ISSN: 0973-7510
E-ISSN: 2581-690X
Utilizing vegetable waste to produce bioactive compounds through microbial consortia represents a holistic solution to waste management challenges. Efficient waste collection systems in major cities ensure proper segregation of vegetable waste and lay the groundwork for resource utilization. Segregating waste at its source enhances waste stream quality and facilitates downstream processing. Research and development efforts investigating tailored microbial consortia seek to optimize waste degradation and bioactive compound yields, thereby unlocking the potential of waste. This approach significantly reduces greenhouse gas emissions by diverting vegetable waste from landfills, thereby mitigating climate change effects and improving air quality. Moreover, it conserves natural resources by reducing the need for virgin materials and promoting biodiversity, conservation, and sustainable resource management. Here, the enzymatic activities and phenolic concentrations derived from the biodegradation of vegetable waste were analyzed over 14 days. Protease activity that plays a vital role in breaking down proteins reached a notable level of 300 µg/ml after 14-day incubation. Lipase activity which is essential for lipid breakdown was observed at a concentration of 6.3 µg/ml. Furthermore, phenolic concentration analysis revealed a significant range, with values ranging from 225 µg/ml to 240 µg/ml after 14 days of incubation. Phenolics are phytochemicals possessing antioxidant properties and potential health benefits. These findings provide valuable insight into the efficacy of utilizing vegetable waste as a substrate for enzymatic and phytochemical production. The observed enzymatic activities and phenolic concentrations highlight the potential of vegetable waste as a valuable resource for the sustainable production of industrially relevant compounds.
Waste Management, Microbial Consortia, Enzymes, Biodegradation, Bioactive compounds
The annual waste production in India is at a staggering 60 million tons, with major metropolitan cities such as Nagpur, Delhi, Mumbai, Chennai, Hyderabad, Bangalore, and Kolkata contributing 10 million tons to this alarming figure. The pressing issue is that landfills in these urban centers have reached their capacity limits, thus compelling experts to advocate innovative waste disposal and recycling methods as alternatives to establishing new landfill sites. The repercussions of inadequate waste segregation are dire and can potentially result in unsanitary conditions, environmental degradation, and rampant spread of diseases. Compounding this challenge is the scarcity of suitable dumping sites, thus emphasizing the urgent need for the implementation of efficient waste segregation and rapid disposal treatment systems. With urbanization continuing to surge and rapid population growth, addressing these pressing waste management concerns is imperative to safeguard public health and ensure environmental sustainability.1
Traditionally, solid waste management techniques encompass a range of methods, including composting, biomethanation, incineration, pyrolysis, and biodegradation. Among these, sanitary landfills have emerged as a modern and efficient waste management approach that has been adopted globally. Biodegradation is a fundamental process influenced by factors such as oxygen availability, temperature, pH, nutrient content, and moisture level and plays a pivotal role in waste decomposition. This natural process involves the breakdown of organic substances by various microbial organisms, including bacteria, algae, fungi, and protozoa. Minimizing household waste through small-scale initiatives within a short time frame is crucial for mitigating environmental impacts and promoting sustainable waste management practices. These initiatives contribute to reducing the burden on landfills and conserving natural resources while fostering environmental stewardship and community engagement.1
The significant contributions of India to global fruit and vegetable production account for 10% and 14%, respectively, and underscore its pivotal role in agriculture. However, organic waste generated from fruits and vegetables poses a significant environmental challenge, particularly in terms of soil and water pollution. Additionally, the decomposition of organic waste contributes substantially to greenhouse gas emissions, ultimately exacerbating environmental pollution and climate change.2
The rapid pace of urbanization in India has further compounded this issue and has led to a massive waste management challenge for the government. With over 377 million urban residents spread across 7,935 towns and cities, the annual generation of municipal solid waste amounts to 62 million tons.3,4 However, the current waste management infrastructure falls short, with only 43 million tons of waste being collected annually and a mere 11.9 million tons of waste being treated. The remaining 31 million tons of waste ends up in landfill sites, ultimately exacerbating environmental degradation and public health risks.5
One of the key issues identified in waste management practices is the inadequate segregation of biodegradable and non-biodegradable waste. Sunita Narain, Director of the Centre for Science and Environment, highlights this challenge while emphasizing the need for municipal authorities to develop effective models for waste segregation.5
In response to these pressing challenges, the present investigation focuses on the treatment of biodegradable waste. By targeting the management and treatment of organic waste from fruits and vegetables, this study aimed to mitigate environmental pollution, reduce greenhouse gas emissions, and alleviate the burden on landfill sites. Through innovative waste treatment techniques such as composting, biomethanation, and microbial degradation, this investigation seeks to transform organic waste into valuable resources such as compost or biogas to thereby promote environmental sustainability and resource efficiency.
Addressing the challenges posed by organic waste from fruits and vegetables requires concerted efforts from governmental authorities, communities, and other stakeholders. By prioritizing the treatment of biodegradable waste and implementing effective waste management strategies, India can move towards a more sustainable and environmentally responsible approach to waste management, ultimately ensuring the well-being of both current and future generations.6
Processing of substrate
The process of utilizing vegetable waste substrates to produce bioactive compounds using microbial consortia begins with the collection and preparation of vegetable waste. The vegetable waste obtained from the kitchen was carefully sieved to remove large particles or contaminants and to ensure the purity of the substrate. Subsequently, the sieved vegetable waste was transferred to sterile glass bottles under aseptic conditions to prevent contamination with unwanted microorganisms. Once the vegetable waste substrate was prepared and placed in glass bottles, microbial consortia specifically tailored for the degradation of vegetable waste and production of the desired bioactive compounds were inoculated into the bottles.7 These microbial consortia may consist of various types of microorganisms, including bacteria, fungi, algae, and protozoa, that are selected based on their ability to efficiently degrade organic matter and produce target compounds. The inoculated glass bottles containing the vegetable waste substrate and microbial consortia were sealed and allowed to stand for 28 days. During the incubation period, the bottles were maintained under aerobic conditions to ensure oxygen availability for microbial activity. Incubation is typically conducted at room temperature to mimic natural environmental conditions and to optimize microbial growth and metabolic activity. Throughout the 28-day incubation period, the microbial consortia degraded the organic components of the vegetable waste substrate, breaking down complex molecules into simpler compounds. Microbial metabolism produces bioactive compounds such as enzymes, phenolics, tannins, and other secondary metabolites as byproducts of the degradation process.8 By allowing the microbial consortia to interact with the vegetable waste substrate during the incubation period, optimal conditions were created for the efficient degradation of waste and the production of the desired bioactive compounds. This approach harnesses the natural processes of biodegradation and microbial metabolism to convert vegetable waste into valuable and beneficial products, thus contributing to waste management and resource utilization. To identify the microorganisms present in the culture, staining procedures were employed to visualize their morphological features and aid in their classification. Microorganisms were inoculated onto selective media tailored to encourage the growth of bacteria, fungi, or other microbes, and the culture characteristics and biochemical properties of the microorganisms were meticulously studied to gain insights into their taxonomy and behavior.9,10
Identification of culture
Test samples labeled as N, BC, ABC, FC, and AFC were subjected to experiments aimed at determining the appropriate outcomes. These experiments involved various biochemical tests such as catalase, oxidase, indole, and citrate utilization tests that were used to assess the metabolic capabilities and physiological characteristics of the microorganisms.11 Additionally, specialized staining techniques such as Gram staining for bacteria and lactophenol cotton blue staining for fungi were used to further elucidate the identity of the microorganisms. By systematically analyzing the cultural and biochemical properties of the microorganisms present in the sample, their taxonomic classification and potential roles in the degradation of vegetable waste substrates were determined. This information is crucial for understanding the composition of microbial consortia and optimizing their performance for efficiently converting vegetable waste into bioactive compounds.12,13
Screening for protease activity
During the screening process for protease activity, protease-producing bacteria and fungi were inoculated onto casein gelatin agar media using the streak plate method.14 This technique involved streaking the surface of the agar plates with the microbial culture and incubation at 37°C for 24 hours (fungi required 3-4 days to grow). During the incubation period, protease-producing microorganisms secrete proteases that act on the proteins present in casein + gelatin agar media. Protease activity was indicated by the formation of a distinct zone of hydrolysis around microbial colonies. The formation of a hydrolysis zone served as a positive indicator of protease activity in the tested microorganisms. This screening method allowed for the rapid and efficient identification of protease-producing bacteria and fungi that could then be further characterized and utilized for the degradation of vegetable waste and the production of bioactive compounds.15
Screening for lipase activity
During screening for lipase activity, lipase-producing bacteria and fungi were inoculated onto 1% tributyrin nutrient agar medium using the streak plate method. The plates were then incubated at 37°C, with bacteria requiring a shorter incubation period of 24 h and fungi requiring 3-4 days to grow. During the incubation period, lipase-producing microorganisms produce lipase enzymes that act on tributyrin that is present in the nutrient agar media. Lipase activity was indicated by the formation of a distinct hydrolysis zone around the microbial colonies. The formation of a hydrolysis zone served as a positive indicator of lipase activity in the tested microorganisms. This screening method allowed for the identification of lipase-producing bacteria and fungi that could then be further characterized and utilized for biodegradation of the substrate and various applications, including bioremediation, food processing, and biofuel production.16
Preparation of microbial consortia
Five milliliters of nutrient broth were dispensed into three separate test tubes. Each test tube was inoculated with a selected pure culture of the microorganism. This step ensured that each microorganism was cultured separately to maintain its characteristics. The inoculated test tubes were then placed into an incubator and incubated for 24 hours at a temperature of 37°C. During this incubation period, the microorganisms multiplied and grew in the nutrient broth.17
After the initial 24-hour incubation period, three millilitres of the culture from each test tube were transferred into 27 mL of freshly prepared broth. The total volume was adjusted to 30 mL by adding additional broth. This step dilutes the culture and provides optimal conditions for further growth and interactions between different microorganisms. The 30-millilitre broth containing the mixed culture was then incubated for another 24 h under the same conditions required for incubation.18,19
After the second incubation, the broth containing the microbial consortia was stored for further use. This prepared microbial consortium was utilized for the biodegradation of organic waste and production of bioactive compounds and could also be used for the bioremediation of contaminated environments.20 This process ensures the development of a diverse and well-adapted microbial community that can effectively perform specific functions.21
Estimation of phenolic content according to the Modified Folin-Ciocalteau Method
The process involves a series of steps that are described below.
By following these steps, the phenolic content of the unknown sample can be accurately estimated using the Modified Folin-Ciocalteu Method, thus providing valuable insights into sample composition and potential application.22,23
- We pipetted varying volumes of 0.1, 0.2, 0.3, 0.4, and 0.5 ml of a working standard solution with concentrations ranging from 20 to 10 µg into a series of test tubes. An unknown sample (0 and 125 ml) was then pipetted into a separate test tube.
- To all test tubes, 0.125 ml of Folin’s reagent was added, and the mixtures were incubated at room temperature for 6 min. Subsequently, 1.25 ml of 7% sodium carbonate was added to each test tube.
- The test tubes were incubated at room temperature for 90 min, and the absorbance was recorded spectrophotometrically at a wavelength of 760 nm.
- We plotted a graph representing the concentration of total phenolic compounds along the x-axis and the corresponding optical density readings along the y-axis. This graph was constructed using the absorbance values obtained from the standard solutions.
- The concentration of total phenolic compounds in the unknown sample was calculated using a standard curve. This was achieved by determining the optical density of an unknown sample and interpolating its concentration from the standard curve.24
Estimation of tannins according to the Modified Prussian Blue Method
Tannin estimation involves following the steps:
- Varying volumes (ranging from 0.1 to 0.5 ml) of the working standard solution were pipetted into a series of test tubes. Additionally, 0.1 ml aliquots of the sample were pipetted into separate test tubes.
- Distilled water was added to each test tube to bring the total volume to 7 ml (including a blank). Potassium ferricyanide (1 ml) and 1 ml of FeCl3 was added to all test tubes, and the contents were thoroughly mixed.
- The absorbance was measured spectrophotometrically at a wavelength of 700 nm for each test tube after thorough mixing.
- We plotted a graph representing the tannin concentration along the X-axis and the corresponding optical density readings along the Y-axis. This graph was constructed using the absorbance values obtained from the standard solutions.
- The concentration of tannins in the unknown sample was calculated using a standard curve. This was achieved by determining the optical density of an unknown sample and interpolating its concentration from a standard curve.
According to these steps, the tannin content of the unknown sample was estimated.25,26
In the investigation, four microorganisms were utilized, Staphylococcus aureus, Bacillus subtilis, Trichoderma viridae, and Aspergillus niger. Each of these microorganisms underwent a comprehensive analysis of their morphological and biochemical characteristics as well as enzymatic activities.
Morphological Characterization- Gram staining and lactophenol cotton blue staining methods were performed to study the morphological characteristics of the microorganism. Gram staining provided information regarding the cell wall composition, while lactophenol cotton blue staining facilitated the observation of fungal structures such as hyphae.27
The streak plate method was applied to identify their colony morphology on selective media. Triple sugar iron agar test and Urease test were carried out to identify their biochemical characteristics. Microbes were also screened quantitatively to observe their enzymatic activities on specific substrate media. Activity Of enzymes such as amylase, cellulase, protease, and lipase are visualized on substrate media containing 1% Starch, Carboxymethyl cellulose, casein/gelatine and Tributyrin, respectively.
Household waste degradation using microbial consortia has emerged as an important method for sustainable waste management. Various waste materials such as peanut shells, onion-garlic skins, dried leaves, and stems of leafy vegetables serve as rich sources of carbon and nitrogen for microbial growth and activity. In this study, microbial consortia were prepared and used for the degradation of household waste. The initial step involved identifying microorganisms using Gram staining and lactophenol cotton blue staining. Using a 45 × lens, a Gram-negative bacterium, Escherichia coli, and two Gram-positive bacteria, Staphylococcus aureus and Bacillus subtilis, were visualized, and this provided insights into their morphological characteristics.
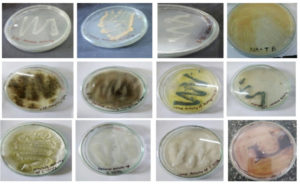
Following identification, the microorganisms were cultured on specific selective media to facilitate growth. Cultural characteristics such as color, shape, size, margin, elevation, and consistency of the colonies were observed. Subsequently, the enzymatic activities of the microorganisms were assessed using substrate media. Staphylococcus aureus and Trichoderma viridae exhibited protease activity on casein gelatin agar, whereas Aspergillus niger demonstrated lipase activity on 1% tributyrin nutrient medium (Table 1 and Figure 1).
Table (1):
Screening of enzyme activity
Enzyme Activity |
Substrate |
Name of Microbes |
Observations |
Result |
|---|---|---|---|---|
Protease Activity |
Casein/ Gelatin Agar |
Staphylococcus aureus |
A zone of Inhibition was observed with yellow-pigmented Irregular Growth On the Surface of the Media |
Positive |
Aspergillus niger |
No zone of inhibition |
Negative |
||
Trichoderma viride |
Zone of inhibition was observed |
Positive |
||
Lipase Activity |
1% Tributyrin + Nutrient Agar |
Bacillus subtilis |
No zone of Inhibition |
Negative |
Aspergillus niger |
Zone of inhibition was observed |
Positive |
||
Trichoderma viride |
No zone of inhibition |
Negative |
Subsequently, the microorganisms were cultured on specific selective media to facilitate growth. Cultural characteristics such as color, shape, size, margin, elevation, and consistency of the colonies were observed (Figure 2).
Experimental test samples were prepared to evaluate the efficacy of microbial consortia for degrading household waste. These included a sample with indigenous microbial activity and four samples containing microbial consortia (BC, ABC, FC, and AFC). Over the course of the experiment, changes in the pH, color, moisture content, and rate of degradation were monitored weekly. This comprehensive analysis provides valuable insights into the effectiveness of microbial consortia for degrading household waste and their potential for implementation in sustainable waste management practices.
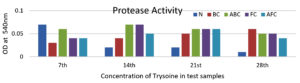
The amounts of enzymes and other nutrients were calculated by various assay methods using standard procedures for all selected samples, and casein + gelatin was used as a substrate to assess protease production. After the analysis of the graphical data (Figure 3 and 4), it was observed that the maximum production of protease occurred after the 7th day of incubation in the N test sample (300 µg/ml), while in the AFC test sample, it was observed as 250 µg/ml after the 21st day. For the sample BC, it was observed as 250 µg/ml after the 28th day of incubation, while ABC- and AFC-supplemented media exhibited a maximum production of 300 µg/ml after the 14th day (Table 2 and 3).
Table (2):
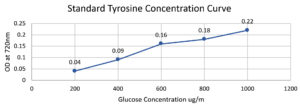
Standard Tyrosine Concentration graph (µg/ml)
Standard Tyrosine |
Optical Density 720nm |
|---|---|
200 µg/ml |
0.04 |
400 µg/ml |
0.09 |
600 µg/ml |
0.16 |
800 µg/ml |
0.18 |
1000 µg/ml |
0.22 |
These data suggest that protease production varied among the different test samples and was influenced by factors such as the composition of the microbial consortia, incubation conditions, and nutrient availability. These findings highlight the importance of optimizing the incubation conditions and microbial consortia composition to maximize enzyme production for various applications.
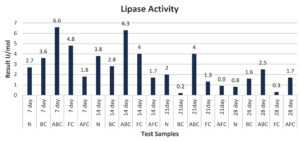
In subsequent studies, lipase activity was investigated using tributyrin as a substrate and was quantified titrimetrically against 50 mM NaOH. The resulting graph indicates that the maximum lipase activity was exhibited by samples ABC, FC, BC, N, and AFC after 7, 14, 21, and 28 d of incubation (Figure 5). Notably, the control sample demonstrated a maximum lipase activity of 6.6 µg/ml after 14 days of incubation (Table 3 and 4).
Table (3):
Protease activity: Optical Density at 540 nm
| Test Samples | Optical Density 540 nm | |||
|---|---|---|---|---|
| 7th Day | 14th Day | 21st Day | 28th Day | |
| Sample N | 0.07 | 0.02 | 0.02 | 0.01 |
| Sample BC | 0.03 | 0.04 | 0.05 | 0.06 |
| Sample ABC | 0.06 | 0.07 | 0.06 | 0.05 |
| Sample FC | 0.04 | 0.07 | 0.06 | 0.05 |
| Sample AFC | 0.04 | 0.05 | 0.06 | 0.04 |
Table (4):
Lipase Activity of Test Samples (µg/ml)
Test Sample |
Incubation period (day) |
Experiment (E) ml |
Control (C) ml |
E-C ml |
Result U/mol |
|---|---|---|---|---|---|
N |
7 |
26.5 |
23.2 |
3.3 |
±2.7 |
14 |
29.1 |
24.5 |
4.6 |
±3.8 |
|
21 |
30.4 |
28 |
2.4 |
±2 |
|
28 |
31.0 |
30 |
1 |
±0.8 |
|
BC |
7 |
27.6 |
23.2 |
4.4 |
±3.6 |
14 |
27.8 |
24.5 |
3.3 |
±2.8 |
|
21 |
28.2 |
28 |
0.2 |
±0.2 |
|
28 |
31.9 |
30 |
1.9 |
±1.6 |
|
ABC |
7 |
31.1 |
23.2 |
7.9 |
±6.6 |
14 |
32.1 |
24.5 |
7.6 |
±6.3 |
|
21 |
32.8 |
28 |
4.8 |
±4 |
|
28 |
33.0 |
30 |
3 |
±2.5 |
|
FC |
7 |
28.9 |
23.2 |
5.7 |
±4.8 |
14 |
29.4 |
24.5 |
4.8 |
±4 |
|
21 |
29.5 |
28 |
1.5 |
±1.3 |
|
28 |
30.4 |
30 |
0.4 |
±0.3 |
|
AFC |
7 |
25.4 |
23.2 |
2.2 |
±1.8 |
14 |
26.5 |
24.5 |
2 |
±1.7 |
|
21 |
29.1 |
28 |
1.1 |
±0.9 |
|
28 |
32.0 |
30 |
2 |
±1.7 |
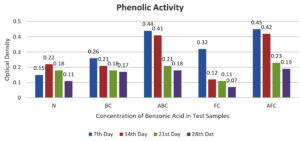
Similarly, the phenolic concentration analysis revealed that the normal sample exhibited a concentration of 225 µg/ml after 14 days of incubation (Figure 6). The graphical observation indicated that samples BC and ABC possessed elevated phenolic concentrations of 260 µg/ml and 440 µg/ml after 7 and 14 days of incubation, respectively (Figure 7 and Table 5 and 6).
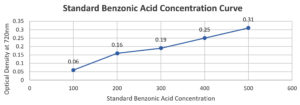
Table (5):
Benzoic Acid Concentration
Test tube |
Standard Solution of Benzoic Acid (µg/ml) |
Optical Density at 720 nm |
|---|---|---|
1 |
100 |
±0.10 |
2 |
200 |
±0.20 |
3 |
300 |
±0.30 |
4 |
400 |
±0.45 |
5 |
500 |
±0.55 |
Table (6):
Optical Density Chart at 720 nm
| Sample | Optical Density at 720 nm | |||
|---|---|---|---|---|
| 7th Day | 14th Day | 21st Day | 28th Day | |
| Sample N | 0.15 | 0.22 | 0.18 | 0.11 |
| Sample BC | 0.26 | 0.21 | 0.18 | 0.17 |
| Sample ABC | 0.44 | 0.41 | 0.21 | 0.18 |
| Sample FC | 0.32 | 0.12 | 0.11 | 0.07 |
| Sample AFC | 0.45 | 0.42 | 0.23 | 0.19 |
These findings underscore the dynamic nature of the enzymatic activity and phenolic concentration over time and highlight the influence of incubation duration on the metabolic processes of the microbial consortia. Moreover, the variation observed among different samples underscores the importance of considering temporal dynamics when assessing biochemical parameters in microbial studies.
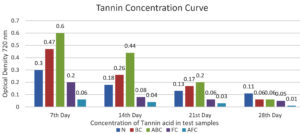
The investigation into bioactive compounds such as tannins revealed concentrations of 475 µg/ml, 750 µg/ml, and 960 µg/ml in the Normal, BC, and ABC test samples, respectively, after 7 days of incubation. These results highlight the varied capacities of different microbial consortia to produce tannins, with sample ABC exhibiting the highest tannin concentration (Figure 8 and 9 and Table 7 and 8).
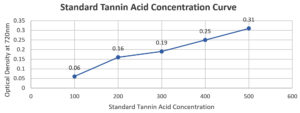
Table (7):
Tannin Concentration
Test tube |
Standard Solution of Benzoic Acid (µg/ml) |
Optical Density at 720 nm |
|---|---|---|
1 |
100 |
±0.06 |
2 |
200 |
±0.16 |
3 |
300 |
±0.19 |
4 |
400 |
±0.25 |
5 |
500 |
±0.31 |
Table (8):
Optical Density at 720 nm
| Sample | Optical Density at 720 nm | |||
|---|---|---|---|---|
| 7th Day | 14th Day | 21st Day | 28th Day | |
| NORMAL [N] | ±0.30 | ±0.18 | ±0.13 | ±0.11 |
| BC | ±0.47 | ±0.26 | ±0.17 | ±0.06 |
| ABC | ±0.60 | ±0.44 | ±0.20 | ±0.06 |
| FC | ±0.20 | ±0.08 | ±0.06 | ±0.05 |
| AFC | ±0.06 | ±0.04 | ±0.03 | ±0.01 |
Conversely, the analysis of glucose concentration indicated maximum levels in samples FC and ABC, where they reached concentrations of 315 µg/ml and 950 µg/ml, respectively, after 7 days of incubation. This suggests that the microbial consortia present in the FC and ABC samples were particularly efficient for metabolizing substrates to produce glucose. These findings emphasize the diverse metabolic capabilities of microbial consortia and their ability to synthesize various bioactive compounds. Additionally, they enhance the importance of understanding and harnessing microbial activity for the production of specific compounds that can be used for various applications, including biodegradation of waste, agriculture, and medicine.
This study yields significant findings regarding the biodegradation of household waste using mixed microbial communities. Mixed microbial communities exhibit higher biodegradation capabilities than single-species cultures. This can be attributed to the diverse genetic information present in different organisms within the community that collectively enables the degradation of complex mixtures of organic compounds in contaminated environments.
Moreover, this investigation demonstrated a promising level of production of industrially important enzymes and phytochemicals by microbial consortia. These enzymes and phytochemicals possess the potential for various industrial applications, including pharmaceutical, nutraceutical, and bioremediation processes.
Furthermore, microbial aerobic degradation of household waste by the microbial consortium was highly significant. This process was observed to reduce the degradation period and offer a more efficient waste management solution.
Household waste degradation using microbial consortia has emerged as a promising approach. Waste materials such as peanut shells, onion-garlic skins, dried leaves, and stems of leafy vegetables are sources of carbon and nitrogen in the medium.
In the present study, for the preparation of the microbial consortia, the first microorganisms were identified by Gram staining and lactophenol cotton blue staining. One gram-negative bacterium, Escherichia coli, and two gram-positive bacteria, Staphylococcus aureus and Bacillus subtilis, were visualized under a 45X microscopic lens.
The microbes were then grown on specific selective media, and their cultural characteristics based on color, shape, size, margin, elevation, and consistency were observed. Protease activity of S. aureus and T. viridae was observed using casein gelatin agar as a substrate. Only A. niger exhibited positive lipase activity in the 1% tributyrin nutrient medium.
The experimental test samples included Normal, BC ABC FC, and AFC. The Normal test sample exhibited indigenous microbial activity, while the remaining four possessed microbial consortia. They were analyzed weekly for changes in pH, color, moisture content, and rate of degradation.
Enzyme and other nutrient contents were calculated according to various assay methods using standard procedures for all selected samples. For protease production Casein. Gelatin was used as a substrate. After the analysis of the graphical data, it was observed that maximum production of protease was observed after the 7th day of incubation in the N test sample (300 µg/ml), while in AFC test sample, it was observed as 250 µg/ml after the 21st day. For sample BC, it was observed as 250 µg/ml after the 28th day of incubation, while ABC- and AFC-supplemented media exhibited a maximum production of 300 µg/ml after the 14th day.
In further experiments, lipase activity was studied, and this requires tributyrin as a substrate and was estimated titrometrically against 50 mM NaOH. Maximum lipase activity was observed on days 7, 14, 21, and 28 in ABC, FC, BG, N, and AFC. The maximum lipase activity by control is 6.6 µg/ml after 14 days of incubation. Similarly, the phenolic concentration observed in the Normal group was 225 µg/ml after 14 days of incubation. From the graphical observation, it was observed that samples BC and ABC possessed high phenolic concentrations of 260 µg/ml and 440 µg/ml after the 7th and 14th day of incubation, respectively.
Assessments of bioactive compounds such as tannins indicated that the levels for the Normal, BC, and ABC test samples were 475 µg/ml, 750l µg/ml, and 960 µg/ml after the 7th day of incubation. Conversely, the glucose concentrations were observed to be maximum in samples FC and ABC at 315 µg/ml and 95 µg/ml after 7 days of incubation.
In the present study, higher biodegradability was observed in mixed communities of microbes, as different genetic information is present in different organisms. This is necessary to degrade mixtures of organic compounds in various contaminated areas. The results also reveal a promising level of production of industrially important enzymes and phytochemicals. Additionally, the microbial aerobic biodegradation of household waste by a microbial consortium is highly significant, as it reduces the time of degradation and produces no foul odor, thus making it environmentally friendly and suitable for urban settings.
Overall, these findings highlight the potential of microbial consortium-based approaches for sustainable waste management, and these approaches offer both environmental benefits and opportunities for industrial applications.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Atlas Bartha, Microbial Ecology fundamentals and application, Fourth edition, Low price edition, Pearson education; 2003
- Bird R, Hopkins RH. The action of some alpha-amylases on amylase. Biochem J. 1954;56(1):86-99.
Crossref - Chapman -Smith A, Cronan JE Jr. Molecular biology of biotin attachment to proteins. J Nutr. 1999;129(2):477-845.
Crossref - Indumathi D. Microbial conversion of vegetable waste for biofertilizer production., Department of Zoology, Vellalar College for Women, Erode-12, Tamilnadu. IOSR J Biotechnol Biochem. 2017.
- Gautam SP, Bundela PS, Pandey AK, Jamaluddin, Awasthi MK, Sarsaiya S. Diversity of cellolytic microbes and the biodegradation of municipal solid waste by a potential strain. Int J Microbiol. 2012;2012:325907.
Crossref - Gautama SP, Bundela PS, Pandey AK, Awasthi MK, Sarsaiya S. Microbial consortium for effective composting of municipal solid waste by enzymatic activities. Journal of Applied Science in Environmental Sanitation. 2010;5(3):301-308.
- Ingale S, Joshi SJ, Gupte A. Production of bioethanol using agricultural waste: Banana pseudo stem. Braz J Microbiol. 2014;45(3):885-892.
Crossref - Joutey NT, Bahafid W, Sayel H, El Ghachtouli N, Biodegradation: involved microorganisms and genetically engineered microorganisms. Biodegradation – Life of Science. 2013.
Crossref - Janarthanan R, Prabhakaran P, Ayyasamy PM. Bioremediation of vegetable waste through bio manuring and enzyme production. Int J Curr Microbial App Sci. 2014;3(3):89-100.
- Laufenberg G, Kunz B, Nystroem M. Transformation of vegetable waste into value added products:: (A) the upgrading concept; (B) practical implementations. Bioresour Technol. 2003;87(2):167-198.
Crossref - Mussatto SI, Machado EMS, Carneiro LM, Teixeria JA. Sugar metabolism and ethanol production by different yeast strains from coffee industry waste hydrolysis. Applied Energy. 2012;92:763-768.
Crossref - Dorothy MH, Gibson AH. Cellulose Decomposition and Associated Nitrogen Fixation by Mixed Cultures of Cellulomonas gelida and Azospirillum Species or Bacillus macerans. Appl Environ Microbial. 1985;50(4):1021-1026.
Crossref - Nelson DL, Cox MM. Principles of Biochemistry fourth edition (Freeman publisher) New York. Editorial team of laboratory Info Gram staining January 19, 2019, laboratory Info.Com. Environ Res. 2004;146 (2016):161-172.
- Novinsak A, Surette C, Allain C, Filion M. Application of molecular technologies to monitor the microbial content of biosolids and composted biosolids. Water Sci Technol. 2008; 57(4):471-77.
Crossref - Pothiraj C, Eyini M. Enzyme activities and substrate degradation by Fungal Isolates on Cassava waste during solid-state fermentation. Microbiology. 2007;35(4):196-204.
Crossref - Panda SK, Mishra SS, Kayitesi E, Ray Ramesh C. Microbial Processing of fruit and vegetable waste for the production of vital enzymes and organic acids, biotechnology, and scopes. Environmental Research, 2016;146-161-72.
- Sarkar P, Meghvanshi M, Singh R, Microbial Consortium: A New approach in effective degradation of organic Kitchen wastes. International Journal of environmental science and development, 2011;2(3):170-174.
- Rao RKM, Sivagnanam SK, Syed FB. Isolation, characterization and identification of predominant microorganisms from agro waste. Der Pharmacia Lettre. 2016;8(5):79-86.
- Steger K, Sjogren AM, Jarvis A, Jansson JK, Sundh I. Development of compost maturity and Actinobacteria populations during full scale composting of organic household waste. J Appl Microbial. 2007;103(2):487-498.
Crossref - Sanmaneet N., Panishkan K., Obsuwan K., Dharmvanij S. Study of compost maturity during humification process using UV-Spectroscopy. World Acad Sci Eng Technol.2011; 80: 403-05
- Payel S, Mukesh M, Rajni S. Microbial consortium: A New approach in effective degradation of organic Kitchen wastes. International Journal of Environmental Science and Development, 2011;2(3).
- Sethi S, Datta A, Gupta BL, Gupta S. Optimization of Cellulase Production from Bacteria Isolated from Soil. ISRN Biotechnol. 2013;2013:985685.
Crossref - Unakal C, Kallur RI, Kaliwal BB. Production of alpha amylase using banana waste by Bacillus subtilis under solid water fermentation. Eur J Exp Biol. 2012;2:1044-1052.
- Umsakul K, Dissara Y, Shrimuang N. Chemical physical and microbiological changes during composting of the water hyacinth. Pak J Biol Sci. 2010;13(20):985-992.
Crossref - Wijngaard HH, Roble C, Brunton N. A survey of Irish fruit and vegetable waste and by products as a source of phenolic antioxidants. Food Chem. 2009;116(1):202-207.
Crossref - Yoshida S, Hiraga K, Takehana T, et al. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science. 2016;351(6278):1196-1199.
Crossref - Chandak AM, Balpande R. Biodegradation of Household Waste Using Micro- Organisms For Production of Important Products. Biosci Biotechnol Res. 2021;14(9):291-298.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.