ISSN: 0973-7510
E-ISSN: 2581-690X
Bacteriocins have attracted much attention in the field of biopreservation and human therapeutics. Therefore a study was carried out to isolate a bacteriocin producing lactic acid bacteria from the fermented appam batter using MRS medium. The bacteriocin produced by the isolate was active against Listeria monocytogenes MTCC 657 and Acinetobacter baumannii MTCC 1425 among the test organisms examined. The bacteriocin producing organism was identified as Pediococcus pentosaceus by standard microbiological methods and 16s rRNA sequencing. Media optimization was carried out by altering the initial pH, time and temperature employed in the production of bacteriocin. In this study, enhanced bacteriocin production was observed at pH 6.0, temp 30 °C and 24 h. The concentration of major carbon and nitrogen sources were also studied for their influence on bacteriocin production. It was found that the bacteriocin activity was increased when the carbon source –dextrose concentration was increased in MRS medium. However, nitrogen source did not have significant impact on bacteriocin production. This indicates the organism P. pentosaceus produced bacteriocin and exhibited significant growth inhibition against the indicator organisms. Therefore, the bacteriocin can be either used as a biopreservative or as an antibiotic to treat clinical pathogens.
Appam batter, Lactic acid bacteria, P. pentosaceus, Bacteriocin, peptide antibiotics
The microbiology of many fermented foods is quite complex and yet to be explored in a greater way. The microbial fermentation process improves the shelf life, texture, taste and aroma of the final products. These properties aid as bio-preservatives in the food industries (Blandino et al., 2003). Indigenous fermented foods have been prepared and consumed for thousands of years. They are strongly linked to culture, traditions and reveal the intellectual richness of indigenous people of the country in terms of their ability to prepare microbial products for varied purposes in addition to food and beverages (Sekar and Mariappan, 2007). However, the preparation of indigenous or “traditional” fermented foods and beverages remain as a household art even today (Larry and Beuchat, 2008).
Lactic Acid Bacteria (LAB) have been used to ferment or culture foods for at least 4000 years. A wide variety of strains are routinely employed as starter cultures in the manufacture of dairy, meat, vegetable and bakery products. One of the most important contributions of these microorganisms is the extended shelf life of the fermented product by comparison to that of the raw substrate. Growth of spoilage and pathogenic bacteria in these foods is inhibited due to competition for nutrients and the presence of starter-derived inhibitors such as lactic acid, hydrogen peroxide and bacteriocins (Ray and Daeschel, 1992).
Bacteriocins become one of the weapons against microorganisms due to the specific characteristics of large diversity of structure and function, natural resource, and being stable to heat. Therefore, bacteriocins may become a potential drug candidate for replacing antibiotics in order to treat multiple drug resistance pathogens in the future (Yang et al., 2014). These bacteriocins are antibacterial proteins vary in spectrum of activity, mode of action, molecular weight, genetic origin and biochemical properties. These substances are produced by various gram-positive and gram-negative bacteria (Moreno et al., 2000). During the past years, antimicrobial compounds produced by Lactic Acid Bacteria (LAB) have been subjected to intensive study because of their potential use for the manufacture of wide variety of traditional fermented foods (Nettles and Barefoot, 1993).
Increasing number of reports on new/novel bacteriocins with unique properties indicate that there is still a large scope to learn about this family of peptide antibiotics. These antimicrobial peptides have huge applications in food preservation and in next-generation antibiotics targeting the multiple-drug resistant pathogens. The unique properties like thermal stability, pH tolerance and no reports on the development of resistant bacteria, made bacteriocins a potential molecule (Perez et al., 2014). Even though bacteriocins are good in controlling the food-borne pathogens, they are naturally safe by losing their activity due to the cleavage of bacteriocins due to gastrointestinal (GI) tract protease (Saavedra et al., 2004).
Many fermented food items are associated with the life style of the people of southern states in India. The present study aims to isolate bacteriocin producing lactic acid bacteria from ‘appam’ batter and optimization of process parameters for enhanced production of bacteriocins with antibacterial activity.
Appam is a type of pancake made from fermented rice batter and coconut milk. It is a common food in the South Indian states of Kerala and Tamil Nadu. This dish is made by steam-cooking the batter, and is eaten most frequently.
Chemicals
de Man, Rogosa and Sharpe (MRS) broth, Nutrient broth, Muller Hinton Agar (MHA), Agar agar type I, Peptone, Tween 20, Tween 80, CTAB SDS, EDTA, Ammonium sulphate and Cellulose nitrate membrane were procured from Hi-Media , Mumbai. All the other chemicals and reagents used in the study were of highest purity available.
Microorganisms
The microorganisms used in this study were Acinetobacter baumannii MTCC 1425, Methicillin-resistant Staphylococcus aureus MTCC 1430 (MRSA) identified as hospital pathogens. Listeria monocytogenes MTCC 657 a food pathogen, Lactococcus lactis subsp.lactis MTCC 440 and Enterococcus faecalis MTCC 31 59 were well known bacteriocin producers. All these cultures were obtained from Microbial Type Culture Collection and Genebank (MTCC), IMTECH, Chandigarh.
Collection and processing of appam batter
‘Appam’ batter was collected from a local market in Malappuram, Kerala and transported to the laboratory in an aseptic container by maintaining low temperature and processed within 24 h. One gram of ‘appam’ batter sample was diluted into 100 ml of 0.1% (w/v) peptone water and incubated at 37 °C under shaking conditions at 100 rpm for 1- 2 h. Later the contents were serially diluted (tenfold serial dilution) with 0.1 % (w/v) peptone water. 100 microlitres of aliquot from 10-7 dilution was spread onto sterile MRS agar plates and incubated at 37 °C for 48 h under anaerobic condition. The colonies grown on MRS agar plates were propagated further separately on fresh MRS agar plates by quadrant streaking. This process was repeated thrice to obtain pure culture. The well grown isolates on MRS agar plates were labeled as AB1, AB2, and AB3 and so on for further references. The labeled individual isolates were stored at 4 °C on MRS agar slants for further studies.
Preparation of cell-free culture filtrates (CCFs)
The selected isolates were inoculated into 100 ml of sterile MRS broth in 250 ml Erlenmeyer flasks and incubated at 37 °C using shaking incubator at 125 rpm for 24 h. The fermented broth was centrifuged at 13,000 rpm for 15 min at 4 °C and the supernatant collected was adjusted to pH 6.5 either using 1 N NaOH or 1 N HCl , and filtered through 0.45 µm filter, known as cell-free culture filtrate (CCF) which would be used for further studies.
Preliminary screening of LAB for antagonistic activities
The CCFs of individual LAB isolates were screened for antagonistic activity against the indicator organisms Acinetobacter baumannii MTCC 1425, Methicillin-resistant Staphylococcus aureus MTCC 1430 (MRSA), Listeria monocytogenes MTCC 657, Lactococcus lactis subsp.lactis MTCC 440 and Enterococcus faecalis MTCC 3159 using MHA plates by well diffusion method as described by Vignolo (1993). The MH agar plates were prepared and seeded with individual indicator organisms. The wells with 6 mm diameter were made on the agar plates, loaded the 50 µl of CCF and incubated at 37 °C for 24 h. The diameter (in mm) of zone of inhibition was measured and the bacteriocin activity was determined. The LAB isolate that showed highest antagonistic activity was selected for further studies.
Examination of CCF for antibacterial activity and bacteriocin assay
The selected LAB isolate was inoculated into 100 ml of sterile MRS broth and the production of bacteriocin was carried out. Then the CCF was processed accordingly.
The assay of bacteriocin activity was carried out as described by Usmiati and Marwati, (2009) by the agar well method using the indicator organisms. Fifty µl of CCF from the selected LAB isolate was loaded into 6 mm diameter wells in Mueller-Hinton agar plates previously seeded with indicator organisms like L. monocytogenes MTCC 657 and A. baumannii MTCC 1425. After 24 h of incubation at 37 °C, the plates were measured for the zone of growth inhibition of indicator organisms. In subsequent assays, we selected L. monocytogenes (food-borne pathogenic bacteria), and A. baumannii (human pathogen) to evaluate the antibacterial effects of CCF of P. pentosaceus. The bacteriocin activity was expressed as arbitrary units per milliliter (AU/ml). One AU of bacteriocin was defined as a unit area of inhibition zone per unit volume of bacteriocin added, in this case mm2 / ml. The bacteriocin activity (AU / ml) was calculated using the following formula:
Arbitrary Unit /ml = Lz – Ls / V
Lz = clear zone area (mm2)
Ls = well area (mm2)
V = volume of sample (ml)
All the assays were carried out in triplicates for individual indicator strains L. monocytogenes MTCC 657 and A. baumannii MTCC 1425.
Morphological characteristics of the bacteriocin producing LAB culture
The selected bacteriocin producing LAB isolate was examined by morphological, physiological and biochemical characteristics according to the criteria of Bergey’s manual of Determinative Bacteriology (1994), and the results were recorded.
Identification of bacteriocin producing LAB isolate by 16S rRNA sequencing and phylogenetic relationship
Genomic DNA from the selected LAB isolate was isolated by the method described by Galvez et al., (2007). The 16S rRNA was amplified using both forward and reverse primers (16S1 : 5’-GCTCACCCTTAACCC-3’ and 16S2: 5’ACCTTCCAAGGGCCTAC-3’) of genomic DNA. The assay was performed by using Taq DNA polymerase and buffers in the thermo cycler for 30 cycles comprising 95 °C denaturation for 30 s, 55 °C annealing for 30 s and 72 °C for extension for 45 s. The PCR amplified rRNA product was purified using the quick PCR purification kit. The analysis of alignment, homology and the construction of phylogenetic tree was performed. The nucleotide sequences determined in this study have been submitted to GenBank for assigning the Accession No.
Optimization of process parameters for bacteriocin production
The selected LAB isolate was used as a starter culture for the optimization of bacteriocin production by conventional method.
Effect of temperature on bacteriocin production
The selected LAB isolate was inoculated into a 250 mL Erlenmeyer flask containing 100 ml of sterile MRS broth (initial pH 6.5±0.2). The flasks were maintained at different temperatures viz 25, 30, 35, 40 and 45 °C for 24 h in a shaker cum incubator (125 rpm). All other parameters like medium components, incubation time and pH were kept constant. After the incubation, the CCF was prepared and subjected to antibacterial activity.
Effect of initial pH on bacteriocin production
The influence on initial pH of MRS medium was analysed for bacteriocin production. MRS medium was prepared and the initial pH was checked and adjusted to 4.5, 5.0, 5.5, 6.0, 6.5, 7.0 and 7.5 using either 1 N NaOH or 1 N HCl. The selected LAB isolate was inoculated into 250 ml Erlenmeyer flasks containing 100 ml of sterile MRS broth with varying pH and incubated at 37 °C for 24 h for the production of bacteriocin under shaking conditions (125 rpm) in a shaker cum incubator. All other parameters like medium components, incubation time and temperature were kept constant. After the incubation, the CCF was prepared and subjected to antibacterial activity.
Effect of incubation time on bacteriocin production
Briefly, 100 mL of MRS broth was prepared, inoculated with the selected LAB and incubated at different time intervals like 0, 12, 24, 36, 48, 60 and 72 h. After the respective time intervals, an aliquot of CCF was collected by centrifugation to measure the optimum time of bacteriocin activity.
Effect of carbon and nitrogen sources on bacteriocin production
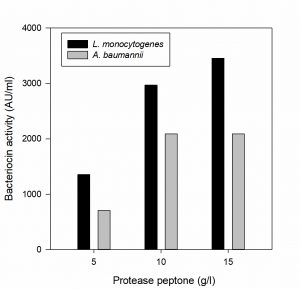
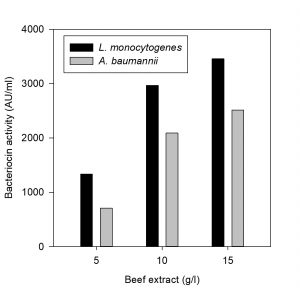
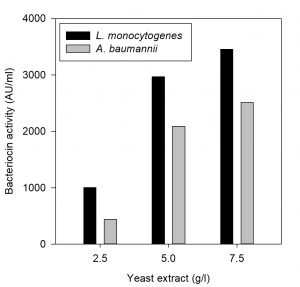
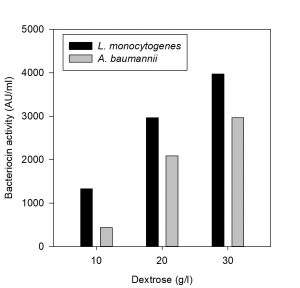
The effect of carbon and nitrogen sources on the production of bacteriocin was evaluated (Hoda et al., 2013). An experimental setup was prepared for the production of bacteriocin by varying the concentrations of carbon and nitrogen sources in the MRS medium (table 1). The MRS broth served as control in this experiment, whereas three sets of run were carried out by varying the concentration of carbon and nitrogen constituents (protease peptone, beef extract, yeast extract and dextrose). All the experiments were carried out by inoculating the selected bacteriocin producing isolate at 37°C in incubator cum shaker (125 rpm) for 24 h. The CCFs obtained were tested for antimicrobial activity against the indicator organisms L. monocytogenes MTCC 657 and A. baumannii MTCC 1425.
Table (1):
Experimental setup for screening the influence of carbon and nitrogen sources for the production of the bacteriocin.
| S.No | Modified ingredient in MRS broth | Concentration (g/l) |
|---|---|---|
| 1 | Protease peptone (g/l) | 5.0 |
| 10.0 | ||
| 15.0 | ||
| 2 | Beef Extract (g/l) | 5.0 |
| 10.0 | ||
| 15.0 | ||
| 3 | Yeast Extract (g/l) | 2.5 |
| 5.0 | ||
| 7.5 | ||
| 4 | Dextrose (g/l) | 10.0 |
| 20.0 | ||
| 30.0 | ||
| 5 | MRS broth | — |
Partial purification of bacteriocin from CCF by ammonium sulphate precipitation and dialysis
The CCF obtained from the selected LAB isolate was saturated with 60, 70 and 80 % solid ammonium sulphate with continuous stirring until dissolving the salt. Then the contents were kept undisturbed at 4 °C overnight with an occasional stirring (Yang et al., 1992). Later the contents were centrifuged at 16,500 rpm at 4 °C for 30 min. The pellet and supernatant were separated. The pellet was reconstituted with sterile water and both supernatant and reconstituted pellet sample were dialysed against 10 mM sodium phosphate buffer (pH 6.5) using a tubular cellulose membrane dialysis bag. The buffer was changed 3-4 times in an interval time of 6-7 h. at 10 °C. Bacteriocin assay was performed in all the fractions after dialysis.
Protein Determination
The amount of protein present in the crude bacteriocin samples like CCFs and the fractions obtained after partial purification process were determined by Lowry’s method (Lowry et al., 1951) using bovine serum albumin fraction-V (BSA) as a standard.
Characterization of partially purified bacteriocin
The partially purified bacteriocin sample from the selected LAB isolate was evaluated for its antimicrobial activity with respect to the influencing factors like temperature, pH, and susceptibility to denaturation by enzymes, surfactants, metals and different concentrations of NaCl (Shiba et al., 2013).
Effect of temperature on bacteriocin activity
Ten milliliters of partially purified bacteriocin sample obtained from the selected LAB isolate was exposed to various temperatures like 20, 35, 50, 65, 80, 95 and 110 °C for 2 h and the fractions from each sample was examined for bacteriocin activity against the indicator organisms by agar well diffusion method.
Effect of pH on bacteriocin activity
Ten milliliters of partially purified bacteriocin sample obtained from the selected LAB isolate was adjusted to pH 3.5, 4.5, 5.5, 6.5, 7.5, 8.5 and 9.5 by adding either sodium hydroxide (1N NaOH) or hydrochloric acid (1N HCl) and incubated for 2 h at room temperature. Residual bacteriocin activity of each of the samples was determined against the indicator organisms by agar-well diffusion method (Rattanachaikunsopon and Phumkhachorn, 2006).
Effect of surfactants on bactericidal activity
The effect of surfactants on the activity of bacteriocin was examined by adding non-ionic (Tween 20: 0.5 % (v/v); Tween 80: 0.5 % (v/v)) and ionic (SDS 0.1 % (w/v), CTAB 0.1 % (w/v)) surfactants to the partially purified bacteriocin. The samples were incubated at room temperature for 2 h and assayed for antimicrobial activity against indicator organisms by well diffusion assay as described by Hoda et al., (2013). The MRS broth with the same concentration of surfactants served as control and the zone of inhibition was measured.
Effect of enzymes on bacteriocin activity
Sensitivity of partially purified bacteriocin was examined by treating the bacteriocin sample with enzymes like trypsin, papain and a-amylase at a final concentration of 1 mg/ml (in phosphate buffer at pH 6.0). The contents were incubated at 37 °C for 2 h and the remaining antimicrobial activity was determined against the indicator organisms (Shiba et al., 2013).
Effect of metal ions on bacteriocin activity
The impact of metal ions on bacteriocin activity was analysed by adding MgSO4 and CuSO4 at 0.5 % (w/v) level to the partially purified bacteriocin and the samples were incubated at room temperature for 2 h. The bacteriocin activity of each sample was determined against the indicator organisms by agar well diffusion assay (Rushdy and Gonnaa., 2013).
Effect of NaCl on bacteriocin activity
The influence of sodium chloride concentration at different levels on bacteriocin activity was performed by adding 2, 4, 6 and 8 % (w/v) NaCl to partially purified bacteriocin samples. After 2 h of incubation at room temperature, the samples were examined for bacteriocin activity against the indicator organisms by agar well diffusion method (Hoda et al., 2013).
Molecular mass determination
The molecular weight of partially purified bacteriocin was determined by tricine-SDS-PAGE gel electrophoresis (Hailer et al., 2007). Ten µg of partially purified bacteriocin mixed with sample loading buffer (15 mM Tris HCl pH 6.8, 2.3 % SDS, 10 mM 2- mercaptoethanol, 20 % glycerol, 1 % bromophenol blue) was loaded in to the wells and was run on 10 % tricine – SDS-PAGE. After the run, the gel was removed and cut into half. The half containing sample and molecular weight marker were stained with coomassie brilliant blue R 250 (Sambrook et al., 1989). The other half containing the samples was processed as described by Mirhosaini et al., (2006). Then the gel was placed in a Petri dish and overlaid with 7 ml of 0.6 % (w/v) agar containing the indicator organisms. Then the plate was incubated at 37 °C for 24 h and the antimicrobial activity of bacteriocin was screened by clear zone of inhibitions.
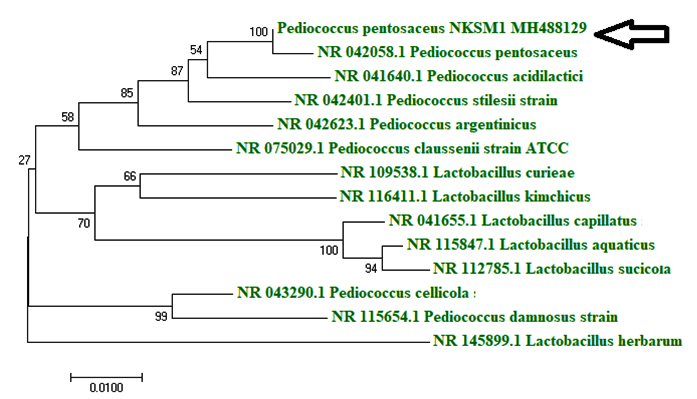
Isolating industrially important microbial strains from natural sources have always proved to be successful and found to possess applications in both food and health care industries (Young et al., 2012). In this study, well developed individual colonies (6 CFU) were observed in MRS agar plate (10-7) from appam batter sample and they were labeled as AB1, AB2, AB3, AB4, AB5 and AB6 and stored at 4 °C on MRS agar slants for further studies. The cell free culture filtrates obtained from the isolated colonies were tested against the indicator organisms like Listeria monocytogenes (food-borne pathogen), Acinetobacter baumannii (human pathogen) and Methicillin-resistant Staphylococcus aureus (MRSA (a multidrug resistant)) on MHA plates, it was found that isolate AB3 exhibited highest (Table 2) antagonistic activity especially against A. baumannii and L. monocytogenes. Upon analyzing the morphological characteristics of AB3, it was observed to be Gram (+) coccus, catalase (-) ve, non-sporulating and non motile. With respect to carbohydrate fermentation pattern, it was noted that sugars were fermented without gas production. Upon examining the characteristics of AB3 isolate, it was confirmed to be lactic acid bacterium Pediococcus pentosaceus and it was characterized based on its morphological, biochemical and molecular characteristics (Bajpai et al., 2016b; Casaburi et al., 2016). Results obtained by 16S rRNA sequencing revealed 100 % homology to P. pentosaceus and the strain was designated P. pentosaceus NKSM 1 (Fig. 1).
Table (2):
Antimicrobial activity (AU/ml)* of CCFs of 6 LAB isolates against the different indicator organisms.
| Indicator strain | LAB isolates from fermented appam batter | |||||
|---|---|---|---|---|---|---|
| AB1 | AB2 | AB3 | AB4 | AB5 | AB6 | |
| Listeria monocytogenes MTCC657 | – | 5105 | – | – | – | |
| Acinetobacter baumannii MTCC 1425 | – | – | 3974 | – | – | – |
| Methicillin-resistant Staphylococcus aureus MTCC 1430 (MRSA) | – | – | – | – | – | – |
| Enterococcus faecalis MTCC3159 | – | – | 204 | – | – | – |
| Lactococcus lactis subsp.lactis MTCC440 | – | – | – | – | – | – |
*Zone of growth inhibition of indicator organisms in diameter (mm) was used to calculate the antimicrobial activity.
According to Devirgiliis et al., (2013) and Yousif (2003), the most important genera of LAB associated with fermented food includes Lactococcus, Pediococcus, Enterococcus, Oenococcus, Leuconostoc, Lactobacillus, Carnobacterium and Weissella. Even in the past, researchers have demonstrated the predominance of LAB on fermented food items such as meat, fish, dairy products, fruits, vegetables, cereals (Abdel gadir et al., 2001; Grosu-Tudor et al., 2014; Hwanhlem et al., 2011; Pringsulaka et al., 2012; Sulieman et al., 2006; Yang et al., 2012). Generally lactobacilli represent a major LAB member within the microorganisms isolated from fermented cereal, meat, dairy and vegetable sources (Corsetti and Settanni, 2007; Devirgillis et al., 2013). Moreover, they play a pivotal role in fermented food as a starter culture (Ratanaburee et al., 2013). Therefore, P. pentosaceus was selected as a good bacteriocin producer and used as a starter culture for the rest of the experiments.
The antibacterial activity of CCF’s of P. pentosaceus against the tested food borne and human pathogenic bacteria were confirmed by the presence or absence of inhibition zones on the agar well plates. Amenu, (2013) reported that other LAB have shown potential antibacterial effects against a number of foodborne pathogens. Also, a variety of pathogenic and foodborne pathogenic bacteria exhibited susceptibility to LAB (Tadesse et al., 2005; Carina Audisio et al., 2011; Darsanaki et al., 2012; Yah et al., 2014).
In the current study, we report the efficacy of bacteriocin produced by P. pentosaceus against the selected the gram negative indicator bacteria A. baumannii. Antimicrobial activity of LAB isolates against Gram-negative bacteria was not previously demonstrated by any of the investigators. The reasons may be attributed to the presence of lipopolysaccharide layer of the cell wall protecting the cell membrane, the site of action of bacteriocins (Stevens et al., 1991). Activity against Gram negative bacteria is an unusual phenomenon which has been rarely documented (Cheikhyoussef et al., 2009; De Kwaadsteniet, et al., 2005; Gong et al., 2010; Todorov and Dicks, 2005). Few other authors like Skytta et al., (1993) reported the inhibitory activity of P. damnosus and P.pentosaceus against a range of gram negative bacteria. The inhibition activity of both gram positive and gram negative bacteria may be resulted due to competition, lactic acid, bacteriocin and hydrogen peroxide production.
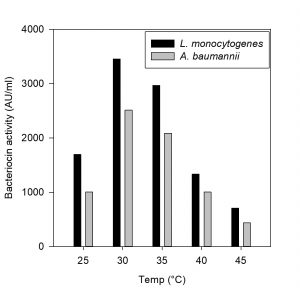
Fig. 2. Effect of temperature on bacteriocin production against L. monocytogenes MTCC657 and A. baumannii MTCC 1425
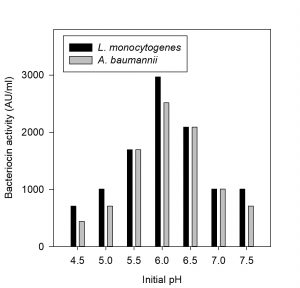
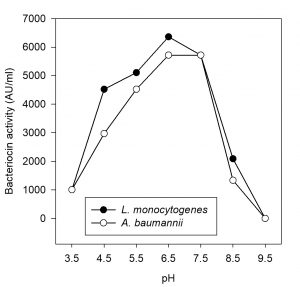
Fig. 3. Effect of different initial pH on bacteriocin production against Listeria monocyto-genes MTCC65 and Acinetobacter baumannii MTCC 1425
Optimization of process parameters for enhanced production of bacteriocin by conventional method
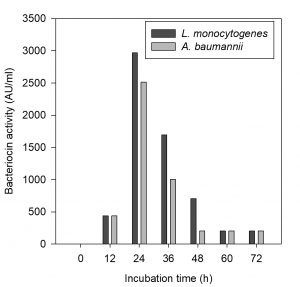
The CCFs of Pediococcus pentosaceus NKSM1 showed maximum bacteriocin production at 30 °C for both Listeria monocytogenes MTCC 657 and Acinetobacter baumannii MTCC 1425 (Fig. 2). Here when the temperature was increased, we could notice that there was a decline in bacteriocin production. Likewise, enhanced bacteriocin production was observed at pH 6.0 (Fig. 3) for both L. monocytogenes MTCC 657 and A. baumannii MTCC 1425. Similarly with respect to incubation time period, among the tested hours, improved production of bacteriocin was noted at 24 h for both L. monocytogenes MTCC 657 and A. baumannii MTCC 1425 (Fig. 4), as the incubation period was increased we observed a decrease in bacteriocin production.
Fig. 4. Production of bacteriocin by Pediococcus pentosaceus at various fermenta-tion time intervals
Optimization of carbon and nitrogen sources in medium constituents
The effect of major carbon and nitrogen sources for the bacteriocin production using P. pentosaceus was evaluated (Hoda et al., 2013) and an experiment was prepared by altering the carbon and nitrogen sources (protease peptone, beef extract, yeast extract, dextrose) in the MRS medium as mentioned in the table 1, presented as Figs. 5-8.
Partial Purification of bacteriocin
The partial purification results showed 70-80% saturation rate of ammonium sulphate increased the possible interactions with proteins which lead to faster elution with higher activity of the bacteriocin. The partially purified bacteriocin from P. pentosaceus exhibited the maximum activity of 8482 AU/mL against L. monocytogenes followed by 7037 AU/mL against A. baumannii. The amount of protein was found to be in the range of 0.84 mg/mL. Similar partial purification procedures were previously employed for bacteriocin produced by L. plantarum and E. mundtii (Todorov et al., 2004; Granger et al., 2005; Todorov et al., 2005).
The partially purified bacteriocin activity was increased by 41.2 % and 45.69 % for Listeria monocytogenes MTCC 657 and Acinetobacter baumannii MTCC 1425 respectively. The fold of purification and the overall yield and specific activity were summarized in tables-3, 3a, 3b, 3c.
Table 3. Partial purification of bacteriocin from Pediococcus pentosaceus isolated from fermented appam batter by ammonium sulphate precipitation method
Table (3a):
Purification table of bacteriocin.
| Indicator strains | Bactericidal activity (AU/ml) | |
|---|---|---|
| Cell-free Culture Filtrate | Partially purified (pellet obtained at 60-80 % saturation of ammonium sul-phate) | |
| Listeria monocytogenes MTCC657 | 3,974 | 8482 |
| Acinetobacter baumannii MTCC 1425 | 2,969 | 7037 |
Table (3b):
Activity against Listeria monocytogenes MTCC 657.
Sample |
Total volume (ml) |
Protein conc. (mg/ml) |
Total proteins (mg) |
Total activity (AU) |
Specific activity (AU/mg) |
Fold of purity |
Act. Yield |
|---|---|---|---|---|---|---|---|
Cell-free culture filtrate (CCF) |
98.3 |
16.2 |
1592 |
390644 |
245.4 |
1 |
100 |
Reconstituted pellet after dialysis |
12.0 |
0.84 |
10.08 |
101784 |
10098 |
41.2 |
39.7 |
Table (3c):
Activity against Acinetobacter baumannii MTCC 1425.
Sample |
Total volume (ml) |
Protein conc. (mg/ml) |
Total proteins (mg) |
Total activity (AU) |
Specific activity (AU/mg) |
Fold of purity |
Act. Yield |
|---|---|---|---|---|---|---|---|
Cell-free culture filtrate (CCF) |
98.3 |
16.2 |
1592 |
291853 |
183.35 |
1 |
100 |
Reconstituted pellet after dialysis |
12.0 |
0.84 |
10.08 |
84444 |
8377.4 |
45.69 |
28.9 |
Characterization of partially purified bacteriocin
The influence of pH, temperature, presence of metal ions, surfactants on the antimicrobial activity of partially purified bacteriocin by P. pentosaceus was examined against the indicator organisms used in this study. The bacteriocin activity was assayed by subjecting the partially purified bacteriocin from P. pentosaceus to different temperatures were shown in table 4.
Table (4):
Effect of temperature on antimicrobial activity of partially purified bacteriocin.
| Temperature (in °C) |
Zone of inhibition (mm) | |
|---|---|---|
| L. monocytogenes MTCC657 | A. baumannii MTCC 1425 | |
| 20 | 2,969 | 2,089 |
| 35 | 7,037 | 5,718 |
| 50 | 5,718 | 5,105 |
| 65 | 3,456 | 2,513 |
| 80 | 440 | 204 |
| 95 | 0 | 0 |
| 110 | 0 | 0 |
The bacteriocin was incubated for 2 h at different conditions and the residual activity was measured. The partially purified bacteriocin was found to be stable at 65 °C, which enables them to be an efficient candidate for industrial applications.
In the present study, bacteriocin from P. pentosaceus was observed to be highly stable at 65 °C with activity of over 3,456 and 2,513 AU/mL for L. monocytogenes and A. baumannii respectively. On the other hand, at 80 °C, a negligible amount of bacteriocin activity was observed whereas at higher temperatures of 95 and 110 °C, bacteriocin activity was completely absent. The results clearly showed that bacteriocin from P. pentosaceus can withstand up to a maximum of 65°C. The bacteriocin was able to withstand pasteurisation which is related to its molecular structure composed of small peptides with no tertiary structure (Parda et al., 2007) which was an important characteristic of a bio- preservative. The stability at broad temperature ranges has been attributed to the unusual amino acids in antimicrobial substances which provide strength to tolerate variations [Bhonsle et al., 2013]
Effect of pH sensitivity on bactericidal activity
The partially purified bacteriocin retained its antimicrobial activity even after incubation for 2 h at pH range of 4.5 to 7.5 and the activity was found to be reduced when the pH was increased to 8.5 and 9.5. However, minimal activity was observed at pH 3.5. This shows that the bacteriocin activities are stable at acidic to neutral pH. The results are shown in Fig. 9. Several bacteriocins produced by Pediococcus spp remained stable at a pH range between 2 to 10 but when these bacteriocins were exposed to high pH (12), there was a loss in activity (Albano et al., 2007). Reduction in activity is ascribed to proteolytic degradation and aggregation of protein (Aasen et al., 2000). Likewise, P. pentosaceus MTCC 5151 showed optimum activity at pH 5.5 (Agarwal and Dharmesh 2012). P. pentosaceus NRC AM1 and P. pentosaceus NRC AM4 grew well at pH 4.0 to 8.0 (Mabrouk et al., 2014).
Effect of surfactants on bacteriocin activity
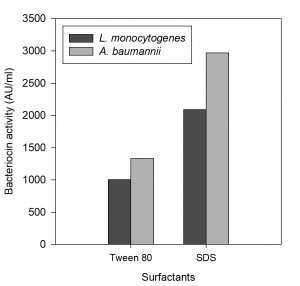
The influence of both non-ionic and ionic surfactants on antimicrobial activity of bacteriocin are depicted in table 5.
Table (5):
Effect of surfactants on the antimicrobial activity of partially purified bacteriocin.
| Surfactant | Zone of growth inhibition (mm) | |
|---|---|---|
| L. monocytogenes MTCC657 | A. baumannii MTCC 1425 | |
| Tween 20 (0.5 % v/v) | – | – |
| Tween 80 (0.5 % v/v) | 1,005 | 1,335 |
| SDS (0.1 % w/v) | 2,089 | 2,969 |
| EDTA Na2 (0.1 % w/v) | – | – |
| CTAB (0.1 % w/v) | – | – |
The anionic surfactant SDS was found to enhance the antimicrobial activity of the partially purified bacteriocin, comparing with the non-ionic surfactant Tween 80 (Fig. 9), whereas other surfactants like tween-20 and CTAB completely suppressed the bacteriocin activity when tested against L. monocytogenes and A. baumannii. The activity of bacteriocin did not decrease when treated with 0.1 % SDS. These results are similar to the findings of Todorov and Dicks, (2005) and Ivanova et al., (2000).
Effect of enzymes on partially purified bacteriocin
The partially purified bacteriocin from P. pentosaceus lost its antimicrobial activity to the enzymes like trypsin, papain and but not for a-amylase. Hence it can be concluded that the bacteriocin produced by P. pentosaceus was proteinaceous nature.
Effect of metal ions on bacteriocin activity
The impact of metal ions on bacteriocin activity was analysed with CuSO4 and MgSO4 at 0.5 % (w/v) concentration and observed that both the metals interfered with the antagonistic activity. Similar observations were reported by Graciela et al. (1995) in L. casei. In an another study, Kabore et al., (2013), has reported that the activity of bacteriocin produced from Bacillus subtilis was completely lost when metal ions such as Fe 2+, Mg 2+ or Mn 2+ was added to growth media. But opposite findings were reported by Von Mollendorff et al., (2006) in which addition of MgSO4 increased bacteriocin activity in case of L.fermentum JW11BZ
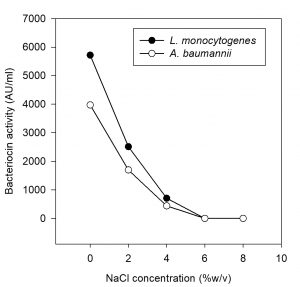
Effect of salt (NaCl) concentration on bacteriocin activity
The effect of salt concentration on the activity of bacteriocin was screened and found that the antimicrobial activity of bacteriocin was reduced even at 4 % of sodium chloride. The growth of LAB is sometimes better in the presence of low salt concentration, usually 1 to 2 % and is inhibited above 3% NaCl, while few LAB are more resistant to NaCl (Altuntas et al., 2010). On the other hand, Sodium chloride at 6 and 8 % completely inhibited the antimicrobial activity. Growth of P. pentosaceus decreased with increase in NaCl concentrations. The current results are in agreement with the Mabrouk et al., (2014). Whereas, Altuntas et al., (2010) have reported that P. acidilactici grew up to 10% NaCl, but grew optimally in the absence of NaCl. According to Delgado et al., (2007) NaCl was required to maintain osmotic pressure in the cells, but not required for the production of bacteriocin.
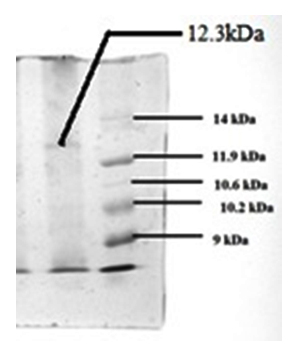
Fig. 12. Separation of bacteriocin by tricine-SDS-PAGE and stained with Coomassie brilliant blue R 250. Lane -1: The protein band with antimicrobial activity against L. monocytogenes and A. baumannii.. Lane-2: Pro-tein molecular weight markers in low range (9-14 kDa)
Molecular weight of bacteriocin
Tricine SDS-PAGE revealed the zone-producing band to a MW of 12.3 kDa (Fig. 12). Similarly, production of bacteriocin having a higher molecular wt of 17.5 kDa by P. pentosaceus was previously reported by Wu et al., (2004). However, production of bacteriocin with a Mw of ~ 4.8 kDa by P. pentosaceus CFR B19 (Venkateshwari et al. 2010) and pediocin PA-1 having a Mw of 2.6 kDa by P. acidilactici PAC 1.0 (Bauer et al. 2005)
The results of these findings demonstrated the distribution of LAB in Indian fermented foods mainly depend on the type of raw materials from which food is prepared as well as the fermentation condition and procedure. The culture conditions and composition of the growth medium were very important for the production of individual bacteriocins. MRS was found to be the best medium for bacteriocin production by Pediococcus pentosaceus. The antimicrobial peptides, bacteriocins, produced by LAB represented unique antimicrobials with high diversity in their structure and physico-chemical properties. In the present study, a bacteriocinogenic LAB isolate, producing novel bacteriocin having broad spectrum of activity against food borne and human pathogenic bacteria was isolated from appam batter. Further characterization of the identified bacteriocin and technological evaluation of the isolate for preparation of fermented food products are needed to be carried out. The current study indicates saprophytic LAB can be an ideal source for the study of new bacteriocins.
ACKNOWLEDGMENTS
The authors are grateful to the Department of Biotechnology, Karunya Institute of Technology and Sciences, Coimbatore, South India and the Department of Biotechnology, Dr. G.R. Damodaran College of Science, Coimbatore, South India for providing necessary facilities to carry out this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Blandino, A., M.E. Al-Aseeri, S.S. Pandiella, D. Cantero and C. Webb, Cereal-based fermented foods and beverages. Food Res. Int., 2003; 36: 527-543.
- Sekar , S., and S. Mariappan. Usage of traditional fermented products by Indian rural folks and IPR. Indian Journal of Traditional Knowledge. 2007; 6(1): 111-120.
- Larry, R,. and G. Beuchat. Chapter: 13, Indigenous Fermented Foods. In: Biotechnology Set, 2008. Second Edition, USA.
- Ray, B. and M. Daeschel. In; Food Biopreservatives of Microbial Origin. Boca Raton, CRC Press, 1992.Florida.
- Yang, S.-C., C. H.Lin, C.T. Sung, and J.-Y. Fang, Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Frontiers in Microbiology. 2014; 5: 241.
- Moreno, I, A S. L. Lerayer, V. L. S. Baldini, and M. F. de F. Leitão, Characterization of bacteriocins produced by Lactococcus lactis strains. Braz. J. Microbiol., 2000; 31: 184-192.
- Nettles. G. C., and Barefoot, F.S., Biochemical and Genetic Characteristics of Bacteriocins of Food-Associated Lactic Acid Bacteria. Journal of Food Protection: 1993; 56(4) : 338-356.
- Perez,R.H., T. T. Zendo and K. Sonomoto. Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact. 2014; 13(1): S3.
- Saavedra, L.C. Minahk, A. P. De R. Holgado, and F. Sesma, Enhancement of the enterocin CRL35 activity by a synthetic peptide derived from the NH2-terminal sequence. Antimicrob. Agents Chemother., 2004; 48: 2778-2781.
- Vignolo, G. M., F. Suriani, A. P. Holgado, and G. Oliver, Antibacterial activity of Lactobacillus strains isolated from dry fermented sausages. Journal of Applied Bacteriology, 1993; 75: 344-349.
- Usmiati. S, and T. Marwati, Selection and optimization processes of bacteriocin production from Lactobacillus sp.. Indonesian Journal of Agriculture. 2009; 2(2): 82-92.
- Holt, JG. NR. Kreing. PHA. Sneath, and JT. Stanely, In : Bergey’s Manual of Determinative Bacteriology. 1994. 9/e, Williams and Wikins, Maryland.
- Galvez, A., H. Abriouel, RL. Lopez, and N. Ben Omar, Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol. 2007; 120(1-2):51-70.
- Hoda Mahrous, Abeer Mohamed, M. Abd El-Mongy, A. I. El-Batal, and H. A. Hamza, Study bacteriocin production and optimization using new isolates of Lactobacillus spp. isolated from some dairy products under different culture conditions. Food Nutr. Sci., 2013;. 4(3): 342-356.
- Yang R., Johnson M C., and Ray B. Novel method to extract large amounts of bacteriocins from lactic acid bacteria, Appl. Environ. Microbiol., 1992; 58(10): 3355-3359.
- Lowry, O.H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., Protein measurement with the folin phenol reagent. J. Biol. Chem., 1951; 193: 265-275.
- Shiba Prosad Banerjee, Krushna Chandra Dora and Supratim Chowdhury, Detection, partial purification and characterization of bacteriocin produced by Lactobacillus brevis FPTLB3 isolated from freshwater fish. J. Food Sci. Technol., 2013; 50(1):17–25.
- Rattanachaikunsopon, P.,and Phumkhachorn, P., Isolation and preliminary characterization of bacteriocin produced by Lactobacillus plantarum N014 isolated from Nham, a traditional Thai fermented pork. J Food Prot., 2006; 69(8):1937–1943.
- Rushdy, A.A. and E.Z. Gomaa, Antimicrobial compounds produced by probiotic Lactobacillus brevis isolated from dairy products. Ann. Microbiol., 2013; 63: 81-90.
- Haider, S.R., H. J. Reid and B. L. Sharp, Chapter-8; Tricine-SDS-PAGE, In: Protein Electrophoresis: Methods and Protocols, Methods in Molecular Biology, Biji T. Kurien and R. Hal Scofi eld (eds.), Humana Press, 2012; 869 :81-91.
- Sambrook, J. E.F. Fritsch, and T. Maniatis, Molecular Cloning: A Laboratory Manual, 2nd ed.; CSH Laboratory Press: 1989. Cold Spring Harbor, NY, USA.
- Mirhosaini, M., I. Nahvi , R. Kasra Kermanshahi and M. Tavassoli , Isolation of Bacteriocin Producing Lactococcus lactis Strain from Dairy Products. International Journal of Dairy Science, 2006; 1: 51-56.
- Bajpai K.Vivek, Jeong-Ho Han, Irfan A. Rather, Chanseo Park, Jeongheui Lim, Woon Kee Paek, Jong Sung Lee, Jung-In Yoon and Yong-Ha Park, Characterization and Antibacterial Potential of Lactic Acid Bacterium Pediococcus pentosaceus 4I1 Isolated from Freshwater Fish Zacco koreanus. Frontiers in Microbiology, 2016; 7(2037): 1-15.
- Casaburi, A., DiMartino, V., Ferranti, P., ,and Picariello. L, Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacturer. Food Control. .2016; 59: 31–45.
- Devirgiliis, C., Zinno, P. and Perozzi, G. Update on antibiotic resistance in foodborne Lactobacillus and Lactococcus species. Frontiers in Microbiology, 2013; 4: 301.
- Yousif, N. M. E., Molecular typing of lactic acid bacteria associated with hussuwa, a Sudanese fermented food. Ph.D. Thesis, 2003. Faculty of Agriculture, University of Khartoum.
- Abdel gadir, W.S., Hamad, S.H., Moller, P.L., and Jakobsen, M. Characterization of the dominant microbiota of Sudanese fermented milk Rob. International Dairy Journal, 2001; 11(1-2): 63–70.
- Grosu-Tudor, S.S, Stancu, M-M., Pelinescu, D. and Zamfir, M. Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World Journal of Microbiology and Biotechnology, 2014; 30(9): 2459-2469.
- Hwanhlem, N., Buradaleng, S., Wattanachant, S., Benjakul, S., Tani, A. and Maneerat, S. Isolation and screening of lactic acid bacteria from Thai traditional fermented fish (Plasom) and production of Plasom from selected strains. Food Control, 2011; 22(3-4), 401-407.
- Pringsulaka, O., Thongngam, N., Suwannasal, N., Attuakor, W., Pothivejkul, K. and Rangsiruji, A. Partial characterisation of bacteriocins produced by lactic acid bacteria isolated from Thai fermented meat and fish products. Food Control, 2012; 23(2): 547-551.
- Sulieman, A.M.E., Ilayan, A.A and El Faki, A. E-A., Chemical and microbiological quality of Garris, Sudanese fermented camel’s milk product. International Journal of Food Science and Technology, 2006; 41(3): 321–328.
- Yang, E., Fan, L., Jiang, Y., Doucette, C., and Fillmore, S. Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMB Express, 2012; 2: 48–59.
- Corsetti, A., and Settanni, L., Lactobacilli in sourdough fermentation. Food Research International, 2007; 40(5): 539-558.
- Ratanaburee, A., Kantachote, D., Charernjiratrakul, W. and Sukhoom, A. Enhancement of g-aminobutyric acid (GABA) in Nham (Thai fermented pork sausage) using starter cultures of Lactobacillus namurensis NH2 and Pediococcus pentosaceus HN8. International Journal of Food Microbiology, 2013; 167(2): 170-176.
- Amenu, D. Antimicrobial activity of lactic acid bacteria isolated from “Ergo”, Ethiopian traditional fermented milk. Curr. Res. Microbiol. Biotechnol. 2013; 1: 278–284.
- Tadesse, G., Ephraim, E., and Aashenafi, M. Assessment of the antimicrobial activity of lactic acid bacteria isolated from Borde and Shameta, traditional Ethiopian fermented beverages,on some foodborne pathogens and effect of growth medium on the inhibitory activity. Int. J. Food Saf., 2005; 5: 13–20.
- Carina Audisio, M., Torres, M.J., Sabate, D.C., Ibarguren,C., and Apella, M.C. Properties of different lactic acid bacteria isolated from A pismellifera L. bee-gut. Microbiol. Res., 2011; 166: 1–13.
- Darsanaki, R.K., Aliabadi, M.A., and Issazadeh, K., Antimicrobial activities of Lactobacillus strains isolated from fresh vegetables. Middle East J. Sci. Res. 2012; 11: 1216–1219.
- Yah, N., Marlida,Y., Arnim, and Yuherman. Antimicrobial activity of lactic acid bacteria thermophilic isolated from hot spring rimbo panti of West Sumatera for food preservatives. Pak. J. Nutri., 2014; 13: 465–472.
- Stevens, K. A., Sheldon, B. W., Klapes, N. A., and Klaenhammer, T. R. Nisin treatment for inactivation of Salmonella species and other Gram-negative bacteria. Applied Environmental Microbiology, 1991; 57: 3613-3615.
- Cheikhyoussef, A., Pogori, N., Chen, H., Tian, F., Chen,W., Tang, J., and Zhang, H. Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances (BLIS) produced by Bifidobacterium infantis BCRC 14602. Food Control, 2009; 553-559.
- De Kwaadsteniet, M., Todorov, S. D., Knoetze, H., and Dicks, L. M. T. Characterization of a 3944 Da bacteriocin, produced by Enterococcus mundtii ST15, with activity against Gram-positive and Gram-negative bacteria. International Journal of Food Microbiology, 2005; 105: 433-444.
- Gong, H. S., Meng, X. and C.and Wang, H.. Plantaricin MG active against Gram negative bacteria produced by Lactobacillus plantarum KLDS1.0391 isolated from “Jiaoke”, a traditional fermented cream from China. Food Control, 2010; 21: 89-96.
- Todorov, S. D., and Dicks, L. M. T. Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzyme and Microbial Technology, 2005; 36: 318-326.
- Skytta, E., Haikara, A., and Mattlla-Sandholm, T., Production and characterization of antibacterial compounds produced by Pediococcus damnosus and Pediococcus pentosaceus. Journal of Applied Bacteriology, 1993; 74: 134-142.
- Todorov, S.D., Vaz-Velho, M., and Gibbs, P., Comparison of two methods for purification of plantaricin ST31, a bacteriocin produced from Lactobacillus plantarum ST31. Brazilian Journal of Microbiology, 2004; 35: 157–160.
- Granger, M., Todorov, S.D., Matthew, M.K.A., and Dicks, L.M.T., Growth of Enterococcus mundtii ST15 in medium filtrate and purification of bacteriocin ST15 by cationexchange chromatography. Journal of Basic Microbiology, 2005; 45: 419–425.
- Todorov, S.D., Wachsman, M.B., Knoetze, H., Meincken, M., and Dicks, L.M.T., An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soy beans. International Journal of Antimicrobial Agents, 2005; 25: 508–513.
- Parada JL, Carolina RC, Adriana BP, Medeiros, and Carlos RS, Bacteriocins from Lactic Acid Bacteria: Purification, Properties and use as Bio preservatives. Brazilian Archives of Biology and Technology, 2007; 50(3): 521-542.
- Bhonsle JB, Clark T, Bartolotti L, and Hicks, R.P, A brief overview of antimicrobial peptides containing unnatural amino acids and ligand-based approaches for peptide ligands. Curr Top Med Chem., 2013; 13: 3205–3224.
- Albano, H., Todorov, S.D., van Reenen, C.A., Hogg, T., Dicks, L.M.T., and Teixeira, P., Characterization of a bacteriocin produced by Pediococcus acidilactici isolated from “Alheira”, a fermented sausage traditionally produced in Portugal. International Journal of Food Microbiology, 2007; 116: 239–247.
- Aasen, I.M., Moreto, T.,Katla, T., Axelsson, L., and Storro, I., Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Applied Microbiology and Biotechnology, 2000; 53:159–166.
- Agrawal R and Dharmesh S. An anti-Shigella dysenteriae bacteriocin from Pediococcus pentosaceus MTCC 5151 cheese isolate. Turk J Biol. 2012; 36(2):177–85.
- Mabrouk AMM, Effat BAM, Sadek ZIM, Tawfiek NF, Hassan ZMR, and Magdoub MNI. Antibacterial activity of some Lactic acid Bacteria isolated from Egyptian Dairy Products. Int J Chem Tech Res., 2014; 6(2):1139–50.
- Ivanova, I., Kabadjova, P., Pantev, A., Danova, S., and Dousset, X., Detection, purification and partial characterization of a novel bacteriocin substance produced by Lactococcus lactis subsp. lactis B14 isolated from boza-Bulgarian traditional cereal beverage. Biocatalis — Vestnik Moskova Uniiversiteta, Seria Kimia, 2000 ; 41: 47–53.
- Graciela M, Vignolo M, Kariruz M, Aida AP, De Ruiz H and Oilver G, Influence of growth conditions on the production of lactocin 705, a bacteriocin produced by L.casei CRL 705. J. Appl. Bacteriol. 1995; 78: 5-10.
- Kabore D, Dennis SN, Hagretou SL, Brehima D, Mamoudou H, Mogens J, and Lin T. Inhibition of Bacillus cereus growth by bacteriocin producing Bacillus subtilis Isolated from fermented baobab seeds (maari) is substrate dependent. International Journal of Food Microbiology, 2013, 162: 114- 119.
- Von Mollendorff JW, Todorov SD, and Dicks LMT. Comparison of Bacteriocins Produced by Lactic Acid Bacteria Isolated from Boza, A cereal Based Beverage from Balkan Peninsula. Current Microbiology. 2006, 54: 209-216.
- Altuntas EG, Cosansu S, and Ayhan K. Some growth parameters and antimicrobial activity of a bacteriocin-producing strain Pediococcus acidilactici 13. Int J Food Microbiol. 2010; 141(1-2):28–31.
- Delgado A, Arroyo Lopez FN, Brito D, Peres C, Fevereiro P, and Garrido- Fernandez A. Optimum bacteriocin production by Lactobacillus plantarum 17.2b requires absence of NaCl and apparently follows a mixed metabolite kinetics. J Biotechnol. 2007; 130(2):193–201.
- Wu CW, Yin LJ, and Jiang ST. Purification and characterization of bacteriocin from Pediococcus pentosaceus ACCEL. J Agr Food Chem. 2004; 52: 1146–1151.
- Venkateshwari, S., Halami, P.M., and Vijayendra, S.V.N., Characterisation of the heat-stable bacteriocin-producing and vancomycin-sensitive Pediococcus pentosaceus CFR B19 isolated from beans, Beneficial Microbes, 2010; 1(2): 159-164
- Bauer R, Chikindas ML, and Dicks LMT. Purification, partial amino acid sequence and mode of action of pediocin PD-1, a bacteriocin produced by Pediococcus damnosus NCFB 1832. Int J Food Microbiol., 2005; 101:17-27.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.