ISSN: 0973-7510
E-ISSN: 2581-690X
Cervical cancer is a notable cause of mortality and morbidity among women of reproductive age. Human papillomavirus (HPV) is the leading cause of cervical cancer among women. Among 170 types of HPV; HPV-16 and -18 are responsible for cervical cancer. The overexpression of oncoproteins E6 and E7 are predominantly responsible for causing neoplasia. The presence of koilocytosis/koilocytotic atypia is the diagnostic point of HPV infection in pap smears. To identify the circulating types of HPV and determine the various risk factors associated with HPV infection, 100 vaginal biopsies or swabs were taken from patients suspected with cervical cancer, and qualitative and semi-quantitative real-time PCR were performed. PCR primers (GP5+/GP6+) based on a conserved region of the HPV-L1open reading frame(ORF) gene were used for the detection of HPV strains, while another set of primers was used for detecting the E6 gene (HPV-16) and E7 gene (HPV-18). The results showed an HPV infection rate of 23%. Furthermore, the prevalent genotype was found to be HPV-16 (73.91%), followed by HPV-18 (26.1%), while mixed infections of both HPV-16 and -18 accounted for 21.74%. In addition, an age of above 45 years, multiple pregnancies, low socioeconomic status, postmenopausal state, anemia, and early coitarche were significantly associated with HPV infection. These results provide the basis for the formulation of an appropriate strategy for disease monitoring to determine the frequency and distribution pattern of HPV infection.
Human Papillomaviruses, Molecular Techniques, HPV16, HPV18, Risk Factors
Recently, cervical cancer has become a burgeoning burden on the health of women. Human papillomaviruses (HPV) infect mucosal and cutaneous epithelium and induce cellular proliferation.1 Over 170 types of HPV circulate worldwide.2 Among these, many types have been found to cause cervical cancer, however, HPV-16 and -18 are the two most high-risk HPV types. The overexpression of two oncoproteins, E6 and E7, are responsible for causing neoplasia. There are many risk factors associated with the development of cervical neoplasia including smoking, being married at early age, multiple pregnancies, early age at first pregnancy, and multiple sexual partners. Vaccination against HPV-16 and -18 infections, that primarily targets adolescent females, are very effective in reducing the incidence of cervical cancer.2
Types of HPV belonging to phylogenetically “high-risk” species of the mucosotropic alpha genus, namely alpha-5,-6,-7,-9, and -11, are known to cause cervical carcinoma. The types most frequently found in cervical cancer are alpha-16, -18,-31,-33,-35, -45, -52, and -58, while rare types include alpha-39, -51, -56, and -59. The remaining types of HPV in the “high-risk” group are classified as “possibly carcinogenic”(group 2: 2A (alpha-68) and 2B (alpha-26, -30,-34, -53, -66, -67, -70, -73, -82, -85, and -97).3
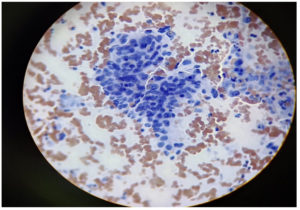
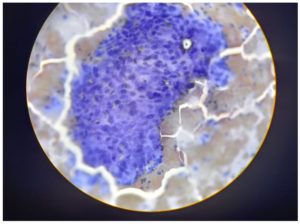
The widespread use of cervical cytology screening to detect both premalignant and malignant lesions has reduced the incidence of cervical cancer globally. The detection of HPV largely depends on cytology, from which abnormal koilocytosis or koilocytotic atypia, comprised of the combination of nuclear atypia and the formation of a perinuclear halo, can be identified. However, the sensitivity of cytology testing depends on the quality of the staining technique and the stage of CIN at which the smear is collected from patients. Nevertheless, cytology methods can often be overburdened by the presence of dyskaryotic smears, which may have a low productive value. This highlights the importance of the molecular detection of HPV as well as the identification of the type of HPV for early detection and treatment. In such scenario, the importance of molecular detection of HPV along with its typing is very essential nowadays. Hence, in this study, we carried out both the cytological study along with molecular detection of HPV side by side.4,5
Type of study
Cross-sectional hospital-based study.
Place of study
Department of Microbiology, Burdwan Medical College and Department of Microbiology, Burdwan University, Burdwan, West Bengal, India, from January 2019 to December 2021.
Ethics committee approval
The Institutional Ethical Committee (IEC) of BMC and IEC of Burdwan University approved the study. Written consent was obtained from each of the patients.
Source of data
A total of 100 vaginal biopsies or swabs were collected in viral transport media at the Department of Gynecology and Obstetrics, Burdwan Medical College and Hospital and sent to the Department of Microbiology, Burdwan Medical College for further laboratory diagnosis in Burdwan Medical College and Hospital.
Study population
Clinically suspected patients of cervical neoplasia, visiting the colposcopy unit for a pap smear or admitted to the Department of Obstetrics and Gynecology in Burdwan Medical College and Hospital.
Data for epidemiological survey
Patients who were clinically suspected to have cervical lesions or cervical cancer were selected and provided a questionnaire to obtain their medical history including clinical signs and symptoms, as well as personal information including age, residency, occupation, income, number of pregnancies, use of contraception, coitarche, menstrual history, and age at the time of marriage. We have also recorded the comorbid conditions including the history of diabetes mellitus, anemia, hypertension, hypothyroidism, and hyperthyroidism.
Pap smear
A sample was taken from the junction of the ectocervix by rotating a wooden Ayre spatula at 360°. The sample was then smeared onto a labelled glass slide and fixed with 95% ethyl alcohol. The smears were then stained using the Papanicolaou method.6 The Bethesda Classification of Cervical Cytology was used for reporting cervical cytology (Figure 1 and 2, Table 1 and 2).7
Table (1):
Bethesda system for reporting cervical cytology (Ref-6)
Pap smear |
Bethesda classification (n=100) (n ,%) |
|---|---|
1. Negative for Intraepithelial lesion or malignancy ) NILM |
84, (84%) |
A. Organisms – |
68, (81%) |
a. Shift in flora suggestive of bacterial vaginosis |
42, (62%) |
b. Trichomonalasvaginalis |
8, (12%) |
c. Fungal organism |
18, (26%) |
d. Actinomycosis |
0, (0%) |
e. HSV |
0, (0%) |
f. CMV |
0, (0%) |
B. Other non neoplastic finding |
16, (16%) |
a. Reactive changes associated with inflammation |
16, (100%) |
b. glandular cells status post hysterectomy |
0, (0%)S |
2. Epithelial cell abnormalities |
16, (16%) |
A. Squamous cell |
16, (100%) |
a. Atypical squamous cells of undermined significance (ASCUS) |
2, (13%) |
b. Atypical squamous cells cannot exclude HSIL (ASC-H) |
2, (13%) |
c. Low grade squamous intraepithelial lesion (LSIL) |
1, (6%) |
d. High grade squamous intraepithelial lesion HSIL |
2, (13%) |
e. Squamous cell carcinoma |
9, (56%) |
B. Glandular cell |
0, (0%) |
a. Atypical glandular cells (endocervical/endometrial and not otherwise specified ) |
0, (0%) |
b. Atypical glandular cells, fever neoplastic (endocervical /NOS) |
0, (0%) |
c. Endocervical adenocarcinoma in situ (AIS) |
0, (0%) |
d. Adenocarcinoma |
0, (0%) |
3. Others |
0, (0%) |
A. Endometrial cells in women >45 years |
0, (0%) |
Table (2):
Distribution of cases according to colposcopic finding (n=100)
Colposcopic findings |
No of cases (n ,%) |
|---|---|
Acetowhite area present |
16, (16%) |
Erosion |
5, (5%) |
Eversion |
3, (3%) |
Growth on anterior lip |
5, (5%) |
Normal |
71, (71%) |
Total |
100, (100%) |
Molecular diagnosis
Primer set design
Studies have shown that the HPV-L1 gene is highly conserved among various HPV genotypes, and a total of 10% of the HPV-L1 sequence can vary in distinct HPV genotypes.8 Moreover, the conserved region of E6 and E7 is comprised of open reading frames (ORFs) for HPV-16 and -18, respectively. The full genome sequences were downloaded from GenBank for alignment. Subsequently, species-specific primer sets for HPV-L1, 16, and 18 were designed using primer 3. Further, the specificity of three pairs of primers was verified using the Primer-BLAST program (Table 3).
Table (3):
Detailed sequences of the designed primer sets
| Gene | Primer | Sequence | Product Length | References |
|---|---|---|---|---|
| L1 gene | GP5+ | 5’-TTTGTTACTGTGGTAGATACTAC-3’ | 142 bp. | (9) |
| GP6+ | 5’-GAAAAATAAACTGTAAATCATATTC-3’ | |||
| E6 gene | Forward | 5’-AGGACCCACAGGAGCGAC-3’ | 126 bp. | (10) |
| Reverse | 5’-AGTCATATACCTCACGTCGCAGT-3’ | |||
| E7 gene | Forward | 5’-TGCCAGAAACCGTTGAATCC-3’ | 268 bp. | (11) |
| Reserve | 5’-TCTGAGTCGCTTAATTGCTC-3’ |
HPV DNA extraction
The viral DNA was isolated using a High Pure PCR Template Preparation Kit (cat. no. 11796828001, according to the manufacturer’s instructions (Roche).
Detection of HPV-L1 gene using qualitative PCR
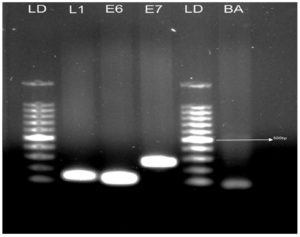
The HPV-L1 gene is conserved in all HPV genotypes. Thus, to cover all of the required positions, the PCR primers GP5+ and GP6+ were used for the amplification of the HPV-L1 gene (Table 3). Qualitative PCR (qPCR) was performed using Emerald Amp® GT PCR Master Mix (RR310B; TAKARA Bio.).Briefly, in a reaction volume of 50 µl, 100 ng of template DNA was mixed with 25 µl of 2׳Emerald Amp GT PCR Master Mix, 0.8 µl of 2 mM MgCl2, and 0.2 µM (final concentration) of forward and reverse primers; the remaining volume was comprised of ultra pure molecular grade water. The PCR cycling conditions were as follows: initial denaturation at 95°C (3 min), 35 cycles including denaturation at 94°C (30 s), annealing at 56°C (30 s), and elongation at 72°C (45 s), followed by 72°C for 5 min. Aliquots (10 µl) of the amplified PCR products were then run in 1.0 % agarose gel stained by ethidium bromide. The suitability of each sample for PCR amplification was verified by the presence of housekeeping gene b-actin, used as a control (Figure 3).
Real-time PCR of HPV-L1 using SYBR Green
Qualitative real-time PCR reactions targeting the L1 region of HPV were performed using TB Green Premix Ex Taq II (TiRNase H Plus). Each reaction consisted of 1xTB Green Premix Master Mix and the Gp5+ and Gp6+ primers in a reaction volume of 25 µl.12,13 Regions showing high rates of homology between different types of HPV were identified and used to design degenerative “broad spectrum” HPV primers. A CFX96 Touch Real-Time PCR Detection System (Bio-Rad) was used for real-time PCR with the following cycling conditions: initial denaturation incubation at 95°C for 2 min, followed by 38 cycles of alternating incubation periods at 95°C for 5 s, at 56°C for 30 s, and at 72°C for 10 s. Fluorescence was detected after each period of extension incubation at 72°C. All the clinical samples were screened using this method to verify the presence of HPV viral DNA. Only one HPV-L1 gene-positive sample (which was identified positive according to the NCBI BLASTN result for human papillomavirus (ID: 10566), was sent for sequencing. This sample was used as positive control. Subsequently, genotyping of HPV-18 and -16 were carried out by using qPCR. β-actin was used as the SYBR Green test control.
HPV-16 and -18 genotyping by qPCR
The L1-positive samples obtained using SYBR Green were further processed for HPV-16 and -18 screening. The E6 and E7 genes were used to classify the HPV-16 and -18 variants present in the clinical samples. Two E6 PCR targets were designed to cover the required positions using the corresponding forward and reverse primers (Table 3). Subsequently, other two primers were used for the detection of HPV E7 gene for the genotyping of HPV-18 types (Table 3).9 qPCR was performed using Emerald Amp® GT PCR Master Mix (RR310B; TAKARA Bio.). Briefly, in a reaction volume of 50 µl, 100 ng of template DNA was mixed with 25 µl of 2׳Emerald Amp GT PCR Master Mix, 0.8 µl of 2 mM MgCl2, and 0.2 M (final concentration) of both forward and reverse primers; the remaining volume was comprised of ultra pure molecular grade water. The cycling conditions for qPCR were as follows: initial denaturation at 95°C (3 min), 35 cycles including denaturation at 94°C (30 s), annealing at 56°C (30 s), and elongation at 72°C (45 s), followed by 72°C in 5 min. Aliquots of the amplified PCR products were run in 1.0% agarose gel stained with ethidium bromide. Since all the amplified products had different lengths, genotypes of the virus were analyzed by using electrophoresis and visualized via an ultraviolet light trap-illuminator.11 Bands of appropriate size were identified by comparing with DNA molecular weight markers. The adequacy of the DNA in each specimen for PCR amplification was determined by the detection of the housekeeping gene b-actin.
Real-time PCR of HPV-16 and -18 using SYBR Green
Positive samples for L1 obtained through a previous round of qPCR were screened for their HPV type since the L1 gene is a conserved region in different HPV types, including HPV-16 and -18.10,11 The quantitative Sybr Green TB Green Premix Ex Taq II (TiRNase H Plus) was used to perform real-time PCR targeting the E6 and E7 regions of HPV. Briefly, in a reaction volume of 25 µl, 1xTB Green Premix Master Mix was supplemented with generic primers of a short sequence of the HPV E6 and E7 genes. HPV16 and HPV18 have highly preserved regions. 10,11 The following cycling conditions were used: initial denaturation at 95°C for 2 min, followed by 38 cycles of alternating incubation periods at 95°C for 5 s, at 56°C for 30 s, and at 72°C for 10 s. After each 72°C extension incubation, fluorescence was recorded. Positive clinical samples derived from HPV-L1 genes were chosen for this approach, confirming the presence of HPV-16 and HPV-18 type viral DNA. As previously mentioned, the adequacy of the DNA in each specimen for PCR amplification was determined by the detection of the housekeeping gen b-actin as control.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences(SPSS) (version 23). Fisher’s exact test was used to analyze categorical data. A p-value of less than 0.05 was used to indicate statistical significance.
The prevalence of HPV infection in 100 samples was 23% (n= 23), among which the most prevalent genotype was HPV-16 (76.91%, n= 17), followed by HPV-18 (26.1%, n=6). Mixed infections of both HPV-16 and -18 were identified in 21.74% (n=5) of the samples. Among the total HPV positive cases, 34.78% patients had reported an underlying comorbidity of anemia, followed by 26.09% with diabetes mellitus (Table 4). Among the patients,60.87% had their first sexual encounter at an age of 18–25 years (Table 5). The rate of HPV positivity was higher in women who had experienced multiple pregnancies (Table 6). Among those patients positive for HPV infection,43.48% were daily workers, followed by 26.1% who were involved in agricultural labor (Table 7). In terms of the monthly income of patients positive for HPV infection, the monthly income of 48% of these patients was ≤10,000 rupees, whereas 35% had a monthly income >10,000-20,000 rupees (Table 8). In terms of the type of contraception method, used by HPV positive women, 52.17% used non barrier methods of contraception, while 34.78% used barrier methods of contraception (Table 9). The highest prevalence (47.83%)of HPV infection was observed among patients aged 41–50 years, followed by 21.74%in patients aged 31-40 years (Table 10). Among the patients positive for HPV, 52.17% reported experiencing white discharge over a long time, followed by 17.39% who reported a history of post-coital bleeding and abdominal pain (Table 11). A comparison between the RT-PCR results and the pap smear samples is provided in Table 12. The sensitivity, specificity, and positive predictive value of RT-PCR method were significantly higher the pap smear (72.53% vs.65.29%, 98.14% vs. 91.76%, and 82.91% vs.70.86%, respectively).
Table (4):
Distribution of cases according to frequency of co-morbidities
| Co-morbidities | According to RT-PCR report | Statistical Test | Cytological findings | Total samples (n=100) n, ( %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HPV positive cases (n=23) (n, %) | Rate of positivity (n, %) | (Negative samples n=77): (n, %) | Fisher’s Exact Test value, df | P value | NILM (n=84) | Epithelial cell abnormalities (n=16) (n, %) | |||
| Organism (n=68): (n, %) | Other non neoplastic findings (n=16) (n, %) | ||||||||
| Hypertension | 5, (21.74%) | 27.78% | 13, (16.88%) | 3.257,4 | 0.583 | 12, (17.64%) | 4, (25%) | 4 ,(25%) | 18, (18%) |
| Diabetes mellitus | 6, (26.09%) | 25% | 18, (23.37%) | 1.354,4 | 0.001* | 16,(23.53%) | 5,(31.25%) | 5, (31.25%) | 24, (24%) |
| Hypothyroidism | 2, (8.7%) | 16.66% | 10, (12.99%) | 5.692,4 | 0.241 | 10, (14.71%) | 1, ( 6.25%) | 1, ( 6.25%) | 12, (12%) |
| Hyperthyroidism | 2, (8.7%) | 40% | 3, (3.9%) | 7.982,4 | 0.369 | 3, 4.41%) | 1, (6.25%) | 1, ( 6.25%) | 5, (5%) |
| Anaemia | 8, (34.78%) | 19.51% | 33, (42.86%) | 1.289,4 | 0.026* | 27, (39.71%) | 5, 31.25%) | 5, (31.25%) | 41, (41%) |
*Significant at p<0.05, Fisher’s Exact Test; No significant difference was observed between the groups HPV-positive cases and negative samples in terms of hypertension, hypothyroidism, and hyperthyroidism. However, a significant difference was observed between the groups HPV-positive cases and negative samples in terms of diabetes mellitus and anemia (p <0.001 and p = 0.026, respectively).
Table (5):
Distribution of cases according to coitarche
| Coitarche (years ) | According to RT-PCR report’s finding | Statistical Test | Cytological findings | Total samples (n=100) n , ( %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HPV positive cases (n=23): (n, %) | Rate of positivity (n, %) | Negative samples (n=77) (n, %) | Fisher’s Exact Test value, df | P value | NILM (n=84) | Epithelial cell abnormalities (n=16) (n, %) | |||
| Organism (n=68) (n, %) | Other non neoplastic findings (n=16) (n, %) | ||||||||
| <18 | 3, ( 13.04 %) | 27.27% | 8, (10.39%) | 7.529,2 | 0.963 | 5, (7.35%) | 4, ( 25%) | 2, ( 12.5%) | 11, (11%) |
| 18-25 | 14, ( 60.87%) | 30.43% | 32, (41.56%) | 1.057,2 | 0.001* | 27, (39.71%) | 8, (50%) | 11, 68.75%) | 46, (46%) |
| >25 | 6, (26.09%) | 13.95% | 37, (48.1%) | 8.524,2 | 0.582 | 36, (52.94%) | 4, (25%) | 3, (18.75%) | 43, (43%) |
*Significant at p<0.05, Fisher’s Exact Test. A significant difference was observed between the HPV-positive cases and negative samples in terms of coitarche (years) for 18–25 years(p <0.001).
Table (6):
Distribution of cases according to the parity
| Parity | According to RT-PCR report’s finding | Statistical Test | Cytological findings | Total samples (n=100) n, ( %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| NILM (n=84) | Epithelial cell abnormalities (n=16): n ( %) | ||||||||
| HPV positive cases (n=23) n, (%) | Rate of positivity (%) | Negative samples (n=77) n, (%) | Fisher’s Exact Test value, df | P value | |||||
| Organism (n=68) n, ( %) | Other non neoplastic findings (n=16) n, ( %) | ||||||||
| P1+0 | 2, ( 8.7 %) | 14.29% | 12, ( 15.58%) | 11.574,3 | 0.452 | 11 ,(16.18%) | 2, ( 12.5%) | 1, ( 6.25%) | 14, (14%) |
| P2+0 | 5, (21.74 %) | 12.82% | 34, (44.16%) | 7.853,3 | 0.836 | 32 ,(47.06%) | 3, (18.75%) | 4, ( 25%) | 39, (39%) |
| P3+0 | 13, ( 56.52 %) | 37.14% | 22, (28.57%) | 8.414,3 | 0.023* | 17, (25%) | 8, (50%) | 10, (62.5%) | 35, (35%) |
| P4+0 | 3, (13.04 %) | 25% | 9,(11.69%) | 4.617,3 | 2.547 | 8, (11.76%) | 3, (18.75%) | 1, (6.25%) | 12, (12%) |
Between the groups in terms of parity for P3+0(p = 0.023).
Table (7):
Distribution of cases according to occupation
| Occupation | According to RT-PCR report’s finding | Statistical Test | Cytological findings | Total samples (n=100) n, ( %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HPV positive cases (n=23) n, (%) | Rate of positivity (%) | Negative samples (n=77) n, (%) | Fisher’s Exact Test value, df | P value | |||||
| NILM (n=84) | Epithelial cell abnormalities (n=16) n, ( %) | ||||||||
| Organism (n=68) n, ( %) | Other non neoplastic findings (n=16) n, (%) | ||||||||
| Agriculture | 6, (26.1%) | 24% | 19, (24.68%) | 5.367,4 | 3.245 | 18, ( 26.47%) | 4, ( 25%) | 4, ( 25%) | 25, (25%) |
| Daily worker | 10, (43.48%) | 27.78% | 26, (33.77%) | 11.257,4 | 1.254 | 25, (36.76%) | 6, (37.5%) | 6, (37.5%) | 36, (36%) |
| Teacher | 2, (8.7%) | 14.29% | 12, (15.58%) | 6.392,4 | 2.581 | 10, (14.71%) | 1, ( 6.25%) | 1, ( 6.25%) | 14, (14%) |
| House wives | 4, (17.39%) | 40% | 6, (7.79%) | 1.257,4 | 0.235 | 2, (2.94%) | 4, ( 25%) | 4, ( 25%) | 10, (10%) |
| Trade | 1, (4.35%) | 6.67% | 14, (18.18%) | 2.365,4 | 0.743 | 13, ( 19.12%) | 1, (6.25%) | 1, ( 6.25%) | 15, (15%) |
*Significant at p<0.05, Fisher’s Exact Test. No significant difference was observed between the groups in terms of occupation.
Table (8):
Distribution of cases according to monthly income
| Monthly income | According to RT-PCR report’s finding | Statistical Test | Cytological findings | Total samples (n=100) n, ( %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| NILM (n=84) | Epithelial abnormalities (n=16): n (%) | ||||||||
| HPV positive cases (n=23) n, (%) | Rate of positivity (%) | Negative samples (n=77) n, (%) | Fisher’s Exact Test value, df | P value | |||||
| Organism (n=68): n (%) | Other non neoplastic findings (n=16) : n ( %) | ||||||||
| <=10000/- | 11,(48%) | 23.91% | 35, (45.45%) | 7.325,3 | 2.014 | 32 ,(47.06%) | 6 ,(37.5%) | 8, (50%) | 46, (46%) |
| >10000-20000/- | 8 ,(35%) | 36.36% | 14, (18.18%) | 2.853,3 | 0.125 | 11, 16.18%) | 6,(37.5%) | 5, (31.25%) | 22, (22%) |
| >20000-30000/- | 3, (13%) | 12.5 % | 21, (27.27%) | 4.258,3 | 0.532 | 19, ( 27.94%) | 3,(18.75%) | 2, ( 12.5%) | 24, (24%) |
| >30000 /- | 1 ,(4.35%) | 12.5% | 7, (9.09%) | 1.246,3 | 0.412 | 6, (8.82%) | 1, ( 6.25%) | 1, ( 6.25%) | 8, (8%) |
*Significant at p<0.05, Fisher’s Exact Test. In addition, no significant difference was observed between the groups in terms of monthly income.
Table (9):
Distribution of cases according to the use of contraceptive methods
| Contraceptive methods | According to RT-PCR report’s finding | Statistical Test | Cytological findings | Total samples (n=100) n, ( %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HPV positive cases (n=23) n , (%) | Rate of positivity (%) | Negative samples (n=77) n, (%) | Fisher’s Exact Test value, df | P value | NILM (n=84) | Epithelial cell abnormalities (n=16) n ( %) | |||
| Organism (n=68) n, ( %) | Other non neoplastic findings (n=16) n, ( %) | ||||||||
| Barrier methods | 8 (34.78%) | 25.81% | 23 (29.87%) | 6.244,2 | 1.249 | 23 (33.82%) | 3 (18.75%) | 5 ( 31.25%) | 31, (31%) |
| Non-barrier methods | 12 (52.17%) | 23.53% | 39 (50.64%) | 3.248,2 | 0.034* | 30, (44.12%) | 11 (68.75%) | 10, (62.5%) | 51, (51%) |
| Not used any contraceptive | 3 (13.04%) | 16.66% | 15(19.48%) | 1.946,2 | 0.001* | 15, (22.06%) | 2 ( 12.5%) | 1 ( 6.25%) | 18, (18%) |
*Significant at p<0.05, Fisher’s Exact Test. However, a significant difference was observed between the HPV-positive cases and the negative samples in terms of the use of non-barrier methods of contraception compared to not–used any method of contraceptive (p = 0.034 and p <0.001, respectively).
Table (10):
Frequency of distribution of cases according to the age group (n=100)
| Age groups in years | According to RT-PCR report’s finding | Statistical Test | Cytological findings | Total Sample (n=100) n, (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HPV positive cases (n=23) n, ( %) | Rate of positivity (%) | Negative samples (n=77) n, (%) | Fisher’s Exact Test value, df | P value | NILM (n=84) | Epithelial cell abnormalities (n=16) n, (%) | |||
| Organism (n=68) n, ( %) | Other non neoplastic findings (n=16) n, ( %) | ||||||||
| <20-30 years | 4 (17.39%) | 14.81% | 23 (29.87%) | 2.148,3 | 2.142 | 22 (32.35%) | 2 (12.5%) | 3 (18.75%) | 27, (27%) |
| 31-40 years | 5 (21.74%) | 19.23% | 21, (27.27%) | 2.127,3 | 0.274 | 21 (30.88%) | 3 (18.75%) | 2( 12.5%) | 26, (26%) |
| 41-50 years | 11 (47.83%) | 28.95% | 27 (35.06%) | 3.582,3 | 0.965 | 19 (27.94%) | 9 (56.25%) | 10 (62.5%) | 38, (38%) |
| >50 years | 3 (13.04%) | 33.33% | 6, (7.79%) | 4.125,4 | 0.716 | 6(8.82%) | 2 ( 12.5%) | 1(6.25%) | 9, (9%) |
*Significant at p<0.05, Fisher’s Exact Test. No significant difference was observed between the groups in terms of age.
Table (11):
Distribution of cases according to frequency of presenting symptoms
| Symptoms | According to RT-PCR report’s finding | Statistical Test | Cytological findings | Total samples (n=100) n, (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| HPV positive cases (n=23) n , ( %) | Rate of positivity (%) | Negative samples (n=77) n, ( %) | Fisher’s Exact Test value, df | P value | NILM (n=84) | Epithelial cell abnormalities (n=16) n, ( %) | |||
| Organism (n=68) n, (%) | Other non neoplastic findings (n=16) n, (%) | ||||||||
| White discharge | 12, (52.17%) | 40% | 30, (38.96%) | 2.654,4 | 0.039* | 26, (38.24%) | 8, (50%) | 8, (50%) | 42, (42%) |
| Post coital bleeding | 4, (17.39%) | 23.53% | 17, (22.08%) | 3.651,4 | 1.022 | 17, (25%) | 2, ( 12.5%) | 2, ( 12.5%) | 21, (21%) |
| Menstrual irregularity | 2, (8.7%) | 28.57% | 7, (9.09%) | 1.247,4 | 0.026* | 7, (10.29%) | 1, ( 6.25%) | 1, ( 6.25%) | 9, (9%) |
| Post menopausal bleeding | 1, (4.35%) | 9.09% | 11, (14.29%) | 1.582,4 | 0.964 | 9, (13.24%) | 2, ( 12.5%) | 1, ( 6.25%) | 12, (12%) |
| Abdominal pain | 4, (17.39%) | 33.33% | 12, (15.58%) | 1.381,4 | 0.001* | 9, (13.24%) | 3, (18.75%) | 4 , (25%) | 16, (16%) |
*Significant at p<0.05, Fisher’s Exact Test. Finally, a significant difference was observed between the HPV-positive cases and the negative samples in terms of white discharge, menstrual irregularity, and abdominal pain (p = 0.039, p = 0.026, and p <0.001, respectively).
Table (12):
Comparison between RTPCR with pap smear (N=100)
| Screening result( n=100) | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (positive predictive value ) (%) | NPV (Negative predicted value ) (%) | ||
|---|---|---|---|---|---|---|---|
| RT PCR | n=100 | ||||||
| Positive | 23 | 72.53% | 98.14% | 96.59% | 82.91% | 96.25% | |
| Negative | 77 | ||||||
| Pap smear | |||||||
| NILM | 84 | 65.29% | 91.76% | 89.47% | 70.86% | 90.19% | |
| Positive (epithelial cell abnormalities ) | 16 | ||||||
Cancer has become one of the largest burdens on public health in recent decades. Among the different types of cancer, 20% are known to originate from viruses, known as oncogenic viruses.1 Oncogenic viruses are responsible for transforming normal cells into malignant cells that, instead of growing in a monolayer, pileup in multilayers, resulting in the formation of tumors. These cancerous cells encode proteins that encourage the transformation of normal cells into cancerous cells, the genetic basis of which is mutations.11
Many types of oncogenic cancer are prevalent worldwide, among which the seventh most common type of cancer is cervical cancer, which is the fourth most prevalent type in women.14 Approximately 85% of cervical cancer cases are found in developing and underdeveloped countries.15 The worldwide prevalence of cervical cancer is 11.7%, with countries including South Africa (17.4.0%), Eastern Africa (33.6%), Eastern Europe (21.4%), Western Europe (9.0%), Eastern Europe (21.4%), and the Caribbean (35.4%) reporting the highest rates of HPV prevalence.16,17 In this study, the prevalence of HPV infection among a cohort of 100 patients clinically suspected of having cervical cancer in Burdwan, India, was 23%. Similarly, another study conducted in Southern India by Franceschi reported a prevalence of HPV infection of 16.9%.18 However, in a study by Senapati et al. in the eastern region of India, an overall prevalence of 60.33% was reported for HPV infection.19
Persistent infection with a high-risk HPV type is a mandatory factor, although not the determining reason, for the development of cervical carcinoma. Premalignant lesions are likely to transform into malignant lesions if they remain unidentified in the cervix for a prolonged time period.3 A study conducted by Centers for Disease Control and Prevention (CDC) in 2008 revealed that, each year, despite a large number of HPV infections (6.2 million), only a small proportion of women infected with a high-risk HPV type developed precancerous lesions, with even fewer developing cervical cancer.20,21 Similar findings were observed in the present study, wherein among a total of 23 patients positive for HPV infection, only 39.13% (n=9) developed cervical cancer.
Various risk factors are known to accelerate this progression into cervical cancer. In this study, several risk factors related to HPV infection and cervical cancer were identified, among which the most important risk factors were age (>45years), multigravida, low socio-economic status, post-menopausal state, anemia, diabetes mellitus, using oral contraceptive pills (OCP) for many years, and long years of coitarche. Lertcharernrit reported similar demographic data, revealing that HPV infection was more common in women under the age of 30, with multiple sexual partners, with a low socioeconomic status, with sexually transmitted diseases, who did not use contraceptives, having had multiple pregnancies, and long years of coitarche and malnutrition.22
In the present study, among the total HPV-positive cases, the leading comorbidities were anemia (34.78%), diabetes mellitus (26.09%), and hypertension (21.74%). Furthermore, as reported in the literature, nutrition and comprised immunity due to other co-morbid conditions may also accelerate the development of precancerous lesions into cervical cancer.23
Lertcharernrit et al. found that early coitarche was associated with the onset of HPV infection.22 Similarly, in the present study, women who had their first exposure to sexual relations at the ages of 18–25 were found to be more likely to be positive for HPV infection. We have also noted that the women that started having sexual relations at an early age were more likely to have many children. These multigravida women were found to be more prone to HPV infection, which suggests that multiparity is proportionately related to HPV infection. As previously suggested, elevated levels of hormones, particularly progesterone, at the time of pregnancy causes alterations to the squamocolumnar junction. This biological phenomenon increases the risk of the direct exposure to HPV, which leads to the persistence of HPV infection, and is eventually responsible for the progression from cervical neoplasia to cancer.24,25Furthermore, during pregnancy, the immune system is suppressed, which may enhance the process of cervical carcinogenesis in individuals infected with HPV.24,25
Nutrition also plays a pivotal role in immunity, which is directly related to HPV infection. In this study, the highest rate of HPV infection was observed in patients with a monthly income <10,000rupees, with some families having a meager monthly income of 2,000–3,000 rupees, most of which were daily workers and farmers, accounting for 43.48% and 26.1% of HPV-positive cases, respectively. Furthermore, nutrition also plays an important role in pregnancy. A low nutritional status is associated with low levels of antioxidants, such as beta-carotene; lycopene; and vitamins A, C, and E (alpha-tocopherol) which can be obtained from fruits and vegetables and which interact with free radicals to prevent cancer.26,27 Thus, in individuals with diets that lackfruit and vegetables, free radicals can damage cell components, including DNA, protein, and cell membranes.28 Conversely, a healthy and well-maintained nutritional status can boost the immune system to counteract this.
Here, we also found that women who use non-barrier methods of contraception (e.g., OCP)were more prone to HPV infection. The daily use of an oral contraceptive for a long period appears to increase the risk of cervical cancer, which suggests that the long-term use of OCP is directly proportional to the risk of cervical cancer. This may be due to the fact that the levels of estrogen and progesterone interact with hormones receptors situated in cervical tissue, thereby playing in role in susceptibility to HPV infection. Sex steroid hormones are considered to be responsible for enhancing the expression of oncogenes (E6 and E7), which triggers the degradation of p53 tumor suppressor genes, there by increasing the ability of viral DNA to transform cells and induce carcinogenesis.29-31
In addition, another physiological factor, age, is known to be closely related to the risk of HPV infection.32 Here, women aged 41–50 years were observed to be more likely to be infected with HPV (47.83%), followed by women aged 31–40 years. In some populations, women aged >55 years have been reported to be prone to HPV infection, a phenomenon that is attributed to weak immunity, the reactivation of latent infection, and birth cohort effects.33 However, many studies have reported HPV infection in younger women, which may be due to the lack of adaptive immune responses at a young age. Moreover, in a study by Trottier, the relatively large area of cervical epithelium transforms into squamous metaplasia in women at a young age, that may increase the chances of HPV DNA infecting the basal cell layer, from which it can proliferate.34
Finally, in this study, approximately 52.17% of HPV-positive women reported experiencing white discharge for an extended period of time, followed by 17.39% who had a history of post-coital bleeding and abdominal pain. Similarly, in a study conducted by Sachan, 36.96% of women found to be HPV-positive complained of white discharge during history taking.6
Concludingly, the patients at the highest risk of HPV infection are multigravida-middle aged Bengali women from a low socioeconomic background with early coitarche, who had the co-morbid conditions of anemia or diabetes mellitus, and who used non-barrier contraceptive methods (e.g. OCP).
This study demonstrates that molecular methods represent a good modality for the diagnosis of HPV infection. If not treated at the earliest, precancerous lesions in patients with HPV infection are likely to develop into cervical cancer. Herein, we observed that pap smears sometimes were unable to reveal cytopathic changes observed in vaginal smears. Conversely, molecular methods were able to identify HPV DNA at an early stage of HPV infection, prior to the onset of cancer.
ACKNOWLEDGMENTS
The authors would like to thank the Medical Technologist (Lab), who diligently helped us to accomplish this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest
AUTHORS’ CONTRIBUTION
NC, SKM and AB conceptualized the study. TB performed literature review. PS helped in the sample collection. NC, SKM, AB, KB and NM performed experiments. AN performed formal analysis. NC, SKM and AB wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated and analysed during the study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethical Committee (IEC) of BMC and IEC of Burdwan University, vide memo no.:BMC-1200, dated 25/05/18 and approval No.:IEC/BU/2019/01, dated 05.07.2019.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology. 2013;445(1-2):224-231.
Crossref - Zur H H, De V EM. Human papillomaviruses. Annual review of microbiology. 1994; 48(1):427-47.
Crossref - Abreu AL, Souza RP, Gimenes F, Consolaro ME. A review of methods for detect human Papillomavirus infection. Virol J. 2012;9:262.
Crossref - Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8(8):CD008587.
Crossref - Cuzick J, Sasieni P, Davies P, et al. A systematic review of the role of human papilloma virus (HPV) testing within a cervical screening programme: summary and conclusions. Br J Cancer. 2000;83(5):561-565.
Crossref - Sachan PL, Singh M, Patel ML, Sachan R. A Study on Cervical Cancer Screening Using Pap Smear Test and Clinical Correlation. Asia Pac J Oncol Nurs. 2018;5(3):337-341.
Crossref - Pangarkar MA. The Bethesda System for reporting cervical cytology. Cytojournal. 2022;19:28.
Crossref - Xu WX, Wang J, Tang HP, et al. Epitomics: IgG-epitome decoding of E6, E7 and L1 proteins from oncogenic human papillomavirus type 58. Sci Rep. 2016;6(1):34686.
Crossref - Manzouri L, Salehi R, Shariatpanahi S, Rezaie P. Prevalence of human papilloma virus among women with breast cancer since 2005-2009 in Isfahan. Adv Biomed Res. 2014;3:75.
Crossref - Yu D, Chen Y, Wu S, Wang B, Tang YW, Li L. Simultaneous detection and differentiation of human papillomavirus genotypes 6, 11, 16 and 18 by All Gloquadruplex quantitative PCR. PLoS One. 2012;7(11):e48972.
Crossref - Settheetham-Ishida W, Kanjanavirojkul N, Kularbkaew C, Ishida T. Human papillomavirus genotypes and the p53 codon 72 polymorphism in cervical cancer of Northeastern Thailand. Microbiol Immunol. 2005;49(5):417-421.
Crossref - Hesselink AT, Berkhof J, van der Salm ML,et al. Clinical validation of the HPV-risk assay, a novel real-time PCR assay for detection of high-risk human papillomavirus DNA by targeting the E7 region. J Clin Microbiol. 2014;52(3):890-896.
Crossref - van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40(3):779-787.
Crossref - Globocan. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122-163.
Crossref - Bruni L. The frequency of HPV infection worldwide On average, 12% of women worldwide had a detectable cervical HPV infection varying by geography and age.2018.
- de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453-459.
Crossref - Franceschi S, Rajkumar R, Snijders PJF, et al. Papillomavirus infection in rural women in southern India. Br J Cancer. 2005;92(3):601-606.
Crossref - Senapati R, Nayak B, Kar SK, Dwibedi B. HPV Genotypes distribution in Indian women with and without cervical carcinoma: Implication for HPV vaccination program in Odisha, Eastern India. BMC Infect Dis. 2017;17(1):30.
Crossref - Jeon S, Lambert PF. Integration of Human Papillomavirus Type 16 DNA into the Human Genome Leads to Increased Stability of E6 and E7 mRNAs: Implications for Cervical Carcinogenesis. Proc Natl Acad Sci U S A. 1995;92(5):1654-1658.
Crossref - Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25(Suppl 1):2-23.
Crossref - Jiraporn L, Sananpanichkul P, Suknikhom W, Bhamarapravatana K, Suwannarurk K, Leaungsomnapa Y. Prevalence and Risk Assessment of Cervical Cancer Screening by Papanicolaou Smear and Visual Inspection with Acetic Acid for Pregnant Women at a Thai Provincial Hospital. Asian Pac J Cancer Prev. 2016;17(8):4163-4167 .
- Chelimo C, Wouldes TA, Cameron LD, Elwood JM. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J Infect. 2013;66(3):207-217.
Crossref - Roura E, Travier N, Waterboer T, et al. The Influence of Hormonal Factors on the Risk of Developing Cervical Cancer and Pre-Cancer: Results from the EPIC Cohort. PLoS One. 2016;11(1):e0151427.
Crossref - Jensen KE, Schmiedel S, Norrild B, Frederiksen K, Iftner T, Kjaer SK. Parity as a cofactor for high-grade cervical disease among women with persistent human papillomavirus infection: a 13-year follow-up. Br J Cancer. 2013;108(1):234-239.
Crossref - Bouayed J, Bohn T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3(4):228-237.
Crossref - Moore MA, Tajima K. Cervical cancer in the asian pacific-epidemiology, screening and treatment. Asian Pac J Cancer Prev. 2004;5(4):349-361.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44-84.
Crossref - International Collaboration of Epidemiological Studies of Cervical Cancer, Appleby P, Beral V, de Gonzalez AB, et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609-1621.
Crossref - Moodley M, Moodley J, Chetty R, Herrington CS. The role of steroid contraceptive hormones in the pathogenesis of invasive cervical cancer: a review. Int J Gynecol Cancer. 2003;13(2):103-110.
Crossref - Gadducci A, Barsotti C, Cosio S, Domenici L, Riccardo Genazzani A. Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature. Gynecol Endocrinol. 2011;27(8):597-604.
Crossref - Dempsey AF. Human papillomavirus: the usefulness of risk factors in determining who should get vaccinated. Rev Obstet Gynecol. 2008;1(3):122-128.
- Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis.2005;191(11):1808-1816.
Crossref - Trottier H, Franco EL. Human papillomavirus and cervical cancer: burden of illness and basis for prevention. Am J Manag Care. 2006;12(17 Suppl):S462-S472.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.