen. | (NH4)2SO4 | 5 | 1.54 ± 0.01 | 21.87 ± 0.06 |
| 6 | 1.54 ± 0.02 | 23.28 ± 0.05 | ||
| 7 | 1.69 ± 0.03 | 25.48 ± 0.06 | ||
| 8 | 1.60 ± 0.04 | 25.01 ± 0.12 | ||
| 9 | 1.51 ± 0.04 | 23.76 ± 0.10 | ||
| Different Metal ions | CaCO3 | 0.1 | 1.40 ± 0.03 | 21.38 ± 0.08 |
| NaCl | 0.1 | 1.39 ± 0.07 | 25.54 ± 0.03 | |
| KCl | 0.1 | 1.32 ± 0.04 | 25.62 ± 0.04 | |
| MnSO4 | 0.1 | 1.30 ± 0.03 | 21.41 ± 0.03 | |
| PbNO3 | 0.1 | 1.29 ± 0.01 | 18.76 ± 0.02 | |
| SnCl2 | 0.1 | 1.40 ± 0.01 | 21.45 ± 0.02 | |
| HgCl2 | 0.1 | 1.21 ± 0.00 | 18.34 ± 0.03 | |
| CaCl2 | 0.1 | 1.31 ± 0.01 | 21.48 ± 0.07 | |
| AgNO3 | 0.1 | 1.32 ± 0.02 | 18.73 ± 0.04 | |
| MgSO4 | 0.1 | 1.32 ± 0.02 | 25.32 ± 0.07 | |
| BaCl2 | 0.1 | 1.33 ± 0.04 | 21.49 ± 0.14 | |
| CuSO4 | 0.1 | 1.37 ± 0.00 | 21.45 ± 0.09 | |
| EDTA | 0.1 | 1.39 ± 0.01 | 21.45 ± 0.12 |
This observation is related to the result of Moubasher et al., who reported that the most suitable temperature is 37 °C for the purified asparaginase enzyme of Lasidiplodia theobromae.61 Furthermore, recent studies by Luhana and Bariya stated the optimum growth situations for the L-asparaginase action by Trichoderma viridae were 37 °C and a medium pH of 8.0. They also showed the influence of different metal ion concentrations, such as zinc, copper, iron, and manganese, and how they affected enzyme production under control conditions.65 Glucose and ammonium sulphate at values of 5 gL-1 and 7 gL-1 maximally influenced the production more than the other tested sources, e.g., maltose, sucrose, fructose, mannitol, xylose, starch, ammonium nitrate, urea, and ammonium chloride. Medium substrate concentration (L-asparagine monohydrate) at a value of 12.5 gL-1 yielded a prominent synthesis (25.54 ± 0.06 UmL-1) of L-asparaginase. Jenila and Gnanadoss also reported glucose and ammonium sulphate as the best carbon and nitrogen sources at a concentration of 3 gL-1 and 20 gL-1, respectively, for the highest production of L-asparaginase by the endophytic Fusarium sp.67 Ruma et al. observed the maximum enzyme activity of 3.58 IU by F. solani with fermentation temperature 35 °C at medium pH 5.0 with 0.002% of magnesium ions, and 1% of sucrose as the best carbon source.68 A lot of studies on L-asparaginase are reported through solid state fermentation only, but here we have adopted submerged fermentation as it is more reliable and stable. The OVAT/OFAT (One Factor At A Time) technique has been adopted by several workers related to the optimum production of L-asparaginase.43,69 120 or even 144 hours of incubation time is found to be effective for L-asparaginase production from Fusarium sp. (F. solani, F. proliferatum, and F. oxysporum) by several authors.20,43,70,71
Values of these most influential factors obtained from the OVAT table 1 were therefore selected as the zero-coded values to determine optimum model values using the Response Surface Methodology technique. The impact of various fermentation factors on the enzymatic action is represented in Figure 3 (a-i). The optimum enzyme production at glucose may be the reason that the isolate MJS2 prefers the simplest sugar (monosaccharide) instead of complex sugars (i.e., disaccharides). In the present study, in most cases, we have seen that L-asparaginase production drops after reaching the peak of any contributing parameters, i.e., incubation time, pH, carbon and nitrogen source, substrate content, etc. This may be driven due to several factors, i.e., limited nutrient availability, accumulation of toxic/inhibitory end products (e.g., ammonia), turbidity of liquid media, interactions of additive nutrients, and change in the pH of culture medium.42,72 A lower pH (<7, i.e., acidic) is not suitable for the enzyme production as it affects the fungal metabolic processes, cell membrane permeability, initiate irreversible modifications in the active site of the enzyme by damaging particular amino acids at the active site, and triggers the hydrolysis of the peptide bond, which initiates irreversible damage to the synthesized enzyme.73
Figure 3. Impact of different fermentation conditions; a- incubation temperature, b- fermentation hours, c- medium pH, d- various sugar components, e- various protein components, f- glucose concentration, g- medium substrate concentration, h-ammonium sulphate concentration, i-different metal ions on the isolate MJS2 in terms of L-asparaginase production. Values placed here are the average along with standard error (SE) of the three trials
RSM-based optimization of L-asparaginase synthesis
A central composite design was adopted to obtain the statistically valid model related to L-asparaginase synthesis using the obtained OVAT data. The polynomial model evaluated the relation of independent variables and enzyme activity. The best model to fit the data was the quadratic model that statistically compared the actual and predicted values at p < 0.0001. Both actual and predicted L-asparaginase activity (U mL-1) were determined using the three most influential variables i.e., glucose, ammonium sulphate, and substrate concentration (L-asparagine) in 20 experimental runs are shown in Table 2. As described in Table 3, quadratic model was recommended to be the best-fit model using the sum of square analysis by comparing the ratio of mean square regression and square residual. This model is best fit in quadratic analysis as the predicted R2 (0.95) and adjusted R2 (0.99) values are high. Adequate Precision for this model has a signal-to-noise ratio value of 104.25 indicating an adequate signal, i.e. this model can be used to navigate the design space.
Table (2):
Tabulated representation of expected and observed values associated with the synthesis of L-asparaginase from MJS2
Trial numbers |
Glucose concen. (gL-1) |
L-asparagine concen. (gL-1) |
Ammonium sulphate concen. (gL-1) |
Expected production of L-asparaginase (U mL-1) |
Observed production of L-asparaginase (U mL-1) |
|---|---|---|---|---|---|
1 |
0 (5) |
1 (13) |
1 (7.5) |
18.96 |
18.91 |
2 |
0 |
1 |
0 (7) |
28.96 |
27.25 |
3 |
0 |
0 (12.5) |
0 |
32.21 |
32.44 |
4 |
-1 (4.5) |
-1 (12) |
1 |
21.84 |
25.17 |
5 |
0 |
0 |
0 |
32.06 |
32.46 |
6 |
0 |
1 |
-1 (6.5) |
24.12 |
22.10 |
7 |
+1 (5.5) |
-1 |
0 |
28.41 |
25.17 |
8 |
-1 |
1 |
-1 |
27.92 |
28.91 |
9 |
0 |
0 |
0 |
32.96 |
32.47 |
10 |
+1 |
0 |
0 |
29.87 |
29.33 |
11 |
1 |
0 |
1 |
27.49 |
29.29 |
12 |
-1 |
1 |
1 |
22.03 |
22.67 |
13 |
-1 |
0 |
0 |
23.42 |
25.17 |
14 |
0 |
0 |
0 |
32.50 |
32.42 |
15 |
+1 |
-1 |
1 |
28.96 |
28.13 |
16 |
-1 |
0 |
-1 |
25.16 |
25.17 |
17 |
-1 |
-1 |
-1 |
20.00 |
19.20 |
18 |
1 |
0 |
-1 |
23.42 |
25.17 |
19 |
1 |
-1 |
1 |
23.97 |
22.33 |
20 |
1 |
1 |
1 |
24.91 |
25.17 |
Table (3):
Representation of ANOVA outcomes related to L-asparaginase production
ANOVA parameters |
Aggregate results of square values |
Degree of freedom |
Values of Mean |
Value of F Distribution |
Probability value |
|---|---|---|---|---|---|
Different model components |
9.49 |
9 |
0.043 |
1127.123 |
0.0005 |
A-Glucose conc. |
1.18 |
1 |
0.511 |
946.179 |
0.0004 |
B-Ammonium sulfate conc. |
5.54 |
1 |
0.354 |
104.52 |
0.0003 |
C-Substrate conc. |
5.04 |
1 |
0.650 |
61.2 |
0.0002 |
AB |
1.37 |
1 |
0.637 |
18.1 |
0.0007 |
AC |
0.68 |
1 |
0.068 |
28.14 |
0.0001 |
BC |
0.78 |
1 |
0.078 |
13.64 |
0.0000 |
A² |
0.57 |
1 |
1.057 |
11.58 |
0.0005 |
B² |
0.30 |
1 |
1.330 |
10.49 |
0.0009 |
C² |
1.74 |
1 |
0.074 |
2.22 |
0.0007 |
Residual |
0.004 |
10 |
0.009 |
1.24 |
|
Lack of Fit |
0.0044 |
5 |
1.089 |
3.179 |
0.0009 |
Pure Error |
5.0060 |
5 |
0.012 |
||
Cor Total |
7.0053 |
19 |
|||
R² |
0.9930 |
||||
Adjusted R² |
0.9927 |
||||
Predicted R² |
0.9537 |
||||
Adeq Precision |
104.2507 |
Table 3 shows the nine variance interactions of three variables that are A, B, C, AB, AC, BC, A2, B2 and C2. Each variance was checked through Analysis of Variance (ANOVA). The high model F-value of 1127.12, suggests its significance. The ANOVA table 3 shows the values of A (value of glucose), B (value of substrate concentration), C (value of ammonium sulphate), A2 (quadratic terms of glucose), B2 (quadratic terms of substrate concentration), and C2 (quadratic terms of ammonium sulphate concentration).
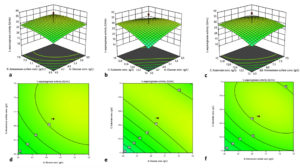
The perturbation plot (Supplementary Figure 1) contrasts the influence of three different factors at a specific point within the design space. A perturbation plot at the center point (glucose content of 4.76 gL-1, ammonium sulphate content of 5.25 gL-1, and substrate concentration of 12.03 gL-1) was obtained to show the interaction of three variables as OVAT on L-asparaginase production. The perturbation plot indicates that B represents L-asparagine concentration, has the most influential impact on L-asparaginase synthesis (steepest slope), and A (glucose) has a lesser effect. Figure 4(a-f) represents the three-dimensional (3D) plots displaying the amalgamated effect of two separate factors for L-asparaginase production, where the third factor remained as a zero coded value.
Figure 4. 3D plot and contour plot elucidating impact of ammonium sulphate and glucose concentration (a, d), substrate concentration and D-glucose concentration (b, e), ammonium sulphate concentration and L-asparagine concentration (c, f) on L-asparaginase synthesis
Based on the above model, the optimum glucose concentration was 4.76 gL-1, ammonium sulphate 5.25 gL-1, and substrate concentration 12.03 gL-1 at 37 °C for a fermentation time of 5 days, resulting in an assumed L-asparaginase activity of 32.47 U mL-1. The expected yields were confirmed under optimized conditions in three independent experiments. Thus, overall, 2.32-fold L-asparaginase activity was observed after adopting the RSM optimization method. Endophytic Fusarium sp. LCJ273 showed an increase in L-asparaginase enzyme production up to 2-fold when 3 gL-1 D-glucose, 20 gL-1 ammonium sulphate, and 2.5 gL-1 wheat bran were used as culture conditions.65 Farag et al. demonstrated the optimised culture conditions for fungal isolate Aspergillus terreus that increased 1.33-fold as compared to unoptimized conditions.74 Here, we have adopted the RSM technique, but some researchers, while working on medicinal plant endophytes (fungi, bacteria, and actinobacteria), adopt Plackett-Burman experiments, Taguchi model, and Central Composite Designs too.61,67,69,75,76 The contemporary reports highlight that the requirement of basic media compositions is higher than our present investigational outcome. Such a lesser requirement of carbon, nitrogen, metal ions, and substrate concentration by endophytic fungi is evident, that it is economically feasible and can be brought forward further for industrial exploitation.
Biochemical characterization of MJS2 produced L-asparaginase
Influence of various substrate concentrations
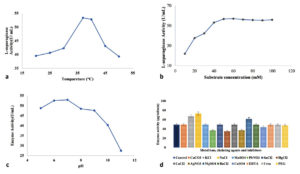
The impact of asparagine monohydrate concentration (10-100 mM) on semi-purified L-asparaginase enzyme was recorded. A change in L-asparagine concentration (from 10 to 50 mM) causes a gradual elevation in the enzymatic action, but after a certain point, the activity remains constant or decreases slightly. The optimum substrate concentration was 50 mM, with the highest activity of 56.69 U mL-1 min-1. The impact of different conditions on the enzymatic action is represented in Figure 5 a-d. An earlier study by Chow and Ting estimated the optimum substrate concentration for L-asparaginase production in the endophyte Penicillium simplicissimum PBL13, which was 30 mM. Another isolate, Fusarium oxysporum MKS1 exhibited maximum response at 10 mM of L-asparagine concentration.77 Kathivaran et al. also reported the positive impact of L-asparagine on enzyme synthesis over other inducer molecules, i.e., L-arginine, L-glutamic acid, L-tyrosine, and L-tryptophan. The maximum L-asparaginase production of 18.3 U mL-1 was achieved at 10 g L-1 of substrate concentration.78 MJS2 exhibits optimum action at a low substrate requirement, which is indicative of the fact that it has a low Km (Michaelis-Menten Constant) value. This highlights that a low substrate concentration is needed to attain the maximum enzymatic action, and it can work in a patient’s body even at lower doses, and also, this chemotherapeutic agent will not get resistant quickly against the cancer cells.8
Figure 5. Impact of different factors a- temperature, b- sulphate concentration, c- pH, d- various salt sources, chelating ions, and inhibitors on the activity of the semi-purified enzyme
Effect of Temperature
The activity and stability of the semi-purified enzyme were studied at seven different temperatures with a 5 °C difference from 20 °C to 50 °C. It was found that 35 °C has the most positive impact on enzymatic activity, and then three other slightly variable temperatures, 36 °C, 37 °C, and 39 °C, also have potent L-asparaginase activity. Figure 3 shows the effect of different incubation temperatures on L-asparaginase production. The maximum L-asparaginase activity was obtained at 37 °C. Darwesh et al. suggested that maximum L-asparaginase action was seen at 30 °C and 37 °C temperatures.64 Type-II L-asparaginase obtained from a rhizospheric microbe Stenotrophomonas maltophilia, also showed maximum activity at 37 °C of medium temperature.79 A maximum/optimum activity at 37 °C provides an opportunity to be used at in vivo systems, i.e., for initial trials in mice/rat models and finally for eradication of ALL in the human body.61 As the majority of the reported L-asparaginase functions at the same temperature (Lasidiplodia theobromine, Aspergillus flavus HK03, A. niger, A. lentulus, Mucor sp., and Rhizopus sp.), and our investigated enzyme follows the same pattern, our result is valid as per contemporary outcomes and bears biotechnological interest.61,65,80,81
Effect of H+ ion concentration
To evaluate the best pH, seven varied pH ranges (5-11) were reacted with the semi-purified enzyme. Figure 4 shows the impact of the various pH values on L-asp action. The highest L-asparaginase action was acquired at pH 7.82 Enzyme from marine Aspergillus flavus was stable at a pH of 7.0 to 8.0.83 The enzyme studied in the present investigation has a wide range of pH adjustability, which makes it suitable for industrial use. The outcomes are similar to the outcomes of Dev et al., who elucidated the enzyme action at pH 7.0 for the bacterial isolate Cupriavidus oxalaticus.84 L-asp from Streptomyces fradie acts at an optimum pH of 8.5.85 Alrumman et al. suggested that the best pH for L-asp activity isolated from Bacillus licheniformis was 6.5.86 Darwesh et al. suggested that the enzyme from Burkholderia pseudomallei works optimum pH 8.64 Rhizomucor miehei, Penicillium sp., Mucor hiemalis, Corynebacterium glutamicum, Bacillus megaterium, Bacillus pumilus synthesised enzymes are known to function at their optimum at pH 7 and it is the mostly physiological pH of human body, so it is a higher chance of working fine when injected into a patient’s body.87-91
Effect of metal ions, chelating agents, inhibitors, and other additives
Among the tested metal and chelating agents, MgSO4, KCl, and NaCl caused the increase of the L-asparaginase activity by 25%, 36.48%, and 48.03%, respectively. These characteristics are crucial to be exploited for the food industry.92 A strain of Aspergillus oryzae was screened for the maximum L-asparaginase producing activity, which revealed the greater effect of KCl and MgSO4 at 0.052% concentration.93 The presence of CaCO3, MnSO4, SnCl2, CaCL2, BaCl2, EDTA, and urea had weak or no effect on L-asparaginase activity. Vala et al. and Monica et al. also showed the decrease in activity in presence of Mn2+ and EDTA in the case of Aspergillus niger and Mucor hiemalis, respectively.94,95 The steep decrease in L-asparaginase action in the presence of PbNO3, HgCl2, and AgNO3 was 25.53%, 29.58%, and 24.83%, respectively. Dias et al. highlighted the impact of different salts and other activators on the asparaginase enzyme-producing ability of Aspergillus spp. MnCl2 highly affected the L-asparaginase production at 1 mM concentration.96 Ei-Naggar et al. showed that Cd+, Pb+, and Hg+ by 145.15%, 143.04%, and 121.53%, respectively, have a negative impact on purified L-asp action synthesised by rhizospheric isolate S. bollosae NEAE-115.85 Monovalent metal, i.e., K+ and divalent ones like Mg2+ and Ca2+ function as activators, whereas Hg2+ and EDTA are enzymatic inhibitors.97 Actually, some of these metal ions function as co-factors (as they act as electron donor or acceptor) for the enzyme and modify its stability, functionality, and turnover number.80
L-asparaginase is in high demand in both the food and pharmaceutical industries, and new sources are to be tested randomly for the same. Here, an endophytic fungus Fusarium oxysporum MJS2, has been projected as an alternative origin related to synthesis of L-asparaginase with an optimization condition of pH 6.8, fermentation temperature 37 °C, fermentation time of 120 h, asparagine monohydrate (50 mM), glucose as a carbon source, and ammonium sulphate as a nitrogen source. In vitro assays confirm the potential of the isolate as a valuable L-asp. producer, and further in vivo screenings are highly solicited to establish this enzyme as an industrially important one.
Additional file: Additional Figure S1.
ACKNOWLEDGMENTS
The authors are thankful to the University Students Instrumentation Centre (USIC) of Vidyasagar University for providing all the necessary instrumentation facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
DB conceptualized the study. SS and HKS performed experiments. DB performed results analysis. SS and HKS wrote the manuscript. DB reviewed, revised and approved the manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request. The information related to the GenBank data can be accessed freely from NCBI database.
ETHICS STATEMENT
Not applicable.
- Varalakshmi V, Raju KJ. Optimization of L-asparaginase production by Aspergillus terreus mtcc 1782 using Bajra seed flour under solid state fermentation. Int J Res Eng Technol. 2013;2(09):2321-7308.
Crossref - Broome JD. Evidence that the L-asparaginase activity of guinea pig serum is responsible for its antilymphoma effects. Nature. 1961;191(4793):1114-1115.
Crossref - Gaffar SA, Shethna YI. Purification and some biological properties of asparaginase from Azotobacter vinelandii. Appl Environ Microbiol. 1977;33(3):508-14.
Crossref - Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects: I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963;118(1):99-120.
Crossref - Pourhossein M, Korbekandi H. Cloning, expression, purification and characterisation of Erwinia carotovora L-asparaginase in Escherichia coli. Adv Biomed Res. 2014;3(1):82.
Crossref - Alam S, Pranaw K, Tiwari R. Recent development in the uses of asparaginase as food enzyme. In: Parameswaran B (ed) Green bio-processes, energy, environment, and sustainability. Springer, Singapore. 2019
Crossref - da Cunha MC, dos Santos Aguilar JG, de Melo RR, et al. Fungal L-asparaginase: Strategies for production and food applications. Food Res Int. 2019;126:108658.
Crossref - Muneer F, Siddique MH, Azeem F, et al. Microbial L-asparaginase: purification, characterization and applications. Arch Microbiol. 2020;202(5):967-981.
Crossref - Nguyen HA, Su Y, Zhang JY, et al. A novel L-asparaginase with low L-glutaminase coactivity is highly efficacious against both T-and B-cell acute lymphoblastic leukemias in vivo. Cancer Res. 2018;78(6):1549-1560.
Crossref - Radadiya A, Zhu W, Coricello A, Alcaro S, Richards NGJ. Improving the treatment of acute lymphoblastic leukemia. Biochemistry. 2020;59(35): 3193-3200.
Crossref - Sobat M, Asad S, Kabiri M, Mehrshad M. Metagenomic discovery and functional validation of L-asparaginases with anti-leukemic effect from the Caspian Sea. Iscience. 2021;24(1):101973.
Crossref - Kumar NSM, Manonmani HK. Purification, characterization and kinetic properties of extracellular L-asparaginase produced by Cladosporium sp. World J Microbiol Biotechnol. 2013;29(4):577-587.
Crossref - Lincoln L, Niyonzima FN, More SS. Purification and properties of a fungal L-asparaginase from Trichoderma viride pers: SF GREY. J Microbiol Biotechnol Food Sci. 2015;4(4):310-316.
Crossref - Patro KR, Basak U, Mohapatra AK, Gupta N. Development of new medium composition for end production of L-asparaginase by Aspergillus f. J Environ Biol. 2014;35(1):295-300.
- Shrivastava A, Khan AA, Shrivastav A, Jain SK, Singhal PK. Kinetic studies of L-asparaginase from Penicillium digitatum. Prep Biochem Biotechnol. 2012;42(6):574-581.
Crossref - Pedreschi F, Kaack K, Granby K. The effect of asparaginase on acrylamide formation in French fries. Food Chem. 2008;109(2):386-392.
Crossref - Friedman M. Acrylamide: inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct. 2015;6(6):1752-1772.
Crossref - Xu F, Oruna-Concha M-J, Elmore JS. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016;210:163-171.
Crossref - Parashiva J, Nuthan BR, Rakshith D, Satish S. Endophytic fungi as a promising source of anticancer L-asparaginase: a review. Curr Microbiol. 2023;80(9):282.
Crossref - Udayan E, Gnanadoss JJ, Isolation and Characterization of L-Asparaginase Producing Endophytic Fungi from Medicinal Plants of Rutaceae Family. Biosci Biotechnol Res Asia. 2023;20(1):241-253.
Crossref - Kayastha S, Rai GB, Dahal S, Subba B. Isolation and Purification of L-Asparaginase Producing Endophytic Fungi from Ocimum tenuiflorum L. Journal of Nepal Biotechnology Association. 2025;6(1):9-14.
Crossref - Cheruiyot DK, Omwenga GI, Njagi ENM. Molecular phylogenetic characterization of L-asparaginase-producing endophytic fungi inhabiting Prunus africana and Periploca linearifolia: Effect of incubation time and pH on enzyme production. Scientific African. 2024;26:e02368.
Crossref - Uzma F, Murthy KN, Srinivas C. Optimization of physiological conditions for L-asparaginase production by endophytic fungi (Fusarium solani) isolated from Tinospora cordifolia (Willd.) Hook. F & Thomson. Eur J Exp Biol. 2016;6(3):37-45.
- Hatamzadeh S, Rahnama K, Nasrollahnejad S, et al. Isolation and identification of L-asparaginase-producing endophytic fungi from the Asteraceae family plant species of Iran. Peer J. 2020;8:e8309.
Crossref - Araujo-Magalhaes GR, Maciel MHC, da Silva LF, et al. Fungal endophytes from leaves of Mandevilla catimbauensis (Apocynaceae): diversity and potential for L-asparaginase production. Braz J Microbiol. 2021;52(3):1431-1441.
Crossref - Arumugam N, Shanmugam MK, Thangavelu P. Purification and anticancer activity of glutaminase and urease free l asparaginase from novel endophyte Chaetomium sp. Biotechnol Appl Biochem. 2022;69(5):2161-2175.
Crossref - Rozina R. Pharmacological and biological activities of Mirabilis jalapa L. Int J Pharmacol Res. 2016;6(5):160-168.
- Mazumder K, Biswas B, Raja IM, Fukase K. A review of cytotoxic plants of the Indian subcontinent and a broad-spectrum analysis of their bioactive compounds. Molecules. 2020;25(8):1904.
Crossref - Al-Snafi AE, Talab TA, Jabbar WM, Alqahtani AM. Chemical constituents and pharmacological activities of Mirabilis jalapa-A review. Int J Pharm Biol Arch. 2021;1(2):034-045.
Crossref - Cammue BPA, De Bolle MFC, Terras FRG, et al. Broekaert. Isolation and characterization of a novel class of plant antimicrobial peptides form Mirabilis jalapa L. seeds. J Biol Chem. 19992;267(4):2228-2233.
Crossref - Hajji M, Jarraya R, Lassoued I, Masmoudi O, Damak M, Nasri M. GC/MS and LC/MS analysis, and antioxidant and antimicrobial activities of various solvent extracts from Mirabilis jalapa tubers. Process Biochem. 2010;45(9):1486-1493.
Crossref - Rahman HS. Preclinical drug discovery in colorectal cancer: a focus on natural compounds. Current Drug Targets. 2021;22(9):977-997.
Crossref - Harun-Or-Rashid M, Akter S, Habiba U, et al. Antioxidant, antibacterial, cytotoxic and thrombolytic activities of flowers of Mirabilis jalapa L: possible role of phenolics and flavonoids. J Agric Food Res. 2023;14:100893.
Crossref - Atrooz OM, Alhmoud JF, Farah HS, Al-Tarawneh FM, Sohemat AA. In vitro Assessment of biological and cytotoxic activity of methanol seed extract of Jordanian Mirabilis jalapa L. Trop J Nat Prod Res. 2024;8(2):6087-6092.
Crossref - Watthanachaiyingcharoen R, Utthasin P, Potaros T, Suppakpatana P. Proteins from Mirabilis jalapa possess anticancer activity via an apoptotic pathway. J Health Res. 2010;24(4):161-165.
- Mishra VK, Passari AK, Chandra P, et al. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD-GC-MS analysis. PloS one. 2017;12(10):e0186234.
Crossref - Prajitha T, Sandhia GS, Subramaniyan S. Isolation, Identification, and Characterization of Endophytic Bacteria from Medicinally Valuable Mirabilis jalapa L. Natil Acad Sci Lett. 2024;47(4):441-445.
Crossref - Gulati R, Saxena RK, Gupta R. A rapid plate assay for screening l asparaginase producing micro organisms. Lett Appl Microbiol. 1997;24(1):23-26.
Crossref - Kumar S, Dasu VV, Pakshirajan K. Purification and characterization of glutaminase-free L-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresource Technology. 2011;102(2):2077-82.
Crossref - Lowry OH, Rosebrough NJ. Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265-275.
Crossref - Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596-1599.
Crossref - Chow Y, Ting ASY. Endophytic L-asparaginase-producing fungi from plants associated with anticancer properties. J Adv Res. 2015;6(6):869-876.
Crossref - Yap LS, Lee WL, Ting ASY. Optimization of L-asparaginase production from endophytic Fusarium proliferatum using OFAT and RSM and its cytotoxic evaluation. J Microbiol Methods. 2021;191:106358.
Crossref - Scheetz RW, Whelan HA, Wriston JC. Purification and properties of an L-asparaginase from Fusarium tricinctum. Archives of biochemistry and biophysics. 1971;142(1):184-189.
Crossref - Ashok A, Doriya K, Rao JV, Qureshi A, Tiwari AK, Kumar DS. Microbes producing L-asparaginase free of glutaminase and urease isolated from extreme locations of Antarctic soil and moss. Sci Rep. 2019;9(1):1423.
Crossref - Chakraborty M, Shivakumar S. Bioprospecting of the agaricomycete Ganoderma australe GPC191 as novel source for L-asparaginase production. Sci Rep. 2021;11(1):6192.
Crossref - de Andrade GA, de Vargas MV, Goulart SN, et al. Screening of endophytic fungi from Antarctic mosses: Potential production for L-asparaginase free of glutaminase and urease activity. J Biotechnol. 2023;377:1-2.
Crossref - Samal S, Rai S, Upadhaya RS. Endophytic fusarium and their association with plant growth. Microbial Endophytes and Plant Growth.2023:259-268.
Crossref - Toghueo RMK. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology. 2020;11(1):1-21.
Crossref - Purushothaman K, Sivasankar E, Sowmeya VG, Sathiavelu M. Bioprospecting Endophytic Fungi from Fusarium Genus as Sources of Bioactive Metabolites. In Fungal Endophytes Volume I: Biodiversity and Bioactive Materials. 2025:207-239.
Crossref - Kumar A, Patil D, Rajamohanan PR, Ahmad A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PloS one. 2013;8(9):e71805.
Crossref - Wang Q-X, Li S-F, Zhao F, et al. Chemical constituents from endophytic fungus Fusarium oxysporum. Fitoterapia. 2011;82(5):777-781.
Crossref - Kour A, Shawl AS, Rehman S, et al. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J Microbiol Biotechnol. 2008;24(7):1115-1121.
Crossref - Asim S, Hussain A, Murad W, et al. Endophytic Fusarium oxysporum GW controlling weed and an effective biostimulant for wheat growth. Front Plant Sci. 2022;13:922343.
Crossref - Frank BH, Pekar AH, Veros AJ, Ho PPK. Crystalline L-Asparaginase from Escherichia coli B: II. Physical properties, subunits, and reconstitution behavior. J Biol Chem. 1970;245(14):3716-24.
Crossref - El-Bessoumy AA, Sarhan M, Mansour J. Production, isolation, and purification of L-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. BMB Reports. 2004;37(4):387-393.
Crossref - Husain I, Sharma A, Kumar S, Malik F. Purification and characterization of glutaminase free asparaginase from Enterobacter cloacae: in vitro evaluation of cytotoxic potential against human myeloid leukemia HL-60 cells. PLoS One. 2016;11(2):e0148877.
Crossref - Kumar S, Darnal S, Patial V, et al. Molecular cloning, characterization, and in-silico analysis of L-asparaginase from Himalayan Pseudomonas sp. PCH44. 3 Biotech. 2022;12(8):162.
Crossref - Aishwarya SS, Selvarajan E, Iyappan S, Rajnish KN. Recombinant L-Asparaginase II from Lactobacillus casei subsp. casei ATCC 393 and Its Anticancer Activity. Indian J Microbiol. 2019;59:313-20.
Crossref - Yap LS, Lee WL, Ting ASY. Bioprocessing and purification of extracellular L-asparaginase produced by endophytic Colletotrichum gloeosporioides and its anticancer activity. Preparative Biochem Biotechnol. 2023;53(6):653-671.
Crossref - Moubasher HA, Balbool BA, Helmy YA, et al. Insights into asparaginase from endophytic fungus Lasiodiplodia theobromae: purification, characterization and antileukemic activity. Int J adv Biol Biomed. 2022;19(2):680.
Crossref - Jenila A, Gnanadoss J, Kengaiah J. Purification, characterization and evaluation of L-asparaginase from endophytic fungi Fusarium sp. LCJ273 for its anticancer property. J Adv Sci Res. 2020;11(01 Suppl 1):305-312.
- Arredondo-Nunez A, Monteiro G, Flores-Fernandez CN, Antenucci L, Permi P, Zavaleta AI. Characterization of a Type II L-Asparaginase from the Halotolerant Bacillus subtilis CH11. Life. 2023;13(11):2145.
Crossref - Darwesh DB, Al-Awthan YS, Elfaki I, Habib SA, Alnour TM, Darwish AB, Youssef MM. Anticancer activity of extremely effective recombinant L-asparaginase from Burkholderia pseudomallei. J Microbiol Technol. 2022;32(5):551-563.
Crossref - Luhana K, Bariya H. Comparative analysis of purified anti-leukemic L-asparaginase enzyme from Trichoderma spp. J Appl Biol Biotechnol. 2023;11(4):185-192.
Crossref - Aghaiypour K, Wlodawer A, Lubkowski J. Structural basis for the activity and substrate specificity of Erwinia chrysanthemi L-asparaginase. Biochemistry. 2001;40(19):5655-5664.
Crossref - Jenila AV, Gnanadoss JJ. Formulation of a suitable medium and its optimization for maximizing L-asparaginase production from endophytic fungi Fusarium sp. LCJ273. Biosci Biotechnol Res Asia. 2018;15(4):887-98.
Crossref - Ruma K, George TK, Aswani P, Jisha MS. Production and Optimization of Extra Cellular L-asparaginase by Fusarium solani Isolated from Withania sominifera. J Biol Act Prod Nat. 2017;7(2):81-88.
Crossref - Vimal A, Kumar A. Optimized production of medically significant enzyme L-asparaginase under submerged and solid-state fermentation from agricultural wastes. Curr Microbiol. 2022;79(12):394.
Crossref - Yadav N, Sarkar S. Production of L-asparaginase by Fusarium Oxysporum using submerged fermentation. Int J Pharm Sci Invent. 2014;3(6):32-40.
- Isaac GS, Abu-Tahon MA. Production of extracellular anti-leukemic enzyme L-asparaginase from Fusarium solani AUMC 8615 grown under solid-state fermentation conditions: purification and characterization of the free and immobilized enzyme. Egypt J Bot. 2016;56(3):799-816.
Crossref - Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv. 2004;22(3):189-259.
Crossref - Pallem C. Solid-state fermentation of corn husk for the synthesis of asparaginase by Fusarium oxysporum. Asian J Pharm Pharmacol. 2019;5(4):678-681.
Crossref - Farag AM, Hassan SW, Beltagy EA, El-Shenawy MA. Optimization of production of anti-tumor L-asparaginase by free and immobilized marine Aspergillus terreus. Egypt J Aquat Res. 2015;41(4):295-302.
Crossref - Abhini KN, Rajan AB, Zuhara KF, Sebastian D. Response surface methodological optimization of L-asparaginase production from the medicinal plant endophyte Acinetobacter baumannii ZAS1. J Genet Eng Biotechnol. 2022;20(1):22.
Crossref - Chergui A, Trabelsi L, Salem-Bekhit MM, et al. Optimization of intracellular L-Asparaginase production by Streptomyces paulus CA01 isolated from wheat bran using the response surface methodology. Biotechnol Biotechnol Equip. 203;37(1):2281493.
Crossref - Chow YY, Ting ASY. Influence of glucose and L-asparagine concentrations on L-asparaginase production by endophytic fungi. J Microbiol Biotechnol Food Sci. 2017;7(2):186-189.
Crossref - Kathiravan A, Udayan E, Gnanadoss JJ. Optimizing the culture conditions for L-asparaginase production from endophytic fungus Curvularia sp. LCJ413 through conventional and statistical approach. J Exp Biol Agric Sci. 2023;11(1):62-74.
Crossref - Abdelrazek NA, Elkhatib WF, Raafat MM, Aboulwafa MM. Production, characterization and bioinformatics analysis of L-asparaginase from a new Stenotrophomonas maltophilia EMCC2297 soil isolate. AMB Express. 2020;10(1):71.
Crossref - El-Gendy MMAA, Awad MF, El-Shenawy FS, El-Bondkly AMA. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J Biol Sci. 2021;28(4):2540-2548.
Crossref - Priya SR, Subhashini A. Characterization and Optimization of Fungal L-Asparaginase Isolated from Soil and Medicinal Plants. J Pure Appl Microbiol. 2022;16(1):453-459.
Crossref - Ohshima M, Yamamoto T, Soda K. Further characterization of glutaminase isozymes from Pseudomonas aeruginosa. Agric Biol Chem. 1976;40(11):2251-2256.
Crossref - Nurce Z, Gezgin Y, Hames EE. antitumor activity of urease-free L-asparaginase with low glutaminase coactivity produced by marine-derived Aspergillus flavus. Biocatalyst and Agril Biotechnol. 2023;54:102958.
Crossref - Dev MJ, Mawal SB, Singhal RS. L-Asparaginase from an acrylamide degrader, Cupriavidus oxalaticus ICTDB921: Production, kinetic modelling, purification and characterization. Biocatal Agric Biotechnol. 2023;53:102871.
Crossref - El-Naggar NE, Deraz SF, El-Ewasy SM, Suddek GM. Purification, characterization and immunogenicity assessment of glutaminase free L-asparaginase from Streptomyces brollosae NEAE-115. BMC Pharmacol Toxicol. 2018;19(1):1-5.
Crossref - Alrumman SA, Mostafa YS, Al-Izran KA, Alfaifi MY, Taha TH, Elbehairi SE. Production and anticancer activity of an L-asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci Rep. 2019;9(1):3756.
Crossref - Kumar DS, Sobha K. l-asparaginase from microbes: a comprehensive review. Adv Biores. 2012;3(4):137-157
- Baghdadi AM, Balobaid AK. Isolation and Molecular Identification of Fungi Producing of L-asparaginase Isolated from Makkah Region Soil. World Journal of Environmental Biosciences. 2021;10(2):42-48
- Huang L, Liu Y, Sun Y, Yan Q, Jiang Z. Biochemical characterization of a novel L-asparaginase with low glutaminase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Appl Environ Microbiol. 2014;80(5):1561-1569.
Crossref - Zhang S, Xie Y, Zhang C, et al. Biochemical characterization of a novel L-asparaginase from Bacillus megaterium H-1 and its application in french fries. Food Res Int. 2015;77(3):527-533.
Crossref - Qeshmi FI, Homaei A, Fernandes P, Javadpoure S. Marine microbial L-asparaginase: biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol Res. 2018;208:99-112.
Crossref - Jia R, Wan X, Geng X, Xue D, Xie Z, Chen C. Microbial L-asparaginase for application in acrylamide mitigation from food: Current research status and future perspectives. Microorganisms. 2021;9(8):1659.
Crossref - da Cunha MC, Aguilar JG dos S, Orrillo LSMDR, de Castro RJS, Sato HH. L-asparaginase from Aspergillus oryzae spp.: effects of production process and biochemical parameters. Prep Biochem Biotechnol. 2022;52(3):253-263.
Crossref - Vala AK, Sachaniya B, Dudhagara D, et al. Characterization of L-asparaginase from marine-derived Aspergillus niger AKV-MKBU, its antip Isolation, purification and characterization of fungal extracellular L-asparaginase from Mucor hiemalis roliferative activity and bench scale production using industrial waste. Int J BIol Macromol. 2018;108:41-46.
Crossref - Monica T, Lincoln L, Niyonzima FN, Sunil SM. Isolation, purification and characterization of fungal extracellular L-asparaginase from Mucor hiemalis. J Biocatal Biotransformation. 2013;9:12-14.
Crossref - Dias FFG, Santos AJG, Sato HH. L-Asparaginase from Aspergillus spp.: production based on kinetics, thermal stability and biochemical characterization. 3 Biotech. 2019;9:289.
Crossref - Emmanuel E, Nzelibe HC, Onyike E. Isolation, partial purification and characterization of L-asparaginase from Hedgehog Serum. J Microb Biochem Technol. 2015;07:404-409.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.