ISSN: 0973-7510
E-ISSN: 2581-690X
Subclinical mastitis (SCM), the asymptomatic inflammation of breast tissue, is the most common form of mastitis in livestock. SCM prevalence and risk factors in dairy animals in Western Chitwan, Bagmati Province, Nepal, were the primary objectives of this study. Out of 243 dairy animals, 104 representing 42.8% were positive for SCM in the California mastitis test (CMT) in the study area. At the quarter level, out of 972 active quarters tested for SCM, 188 (19.3%) were positive to CMT test. The prevalence of Staphylococcal SCM was 39.92% (97/243) and 18.21% (177/972) at animal level and quarter level, respectively. Coagulase Negative Staphylococcus (CNS) (46.33%) was the most prevalent Staphylococcus to cause SCM at quarter level. While at animal level, SCM due to occurrence of both S. aureus and CNS (36.08%) in an individual was more common. High susceptibility towards Amikacin, Ceftriaxone and Gentamicin was seen against both isolates. Low resistance against Amikacin and Ceftriaxone was seen against both isolates. Ciprofloxacin (41.2% vs 27.4%) and Gentamicin (37.8% vs 23.2%) were more resistance towards CNS whereas Enrofloxacin (41.1% vs 25.2%) and Tetracycline (36.8% vs 15.1%) were more resistant towards S. aureus. Older aged, multiparous and late lactating animals had the highest prevalence of staphylococcal SCM i.e., 58.1%, 56% and 52.6%, respectively. The study concludes that there is a high prevalence of Staphylococcal SCM in Western Chitwan, Nepal. CNS is the most common mastitis pathogen. Increased antimicrobial resistance to S. aureus and CNS could be the result of the indiscriminate use of antibiotic drugs without an antibiotic susceptibility test (AST). This study emphasizes the importance of ongoing antibiotic surveillance, excellent farm and animal hygiene, and suitable housing and feeding management.
Risk factors, Antibiogram, Coagulase Negative Staphylococcus, S. aureus, Subclinical mastitis, Nepal
Mastitis is a significant infectious disease among dairy animals, present in both clinical and subclinical forms, causes heavy economic losses, and various efforts and advances are being made for its adequate management and treatment.1-3 Economic loss is due to decreased in production and quality, increase in labor and veterinary cost as well as increase in rate of culling.4-8 Subclinical mastitis (SCM), the asymptomatic inflammation of mammary tissue, is the most common form of mastitis.9-11 It is caused by wide ranges of causative organisms like bacteria, fungi, algae and mycoplasmas. Although milk does not vary significantly, SCM is 40 times more common than clinical mastitis and causes higher economic losses because of increased SCC, which is more difficult to detect.12 SCM is more costly since it raises the risk of clinical mastitis as well as milk production loss.13 According to Singh & Singh14 SCM causes more than three times losses as compared to clinical mastitis. Treatments, preventive measures and increased labor costs are the actual expenses that result from decreased milk production, whereas the losses are represented by the reduction in milk production.15 According to Dhakal and Tapa,16 mastitis costs Nepalese farmers Rs. 4287 or $ 63 per lactating buffalo. In India, INR 60532.2 million was estimated amount of annual economic loss due to mastitis in which 70-80% (INR 43653.2 million) accounts for sub-clinical mastitis.17,18
Nepal is an agricultural developing country. A big part of Nepal’s economy is dependent on agriculture, and livestock play an important role in that sector. About 27.1% of total Gross Domestic Product (GDP) is contributed by Agriculture and Forestry.19 Livestock and dairy sector contribute 32% of agricultural GDP and 11% of national GDP.20,21 Cattle and buffaloes are considered to be major livestock reared in Nepal. Livestock is an integral part of Nepalese farming, providing milk, meat, draught power, fertilizer (manure) and household fuel. It also carries great religious significance.22 Rural people’s living standards have been boosted by the dairy industry’s ability to bring money from the city to the countryside. Cattle and buffaloes are a vital source of revenue flow for smallholder farmers.9,10 According to Agriculture and Livestock Diary 2079, there are 7,466,841 cattle and 5,159,931 buffalo in Nepal and total milk production is 2,479,899 metric tons.22 Chitwan district is the largest producer of milk in the country which produces 290,000 L of milk daily.4
The most prevalent and well-known cause of mastitis in dairy cows is Staphylococcus aureus.23 Staphylococcal SCM is very common in Asian countries24-27 as well as different parts of Nepal like Chitwan,14,28 Pokhara,29 Lamjung13 and Bhaktapur.30 Economic impact of the Staphylococcal SCM is one part, the other is potential zoonotic risk brought about by contaminated raw milk and antibiotic residues as a consequence of drug therapy.31 Identification of Staphylococcus and its antibiotic resistance are necessary for efficient control of mastitis implementation. For the sake of public health in Nepal and other developing nations, this information is critical for treating and preventing mastitis. The Chitwan district needs to have baseline data on SCM prevalence and understand the related risk factors in order to reduce its prevalence. Thus, the current study was designed to investigate the prevalence and risk factors of Staphylococcal mastitis in dairy animals in western Chitwan, Nepal, as well as the antibiotic resistance of Staphylococcal isolates.
Study Area
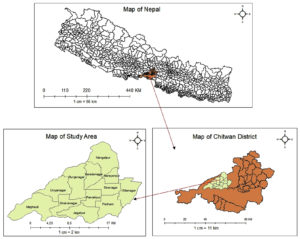
This study was conducted in 12 different localities; Mangalpur, Saradanagar, Fulbari, Sibanagar, Gitanagar, Gunjanagar, Parbatipur, Patihani, Dibyanagar, Sukranagar, Jagatpur and Meghauli of Western part of Chitwan district, Bagmati Province, Nepal. The benchwork was conducted in the laboratory of National Cattle Research Program, Nepal Agricultural Research Council (NARC), Rampur, Chitwan, Nepal (Fig. 1).
Study Design
Investigation participants were surveyed from January to March 2020 in a cross-sectional study. This information was gathered using a standardized questionnaire with open and closed ended questions for each animal that was tested. One to one interview was conducted among the farmers in local Nepali language after explaining the objective of this study and securing the agreement of each individual participant. First-hand information was obtained with the farmer. The questionnaire was divided into three parts; first section was about the demographic character of the farmers; second part was related to the individual animal and third section was focused on management and hygiene of the farm. A number of potential risk factors for SCM were recorded. The factors assessed were frequency of milking, time of milking, feeding practice, housing practice, handwashing before and after milking, udder washing before and after milking, and barn cleaning. Age, breed, parity, stage of lactation, milk output, presence of teat injuries and past mastitis history were documented at the cow level. It was also confirmed that there was no evidence of any other diseases or health issues. The color, smell, and consistency of the milk were all closely monitored. The presence of clots, flakes, blood, and other consistency alterations were among the signs of clinical mastitis that were rejected. Milk samples from cattle and buffalo were aseptically collected in the labelled universal sample container for the study.
Sampling method and sample size determination
The sample size in particular was generated from EpiTools Epidemiological Calculators by Ausvet, in assumption of prevalence of Staphylococcal SCM in Ratnanagar Municipality to be 18.06% and expected precision of 5% and confidence level of 95%.32 And considering the sensitivity and specificity of Mannitol Salt Agar to be 97.86% and 100% respectively.33 The estimated sample size for true prevalence was 243 all over Western Chitwan. Samples from separate quarters were collected in the labelled universal sample container. Two staged random sampling was done. Samples from dairy animals both buffaloes and cattle in the area were collected using probability proportion to size (PPS).
Collection and transportation of sample
Cattle and buffaloes were milked in Western Chitwan and milk samples were taken before milking. Before collecting samples, the udder and specifically the teats were thoroughly cleansed and dried. Teats that were closer to the sample collection were sampled first, followed by those that were further away. After discarding the first few streams, 10 mL of milk were collected in sterile universal sample collection bottles and labelled. In order to further isolate and identify the microorganisms, the samples were transported to the National Cattle Research Program Microbiology Laboratory in an icebox. A maximum of 24 hours was allowed for the samples to be cultured on standard bacteriological media before being stored at 4°C.
California Mastitis Test (CMT)
SCM screening and detection can be greatly improved with the use of CMT.34 A quantity of 2 mL milk were obtained from each quadrant and evaluated visually with the naked eye for any anomalies in color, consistency, blood particles, etc. in four shallow cups of CMT paddle. Each cup received the same amount of commercial CMT reagent. For 15 seconds, the mixtures were agitated with a gentle circular motion in a horizontal plane. Based on gel formation, the CMT results were graded as follows: 0 for negative, 1 for trace, 2 for weak positive, 3 for distinct positive, and 4 for strong positive. There was no difference between CMT scores of zero (0) and those of 1, 2, 3, and 4 indicating subclinical mastitis. Positive cows had a CMT score of 1 or higher in at least one quarter of their herd.
Isolation of Staphylococcus aureus
For isolation of Staphylococcus species, all the CMT positive animals were selected. CMT negative dairy animals were not included in further milk subculture. To comply with specific objective of the study, bacteriological examination was focused only on identification and isolation of Staphylococcus species, which is the most common cause of contagious mastitis in dairy herds worldwide.
Test tubes with 8 mL of buffered peptone water (HiMedia, Mumbai, India) were filled with around 2 mL of CMT positive sample. Warming milk samples at 25°C for 15 minutes dispersed bacteria and fat globules that might be concentrated in the cream layer and bound together by clumps of fat globules in the samples. After 24 to 48 hours of aerobic incubation at 37°C, the test tubes were discarded. Standard microbiological methods were followed in the isolation of Staphylococcus aureus. It was mixed completely and a standard loop (0.01 mL) of milk sample was streaked on Mannitol Salt Agar (MSA) (HiMedia, Mumbai, India), which was incubated for 24 hours. For 24 hours, the petri plates were incubated aerobically at 37°C with the sample identity and teat level clearly marked. The growth and morphological characteristics of the colonies, such as size, shape, and color, were analyzed on the plates. Nutrient agar (HiMedia, Mumbai, India) was used to separate the presumed colonies and incubate them for 24–48 hours at 37°C in order to obtain a clean culture. The colonies were selected on the basis of mannitol fermentation (golden yellow and pink). Nutrient broth was prepared and 500 μL was taken on 1.5mL labelled Eppendorf tube and pure colony isolated from Nutrient Agar was transferred to the tube using inoculating loop. Each sample was stored in three Eppendorf tubes and incubated for 24 hours at 37°C. 500 μL of 50% glycerol was placed on the Eppendorf tube to make 25% dilution and stored in defreeze according to the protocol by Addgene.
Identification of S. aureus and Coagulase Negative Staphylococcus (CNS)
The preserved samples from Eppendorf were streaked over mannitol salt agar (MSA) using a sterile inoculation loop to obtain a pure colony, and then incubated at 37°C for 24 hours. Identification of Staphylococcus was done by gram staining (purple grapelike cluster) and various biochemical tests like catalase test and oxidase test. Slide coagulase test was done to identify S. aureus and CNS. In order to do so, bacterial colony from MSA was first transferred to Nutrient Agar and incubated at 37˚C for 24 hours. Then slide coagulase test was performed. Similarly, CNS was further confirmed by Novobiocin susceptibility test.
Antibiotic Susceptibility Test
Antibiotic susceptibility test was done for the confirmed samples of Staphylococcus according to Kirby Bauer disk diffusion method. Muller Hilton Agar (MHA) (HiMedia, Mumbai, India) plates were inoculated with 0.5 McFarland of bacterial suspension. This study utilized Tetracycline (30 μg), Enrofloxacin (5 μg), Gentamicin (10 μg), Amikacin (30 μg), Ceftriaxone (30 μg) and Ciprofloxacin (5 μg) antibiotic discs. These antibiotics were selected because they were the most readily available, used, and prescribed antibiotics in Nepal. A zone scale was used to assess the inhibition zone after 18 hours of incubation at 37°C. Antibiotics were classified as resistant, intermediate sensitive and sensitive on the basis of zone of inhibition produced by each antibiotic disk according to Clinical Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) interpretation.
Data Analysis
Statistical Package for the Social Sciences (SPSS) version 20.0 was used to analyze and summarize the data (SPSS Inc., USA). The descriptive data were summarized using frequency and proportion. Testing for a connection between independent variables (farm management and hygiene) and an outcome variable (Staphylococcal SCM) was done using the chi-squared test at 95% confidence interval and p<0.05.
Features of sampled animals
Out of total 243 sampled dairy animals, 50 (20.6%) (95% CI: 0.261-1.017) were buffalo and 193 (79.4%) were cattle. The variation showed higher number of cattle holdings in the western Chitwan than compared to buffaloes. Among 193 cattle 47 (24.4%) were Holstein Friesian cross, 119 (61.7%) were Jersey-cross and 27 (13.9) were local. Cross-breeds were popular among the farmers in the research area. Majority of animals (79.4%) were in their 1st to 3rd parity. More than 50% animals yield 7 to 14 L milk per day while maximum number (65.4%) of animals did not have history of mastitis (Table 1).
Table (1):
Descriptive features of sampled animals.
| Variables | Cattle (n=193) n (%) |
Buffalo (n=50) n (%) |
Total n (%) |
|
|---|---|---|---|---|
| Age | >2 | 63 (32.6) | 7 (14.0) | 70 (28.8) |
| 4 to 8 | 109 (56.5) | 33 (66.0) | 142 (58.4) | |
| >8 | 21 (10.9) | 10 (20.0) | 31 (12.8) | |
| Parity | 1st to 3rd | 156 (80.8) | 37 (74) | 193 (79.4) |
| 4th and above | 37 (19.2) | 13 (26) | 50 (20.6) | |
| Lactation | Early | 73 (37.8) | 19 (38) | 92 (37.9) |
| Mid | 71 (36.8) | 23 (46) | 94 (38.7) | |
| Late | 49 (25.4) | 8 (16.0) | 57 (23.5) | |
| Milk Yield | <7 L | 50 (20.6) | 23 (46.0) | 73 (30.0) |
| 7 to 14 L | 117 (60.6) | 26 (52.0) | 143 (58.8) | |
| >14 L | 26 (13.5) | 1 (2.0) | 27 (11.1) | |
| History of Mastitis | Yes | 73 (37.8) | 11 (22.0) | 84 (34.6) |
| No | 120 (62.2) | 39 (78.0) | 159 (65.4) | |
| Teat injury | Yes | 21 (10.9) | 5 (10.0) | 26 (10.7) |
| No | 172 (89.1) | 45 (90.0) | 217 (89.3) | |
Management and hygiene practices
Semi-intensive housing system was popular in western Chitwan area. Among the feeding practice, most of the dairy animals were fed before milking (57.2%) (95% CI: 1.159 -3.369). Most of the cattle about 54.4% were fed before and 45.6% were fed after. Likewise, barn cleaning once a day was done by 42.4% of the farmers, twice a day by 49.8% of them and once in 2-3 days by 7.8%. 58.4% and 41.6% of the farmers rearing dairy animals used steel and plastic container for milking respectively (Table 2).
Table (2):
Management and hygiene practices by cattle and buffalo farmers.
| Variables | Cattle (n=193) n (%) |
Buffalo (n=50) n (%) |
Total n (%) |
|
|---|---|---|---|---|
| Housing | Intensive | 78 (40.4) | 21 (42.0) | 99 (40.7) |
| Semi-Intensive | 115 (59.6) | 29 (58.0) | 144 (59.3) | |
| Feeding | Before | 105 (54.4) | 34 (68.0) | 139 (57.2) |
| After | 88 (45.6) | 16 (32.0) | 104 (42.8) | |
| Udder cleaning before | Yes | 190 (98.4) | 48 (96.0) | 238 (97.9) |
| No | 3 (1.6) | 2 (4.0) | 5 (2.1) | |
| Udder cleaning after | Yes | 46 (23.6) | 7 (14.0) | 53 (21.8) |
| No | 147 (76.2) | 43 (86.0) | 190 (78.2) | |
| Barn Cleaning | Once a day | 84 (43.5) | 19 (38.0) | 103 (42.4) |
| Twice a day | 93 (48.2) | 28 (56.0) | 121 (49.8) | |
| once in 2-3 days | 16 (8.3) | 3 (6.0) | 19 (7.8) | |
| Milking utensil | Plastic | 85 (44.0) | 16 (32.0) | 101 (41.6) |
| Steel | 108 (56.0) | 34 (68.0) | 142 (58.4) | |
Prevalence of subclinical mastitis (SCM)
A total of 243 samples were collected all around Western Chitwan, Nepal. All 972 teat were subjected to the CMT test and scored (0, +1, +2, +3 or +4) on basis of gel formed. Out of 243 dairy animals 104 were CMT positive and 139 were negative, i.e., 42.8% and 57.2% respectively. Overall prevalence of subclinical mastitis in Western Chitwan, Nepal was found to be 42.8% in animal level. Similarly, 188 out of 972 teats were CMT positive, depicting the quarter-wise prevalence of SCM in Western Chitwan, Nepal to be 19.3%.
Prevalence of Staphylococcal SCM
Out of 188 teats of 104 CMT positive animals, very high prevalence of Staphylococcus was seen, i.e., 93.27% (97/104) in animal level and 94.14% (177/188) in quarter-level. The overall prevalence of staphylococcal SCM was found to be 39.92% (97/243) and 18.21% (177/972) in animal and quarter-level respectively in Western Chitwan, Nepal.
Antibiotic Susceptibility Test (AST)
Altogether 177 quarter with positive isolates of Staphylococcus were reported. Out of which S. aureus was isolated from 58 teats, CNS was isolated from 82 teats whereas 37 teats showed both S. aureus and CNS isolates. Thus, making the overall S. aureus and CNS isolates to be 95 and 119 respectively. So, total 214 isolates were subjected to AST on Muller Hinton agar against six different antibiotics for AST.
AST of S. aureus isolates
AST of 95 S. aureus isolates was performed. Amikacin (91.6%) was the most susceptible antibiotic. Ciprofloxacin showed highest intermediate susceptibility i.e., 56.8% susceptibility, followed by Enrofloxacin and Tetracycline each being 54.7%. Gentamicin did not have the intermediate range but S. aureus were more resistant to Enrofloxacin (41.1%) and Tetracycline (37.8%) (Table 3).
Table (3):
Antibiotic Susceptibility Test (AST) of S. aureus isolates.
| Antibiotics |
Susceptible | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|
| N | % | n | % | n | % | |
| Tetracycline | 8 | 8.4% | 52 | 54.7% | 35 | 36.8% |
| Gentamicin | 73 | 76.8% | – | – | 22 | 23.2% |
| Enrofloxacin | 4 | 4.2% | 52 | 54.7% | 39 | 41.1% |
| Amikacin | 87 | 91.6% | 5 | 5.3% | 3 | 3.2% |
| Ceftriaxone | 76 | 80.0% | 15 | 15.8% | 4 | 4.2% |
| Ciprofloxacin | 15 | 15.8% | 54 | 56.8% | 26 | 27.4% |
AST of coagulase negative Staphylococcus (CNS) isolates
Ceftriaxone was the most susceptible antibiotic whereas Ciprofloxacin was the most resistant antibiotic. Enrofloxacin showed highest among intermediate susceptibility with 68.1% susceptibility. Gentamicin did not have the intermediate range and no isolates showed intermediate susceptibility towards Ciprofloxacin. CNS were most resistant to Ciprofloxacin (41.2%) (Table 4).
Table (4):
Antibiotic Susceptibility Test (AST) of Coagulase Negative Staphylococcus (CNS) isolates.
| Antibiotics | Susceptible | Intermediate | Resistant | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Tetracycline | 84 | 70.6% | 17 | 14.3% | 18 | 15.1% |
| Gentamicin | 74 | 62.2% | – | – | 45 | 37.8% |
| Enrofloxacin | 8 | 6.7% | 81 | 68.1% | 30 | 25.2% |
| Amikacin | 85 | 71.4% | 27 | 22.7% | 7 | 5.9% |
| Ceftriaxone | 93 | 78.2% | 20 | 16.8% | 6 | 5.0% |
| Ciprofloxacin | 70 | 58.8% | 0 | 0.0% | 49 | 41.2% |
Risk factors of Staphylococcal SCM
Testing for a connection between independent variables (farm management and hygiene) and an outcome variable (Staphylococcal SCM) was done using the Chi-squared test, at 95% confidence interval and p<0.05. Species, age, parity, stage of lactation, milk yield per day, history of mastitis, teat injury, housing and feeding practice were considered as potential risk factors of Staphylococcal SCM. Among them species, age, parity, stage of lactation, history of mastitis, teat injury and feeding practice were statistically significant at p-value < 0.05. Dairy animals with age more than 8 years were 3.462 (95% CI:1.433-8.362) times more likely to acquire Staphylococcal SCM that animal of age 2-4 years. Animals with history of mastitis were 3.796 times risk of acquiring the disease that with no history similarly animals with teat injury were 2.686 times riskier than animal with normal teats (Table 5).
Table (5):
Chi-squared Test for risk factors associated with staphylococcal SCM in dairy animals of Chitwan, Nepal.
| Risk Factors | No. of Animals (%) | SCM | Staphylococcal Sub Clinical Mastitis | |||
|---|---|---|---|---|---|---|
| No. (%) positive for SCM | No. (%) positive for Staphylococcal SCM | OR (95% Cl) | p-value | |||
| Species | Cattle | 193 (79.4) | 104 (53.9) | 83 (43%) | REF | 0.049* |
| Buffalo | 50 (20.6) | 35 (70.0) | 14 (28%) | 0.515 (0.261 – 1.017) | ||
| Age | 2-4 years | 70 (28.8) | 21 (30.0) | 20 (28.6) | REF | 0.016* |
| 4-8 years | 142 (58.4) | 64 (45.1) | 59 (41.5) | 1.777 (0.959 – 3.293) | ||
| >8 years | 31 (12.8) | 19 (61.3) | 18 (58.1) | 3.462 (1.433 – 8.362) | ||
| Parity | 1st to 3rd | 193 (79.4) | 75 (38.9) | 69 (35.8) | REF | 0.01* |
| 4th and above | 50 (20.6) | 29 (58.0) | 28 (56.0) | 2.287 (1.217 – 4.300) | ||
| Stage of Lactation | Early | 92 (97.9) | 30 (32.6) | 29 (31.5) | REF | 0.038* |
| Mid | 94 (38.7) | 43 (45.7) | 38 (40.4) | 1.474 (0.807 – 2.693) | ||
| Late | 57 (23.5) | 31 (54.4) | 30 (52.6) | 2.141 (1.222 – 4.769) | ||
| Milk Yield Per Day | Low | 73 (30.0) | 24 (32.9) | 23 (31.5) | REF | 0.104 |
| Mid | 143 (58.8) | 71 (49.7) | 65 (45.5) | 1.822 (1.001 – 3.279) | ||
| High | 27 (11.1) | 9 (33.3) | 9 (33.3) | 1.087 (0.424 – 2.783) | ||
| History of Mastitis | Absent | 159 (65.4) | 51 (32.1) | 46 (28.9) | REF | <0.001* |
| Present | 84 (34.6) | 53 (63.1) | 51 (60.7) | 3.796 (2.177 – 6.620) | ||
| Teat Injury | Absent | 217 (89.3) | 88 (40.6) | 81 (37.3) | REF | 0.019* |
| Present | 26 (10.7) | 16 (61.5) | 16 (61.5) | 2.686 (1.164 – 6.202) | ||
| Housing | Intensive | 99 (40.7) | 44 (44.4) | 42 (42.4) | REF | 0.509 |
| Semi-Intensive | 144 (59.3) | 60 (41.7) | 55 (38.2) | 0.839 (0.498 – 1.413) | ||
| Feeding Practice | After Milking | 104 (42.8) | 37 (35.6) | 32 (30.8) | REF | 0.011* |
| Before Milking | 139 (57.2) | 67 (51.5) | 65 (50.0) | 1.976 (1.159 – 3.369) | ||
The prevalence of SCM by CMT was found to be 42.8% at animal level and 19.3% at quarter level, in Western Chitwan, Nepal. This finding was nearly similar to the findings of Khanal and Pandit13 where the prevalence of SCM was 46.1% at animal level and Shrestha et al.,28 where prevalence was 41.5%. Comparing with other studies in Nepal, animal level prevalence of our finding was lower than the study of Shrestha and Bindari30 and Lamsal,31 while it was higher than the study of Dhakal.1 Out of the total CMT positive subcultures, Staphylococcus species were found in 93.27% and 94.14% of animals and quarters, respectively. In Western Chitwan, 39.92% of animals tested positive for SCM caused by Staphylococcus species, while 18.27% of quarters tested positive. As per Khanal and Pandit,13 Dhakal et al.,14 Shrestha et al.,28 Pandit,32 and Mekibib et al.,37 Staphylococcus species was the most common pathogens responsible for sub clinical mastitis. Among the positive Staphylococcal isolates, after the identification three types of Staphylococcus species were observed. In quarter level, the highly prevalent was CNS (46.33%), followed by S. aureus (32.77%) and less common but considerable infection of both S. aureus and CNS (20.9%) in a single quarter. Dieser et al.,38 also observed that CNS was the most often isolated pathogen (52.1%), followed by S. aureus (21.3%). Similar results were obtained during a study conducted in 2001, which reported 49.6% CNS, outnumbering all other isolated pathogens. It is possible that the differences in prevalence of SCM and Staphylococcus between studies could be related to differences in sample size and sampling procedures. Also, variations may be due to geographic location, climate, livestock rearing system, husbandry practices and hygiene.34
One or more antibiotic-resistant strains of S. aureus and CNS were found to exist in this study’s AST results. In this study, tetracycline was found to be resistance, 36.8% in case of S. aureus whereas in case of CNS it was highly susceptible (70.6%). Gentamicin was highly susceptible (76.8%) against S. aureus whereas it was 37.8% resistant against CNS. Enrofloxacin was highly resistant (41.1%) against S. aureus whereas it was 25.2% resistant in CNS. Amikacin, third generation aminoglycoside, was sensitive to both S. aureus (91.6%) and CNS (71.4%). Ceftriaxone, being a third generation β-lactam antibiotic, it was highly susceptible against both S. aureus (80%) and CNS (78.2%). Resistance against ciprofloxacin was more in CNS (41.2%) than compared against S. aureus (27.4%). In a study in Chitwan, Nepal, S. aureus (48.3%) and CNS (52.5%) were found to be sensitive to Tetracycline.28
Similarly, the overall antibiotic susceptibility pattern of western Chitwan against Staphylococcus causing SCM were highly susceptible to amikacin (80.4%) and ceftriaxone (79%). Amikacin and ceftriaxone being third generation drugs were more susceptible, while ciprofloxacin (35%), enrofloxacin (32.2%) and gentamicin (31.3%) were resistant in AST. However, Pandit32 showed the susceptibility of Enrofloxacin to be 73.33%. Intermediate resistance of enrofloxacin (69% vs 16%) and resistivity against gentamicin (31% vs 13%) was found way more than the findings by Pandit.32
Looking at the resistance patterns and a larger percentage of intermediate susceptibility of drugs against both S. aureus (tetracycline, enrofloxacin, ceftriaxone) and CNS (enrofloxacin), and also the fact of decreased efficacy of the lower tier antibiotics (ciprofloxacin against both S. aureus and CNS, gentamicin against CNS) suggests the hazardous use of antibiotics. A prescription is not required for farmers to get antibiotics at the pharmacy. This laxity has increased the likelihood of antibiotic overuse, which in turn has contributed to the rise of antibiotic resistance.
The prevalence of SCM was higher in older aged (>8 years old) than compared to younger animals. Similarly, the prevalence of staphylococcal mastitis was highest among the older aged (>8 years) animals followed by animals of 4-8 years age and the least in animals from age group <4 years. This result is similar to that of Kayesh et al.,40 where highest prevalence (37.50%) in the age group of 9-12 years and also supported the point that with the increase in age the incidence subclinical mastitis is increased. SCM is more common in older cows due to the weakening of the sphincter muscles and the deterioration of the vaginal canal walls and udder tissue that occurs as the animal ages.41-43 Our results however contradicted with Maheshwari et al.,44 who found highest prevalence of staphylococcal SCM (25.97%) in cattle of age group 5-7 years. Since there was significant difference between species among various age categories, buffaloes (20%) were more prone to Staphylococcal mastitis than cattle (10.9%) in the present study. Likewise, the prevalence of SCM due to S. aureus was similar among the age category of 4-8 years and older age (>8years). However, prevalence of SCM due to CNS was highest among younger animals <4 years which was similar to the finding of Bochniarz et al.45 Likewise, the prevalence of SCM due to mixed species of Staphylococcus was highest in older animals. This might be due to greater length of exposure time.
The prevalence of SCM was higher in the animals from 4th parity and above (58%) (95% CI:1.217-4.300) according to the CMT results. The prevalence of SCM due to Staphylococcus species (56%) was higher in animals from 4th parity and above. In this study, parity had no relationship with the incidence of SCM among cattle and buffalo. The increased incidence of mastitis seen in this study coincided with the findings of Rabbani and Samad.41
The prevalence of SCM was highest on the animals in late lactation (>6 months), followed by mid (3-6 months) and least in early lactating animal, i.e., 54.4% (95% CI: 1.222-4.769), 45.7% (95% CI: 0.807-2.693) and 32.6% respectively. Likewise, the prevalence of Staphylococcus causing SCM was also the same, highest in late lactating and least in the early lactating. This result was similar to the results by Khanal and Pandit13 where prevalence was 58.5% in late lactation compared to 41.3% in early lactation. Study by Pandit27 also supports our study.27 As per results by Bochniarz et al.,45 highest numbers of CNS mastitis cases were observed during the first and second lactation. Likewise, the prevalence of SCM due to combined infection of both pathogens was higher in late lactating (24.6%) animals than mid (13.8%) and early lactating (8.7%) animals.
In this study, the prevalence of SCM on basis of CMT was found high among medium yielders (7-14 liters/day), followed by high yielders (>14 liters/day) and low yielders (<7 liters/day). Our findings contrast with the study of Kayesh et al40 which showed higher prevalence of SCM in low yielding cows (<7litres/day). The difference in incidence might be due to variation intrinsic factors (breed, stage of lactation, parity, etc.) and also extrinsic factors (environment, hygiene, feeding, housing, etc.). There was significant difference in distribution of species among various milk yielding categories. Medium yielding cattle were at more risk of SCM than buffaloes within the same yielding category. This might be attributed to the tighter teat sphincter of buffaloes as compared to that of cows.
The prevalence of SCM caused by Staphylococcus species among three categories of milk yield per day was not found significantly different. The prevalence of SCM due to S. aureus was highest among medium yielders and lowest in low yielder. According to Pandit32 the prevalence rate was also lowest among the yielders, which is similar to our finding.
According to the results of the CMT, SCM was more prevalent on animals with a history of mastitis (63.1%) (95% CI: 2.177- 6.620) than those without a history of mastitis (32.1%). Previously infected animals were at greater risk of staphylococcal SCM (OR=3.796; 95% CI, 2.177 – 6.620), implying that repeated exposure to microorganisms and other stress factors may increase the likelihood of re-infection in the mammary glands. Research reports by Mekibib et al37 supported the findings of this study. Likewise, prevalence of SCM on basis of CMT was highest among the animals having teat injury (61.5%) (95% CI: 1.164- 6.202). Animals with staphylococcal SCM had more chances of concurring with teat injury (61.5%). Risk of staphylococcal SCM in animals with teat injury was comparatively higher (OR=2.686; 95% CI, 1.164-6.202).
In the study almost similar prevalence of staphylococcal SCM was seen in both cattle and buffaloes on the basis of housing system according to CMT results. In western Chitwan, there was significant difference in the prevalence of staphylococcal SCM in dairy animals with feeding practice before milking (51.5%) and after milking (35.6%). The risk of having SCM was higher in the animals fed before milking. To avoid mastitis, it is recommended that cows be fed immediately after milking, since this helps to keep the cows from lying down and leaving their teat-ducts exposed to microorganisms from the environment.
SCM is quite common in Nepalese dairy cattle and buffaloes, resulting in significant financial losses due to decreased milk yield, increased medical costs, and increased labor. Poor cleanliness, inadequate sanitation, lack of early diagnosis, lack of good veterinary service, and most crucially, lack of awareness among farmers have all been identified as major contributors to the high frequency of SCM. Infected milk creates health problems in humans, therefore sub-clinical mastitis has zoonotic potential. As a result, the issue of bovine SCM is important from the perspective of consumers. Early detection by laboratory diagnosis and proper treatment by antibiotic sensitivity test is still a difficult task in developing countries like Nepal, where uneducated farmers, subsistence farming, and a lack of effective government efforts make early detection by laboratory diagnosis and proper treatment by antibiotic sensitivity test a difficult task. It is crucial to remember that production-related diseases like mastitis are not given much attention in national programs, despite the fact that they are common in small-scale dairy operations. To do this, the government, non-governmental organizations (NGOs), international non-governmental organizations (INGOs), and all other relevant parties must commit to bringing mastitis control plans to fruition. These programs must concentrate on prevention and control of SCM through excellent management practices and education on the condition through training, seminars, and workshops. Farmers must be educated about the prevention and treatment of SCM through an effective extension program. Farmers should also have easy access to a fast test kit for mastitis diagnosis. Farmers need effective veterinarian services for early diagnosis and treatment of SCM, and veterinary hospitals must offer them. More research is needed on the status, economic aspects, prevention, and control strategy of SCM in Nepalese cattle, which will aid in the planning and execution of various mastitis control initiatives in the country.
ACKNOWLEDGMENTS
The authors would like to acknowledge all the participants of the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
BBT and GP Conceptualize the study. BBT, DS, SB, PS, CRP and GP designed the methodology. BBT and DS wrote the original draft. BBT, DS, DC, PM, KD and GP wrote, reviewed and edited the final manuscript for publication. All authors read and approved the final manuscript for publication.
FUNDING
This research was funded by National Cattle Research Program, Nepal Agricultural Research Council.
ETHICS STATEMENT
This study was approved by Internship Advisory Committee of Veterinary Teaching Hospital, Paklihawa Campus, Institute of Agriculture and Animal Science, Tribhuvan University, Nepal Agricultural Research Council (NARC) and by the ethical review board of the Nepal Veterinary council with reference number Ethical. 03/2077.78
AVAILABILITY OF DATA
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Dhakal IP. Normal somatic cell count and subclinical mastitis in Murrah buffaloes. Journal of Veterinary Medicine, Series B. 2006;53(2):81-86.
Crossref - Deb R, Kumar A, Chakraborty S, et al. Trends in diagnosis and control of bovine mastitis: A review. Pak J Biol Sci. 2013;16(23):1653-1661.
Crossref - Sharun K, Dhama K, Tiwari R, et al. Advances in therapeutic and managemental approaches of bovine mastitis: a comprehensive review. Vet Q. 2021;41(1):107-136.
Crossref - Bhandari S, Subedi D, Tiwari BB, Shrestha P, Shah S, Al-Mustapha AI. Prevalence and risk factors for multidrug-resistant Escherichia coli isolated from subclinical mastitis in the western Chitwan region of Nepal. J Dairy Sci. 2021;104(12):12765-12772.
Crossref - Lamey AE, Ammar AM, Zaki ER, Khairy N, Moshref BS, Refai MK. Virulence factors of Escherichia coli isolated from recurrent cases of clinical and subclinical mastitis in buffaloes. Int J Microbiol Res. 2013;4(1):86-94.
Crossref - Lejaniya AS, Chandran D, Geetha R. Recent trends in application of lactic acid bacteria (LAB) in dairy and biomedical industry: A critical review. World Journal of Pharmaceutical Research. 2021;10(12): 577-591.
- Chandran D, Radhakrishnan U, Eldho L. Characterization of Malabari goat lactoferrin and its pepsin hydro-lysate. J Vet Anim Sci. 2020;51(1):40-47.
- Chandran D, Radhakrishnan U. Lactoferrin: A General Review. Int J Pharma Sci Rev Res. 2019;58(2):65-75.
- Salvador RT, Beltran JM, Abes NS, Gutierrez CA, Mingala CN. Prevalence and risk factors of subclinical mastitis as determined by the California Mastitis Test in water buffaloes (Bubalis bubalis) in Nueva Ecija, Philippines. J Dairy Sci. 2012;95(3):1363-1366.
Crossref - Mpatswenumugabo JP, Bebora LC, Gitao GC, et al. Prevalence of subclinical mastitis and distribution of pathogens in dairy farms of Rubavu and Nyabihu districts, Rwanda. J Vet Med. 2017;217: 8456713.
Crossref - Ebrahimie E, Mohammadi-Dehcheshmeh M, Laven R, Petrovski KR. Rule discovery in milk content towards mastitis diagnosis: Dealing with farm heterogeneity over multiple years through classification based on associations. Animals. 2021;11(6):1638.
Crossref - Ebrahimie E, Ebrahimi F, Ebrahimi M, Tomlinson S, Petrovski KR. A large-scale study of indicators of sub-clinical mastitis in dairy cattle by attribute weighting analysis of milk composition features: highlighting the predictive power of lactose and electrical conductivity. J Dairy Res. 2018;85(2):193-200.
Crossref - Khanal T, Pandit A. Assessment of sub-clinical mastitis and its associated risk factors in dairy livestock of Lamjung, Nepal. Int J Infect Microbiol. 2013;2(2):49-54.
Crossref - Dhakal IP, Dhakal P, Koshihara T, Nagahata H. Epidemiological and bacteriological survey of buffalo mastitis in Nepal. J Vet Med Sci. 2007;69(12):1241-1245.
- McInerney JP, Howe KS, Schepers JA. A framework for the economic analysis of disease in farm livestock. Prevent Vet Med. 1992;13(2):137-154.
Crossref - Dhakal IP. Economic impact of clinical mastitis in the buffaloes in Nepal. Buffalo Journal. 2002;2:225-234.
- Oliveira FG, Cook RF, Naves JH, et al. Equine infectious anemia prevalence in feral donkeys from Northeast Brazil. Prev Vet Med. 2017;140:30-37.
Crossref - Bhakat C, Mohammad A, Mandal DK, et al. Readily usable strategies to control mastitis for production augmentation in dairy cattle: A review. Vet World. 2020;13(11):2364-2370.
Crossref - Poudel U, Dahal U, Dhakal S. Review of poultry production and poultry vaccine manufacture in Nepal. Global Journal of Agricultural and Allied Sciences. 2021;3(1):1-7.
Crossref - Tiwari I, Shah R, Kaphle K, Gautam M. Treatment approach of different hormonal therapy for repeat breeding dairy animals in Nepal. Arch Vet Sci Med. 2019;2(3):28-40.
Crossref - Subedi D, Bhandari S, Pantha S, et al. Epidemiology of African Swine Fever and Its Risk in Nepal. Microbiol Res. 2021;12(3):580-90.
Crossref - Maharjan K, Chaudhary D. Scenario and policy of decent nutrition and food security in the post-covid-19 in Nepal. Journal La Sociale. 2021;2(1):10-17.
- Cvetnic L, Samardzija M, Duvnjak S, et al. Multi-locus sequence typing and spa typing of Staphylococcus aureus isolated from the milk of cows with subclinical mastitis in Croatia. Microorganisms. 2021;9(4):725.
Crossref - Sharma N, Maiti SK, Sharma KK. Prevalence, etiology and antibiogram of microorganisms associated with Sub-clinical mastitis in buffaloes in Durg, Chhattisgarh State (India). Int J Dairy Sci. 2007;2(2):145-151.
Crossref - Islam MA, Islam MZ, Rahman MS, Islam MT. Prevalence of subclinical mastitis in dairy cows in selected areas of Bangladesh. Bangladesh Journal of Veterinary Medicine. 2011;9(1):73-78.
Crossref - Annamanedi M, Sheela P, Sundareshan S,et al. Molecular fingerprinting of bovine mastitis-associated Staphylococcus aureus isolates from India. Sci Rep. 2021;11(1):1-5.
Crossref - Hoque MN, Das ZC, Rahman AN, Haider MG, Islam MA. Molecular characterization of Staphylococcus aureus strains in bovine mastitis milk in Bangladesh. Int J Vet Sci Med. 2018;6(1):53-60.
Crossref - Shrestha A, Bhattarai RK, Luitel H, Karki S, Basnet HB. Prevalence of methicillin-resistant Staphylococcus aureus and pattern of antimicrobial resistance in mastitis milk of cattle in Chitwan, Nepal. BMC Vet Res. 2021;17(1):1-7.
Crossref - Joshi LR, Tiwari A, Devkota SP, Khatiwada S, Paudyal S, Pande KR.Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in Dairy Farms of Pokhara, Nepal. Int J Vet Sc. 2014;3(2):87-90.
- Shrestha S, Bindari YR. Prevalence of sub-clinical mastitis among dairy cattle in Bhaktapur District, Nepal. Int J Agric Biosci. 2012;1(1):16-19.
- Khazaie F, Ahmadi E. Bovine subclinical mastitis-associated methicillin-resistant Staphylococcus aureus, selective genotyping and antimicrobial susceptibility profile of the isolates in Kurdistan province of Iran. Iran J Microbiol. 2021;13:65.
Crossref - Pandit, K. Assessment of sub-clinical mastitis using CMT, cultural examination and antibiogram in commercial dairy farms of Ratnanagar municipality, Chitwan, Institute of Agriculture and Animal Science, Tribhuvan University, 2018.
- Subramanian A, Chitalia VK, Bangera K, et al. Evaluation of HiaureusTM coagulase confirmation kit in identification of Staphylococcus aureus. J Clin Diagn Res. 2017;11(2):DC08-DC13.
Crossref - Fosgate GT, Petzer IM, Karzis J. Sensitivity and specificity of a hand-held milk electrical conductivity meter compared to the California mastitis test for mastitis in dairy cattle. Veterinary Journal. 2013;196:98–102.
Crossref - Acharya KP, Wilson RT. Antimicrobial resistance in Nepal. Front Med. 2019;6:105-110.
Crossref - Lamsal P. Cattle hygiene status and its relation with subclinical mastitis: A study in commercial farms in Rampur, Nepal. Int J Appl Sci Biotechnol. 2018;6:252–254.
Crossref - Mekibib B, Furgasa M, Abunna F, Megersa B, Regassa A. Bovine mastitis: Prevalence, risk factors and major pathogens in dairy farms of holeta town, central Ethiopia. Veterinary World. 2010;3:397–403.
Crossref - Dieser SA, Vissio C, Lasagno MC, Bogni CI, Larriestra AJ, Odierno LM. Prevalence of pathogens causing subclinical mastitis in Argentinean dairy herds. Pak Vet J. 2014;34(1):124-126.
- Roche JR, Dillon PG, Stockdale CR, Baumgard LH, VanBaale MJ. Relationships among international body condition scoring systems. J Dairy Sci. 2004;87(9):3076-3079.
- Kayesh M, Talukder M, Anower A. Prevalence of subclinical mastitis and its association with bacteria and risk factors in lactating cows of Barisal district in Bangladesh. Int J Biol Res. 2014;2:35-38.
Crossref - Rabbani AF, Samad MA. Host determinants based comparative prevalence of subclinical mastitis in lactating Holstein-Friesian cross cows and Red Chittagong cows in Bangladesh. Bangladesh Journal of Veterinary Medicine. 2010;8(1):17-21.
Crossref - Park B, Choi TJ, Park MN, Oh SH. Estimation of environmental effects and genetic parameters of carcass traits on Chikso (Korean brindle cattle). Asian-Australas J Anim Sci. 2020;33(4):525.
- Abrahmsen M, Persson Y, Kanyima BM, Bage R. Prevalence of subclinical mastitis in dairy farms in urban and peri-urban areas of Kampala, Uganda. Trop Anim Health Prod. 2014;46(1):99-105.
Crossref - Maheshwari P, Shukla PC, Swamy M, Sharma V. Occurrence of staphylococcal subclinical mastitis in cattle in and around Jabalpur (MP). J Anim Res. 2016;6(5):985.
Crossref - Bochniarz M, Wawron W, Szczubiał M. Coagulase-negative staphylococci (CNS) as an aetiological factor of mastitis in cows. Pol J Vet Sci. 2013;16(3):487-492.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.