ISSN: 0973-7510
E-ISSN: 2581-690X
Bacteria control gene expression by quorum sensing (QS) mechanism owing to producing small signal molecules associated with population density. Both gram-positive and gram-negative bacteria use QS to manage various physiological characteristics, including bioluminescence, virulence gene expression, biofilm formation, and antibiotic resistance. Impatience balsamina is a flowering, perennial and annual herb indigenous to southern Asia in India. All parts of Impatience balsamina have a therapeutic effect on different diseases. This study evaluated the anti-quorum sensing activity of leaf extract of Impatience balsamina by examining its action on Violacein production by Chromobacterium violaceum, a biosensor strain, and Biofilm, Pyocyanin, Protease, and Chitinase production by the reference strain Pseudomonas aeruginosa PA 01. Minimum inhibitory concentration (MIC) for Pseudomonas aeruginosa PA 01was 3.125mg/ml. A concentration of 1.563mg/ml (sub-MIC) showed inhibition of 100% on Las A protease, 78.42% on chitinase, 30.75% on biofilm, and 93.33% on pyocyanin production by Pseudomonas aeruginosa PA 01. This article displayed the quorum quenching activity of Impatience balsamina by hindering the quorum-sensing controlled characteristics of bacteria without killing it, which reduces the proneness of drug resistance in bacteria, a globally accepted emerging problem in the medical field.
Impatience balsamina, Quorum Sensing, Pyocyanin, Biofilm
Medicinal plants have been used for treating diseases in traditional medicine for several generations worldwide. The application of herbal medicines has been practiced in India for numerous years as a medicament for numerous diseases and physiological conditions in conventional systems, namely Ayurveda, Unani, and Siddha, and continues the same even now.1 Plant-derived crude extracts have been used as medicines, and it has historical involvement in the progression of humans. Plants are well known in traditional medical practices; hence, their activity has been studied to isolate lead molecules.
Impatience balsamina is a species of Impatience to be included in the family Balsaminaceae. Impatience balsamina (English name: Rose balsam, Hindi name: Gulmehendi) is locally known as balsam in Kerala, South India. The two important genera of Balsaminaceae are Impatience Linnaeus Hydrocera wight and Arnott, and it includes more than 1000 species belonging to the family Balsaminaceae.2
Impatience balsamina L(Balsaminaceae) is a curative plant; all the parts of the plant have a therapeutic effect on disparate types of diseases such as thorn or glass puncture wounds, abscesses, chronic ulcers, warts, snake bites, rheumatism, fractures, and fingernail inflammation, jaundice, digestive disorders and so on. It has antimicrobial, antirheumatic, antipruritic, and antitumoral effects and has also been used to treat strenuous labor and puerperal pain in Chinese medicines for innumerable years.3 In addition to that, the leaves, seeds, and stems are consumable if cooked.
Pseudomonas aeruginosa is a potentially pathogenic bacterium due to the production of multiple virulence factors such as proteases, hemolysins, exotoxin A, exoenzyme S, and pyocyanin.4 It leads to multidrug resistance in bacteria and is one of the most significant risks to public health as it increases morbidity, mortality, and cost of health care setup.5 Biofilms are the complex communities of different organisms in a self-produced matrix and are attached to living and non-living surfaces. Biofilm formation is one of the main reasons for the emergence of antibiotic-resistant strains.6 This phenomenon protects bacteria from the host’s ultraviolet radiation, desiccation, chemicals, and defense mechanisms. Therefore, recrudescent and dreadful infections are often interconnected to biofilm formation.7 The degree of resistance to antimicrobial agents is 1000 times higher in biofilm-grown cells than in their absence.8 Pyocyanin is a bluish-green pigment and is one of the principal virulence factors of Pseudomonas aeruginosa; it is produced by most of the isolates of Pseudomonas aeruginosa.9
The quorum-sensing mechanism of bacteria controls gene expressions in agreement with population density by secreting small signal molecules; thereby, it regulates particular phenotypic expressions, namely biofilm formation, pyocyanin production, etc.10 It toughens the biofilm formation of Pseudomonas aeruginosa and makes it incurable as biofilms are unaffected by antibiotics.11
Quorum sensing is a method through which a single cell can detect communication from neighboring cells by accumulating signaling chemicals. Bacteria communicate with one another by creating signaling molecules, and they can only demonstrate quorum sensing when the chemicals reach the desired concentration. Quorum sensing (QS) is a bacterial communication mechanism that permits specialized processes like biofilm formation, virulence factor expression, secondary metabolite production, and stress adaptation mechanisms like bacterial competition systems, including secretion systems, to be controlled (SS). P. aeruginosa produces a remarkable display of both cell-associated and extracellular virulence factors. Quorum sensing is the mechanism of cell-to-cell interaction in a bacterial population, where bacteria produce signal molecules that other individuals can detect. There are two QS systems in P. aeruginosa which are well studied. The las system consists of the LasR transcriptional regulator and the LasI synthase protein. The LasI is essential for the production of the AHL signal molecule N-(3-oxododecanoyl)-l-homoserine lactone (3O-C12-HSL).12 Acyl homoserine lactones (AHLs) are the signal molecules produced in P. aeruginosa. In order to become an active transcription factor, LasR requires 3O-C12-HSL. LasR forms multimers that can bind DNA and regulate the transcription of multiple genes.13 The second QS system includes RhlI and RhlR proteins. The RhlI synthase produces AHL N-butyryl-l-homoserine lactone (C4-HSL), and RhlR is the transcriptional regulator. The expression of several genes is regulated when RhlR is complexed with C4-HSL.14 QS in P. aeruginosa regulates the virulence genes during infections in humans.
This study aimed to determine the chemical composition and anti-quorum sensing activity of acetone extract of leaf of Impatience balsamina.
Plant Material
The branches of the Balsam plant were collected from the local garden, Udupi. The plant sample was identified and deposited at Calicut university herbarium for reference purposes (located Account number is 7084.). The collected samples were thoroughly washed with running tap water, and leaves were separated; shade dried for two days. Further dried in a hot air oven at 500C and then powdered with the help of a mechanical blender and stored in an airtight container for further use. Both the fresh and dry weights of the samples were noted.
Preparation of Solvent Extract
Initial extraction was carried out using dried leaf, stem, and root powdered material of Impatience balsamina. Three-step sequential extractions were performed with Chloroform, Acetone, and Ethanol. A dry powder sample (25 gram) was transferred to a filter paper cone, sealed, and inserted into the Soxhlet thimble. Chloroform (200 milliliters), the nonpolar solvent, was taken in a round bottom flask, and a thimble containing the sample was inserted into it. The Soxhlet condenser was fixed to the thimble, and continuous cold-water flow was maintained. Soxhlet extraction was made for 6 hours to extract most of the nonpolar phytochemicals of Balsam leaves. Later the solvent was evaporated by rotary vacuum evaporator, and the insoluble sample in the thimble was dried in a hot air oven and re-extracted with polar solvent Acetone for 8 hours. Later the solvent was evaporated by a rotary vacuum evaporator. After the solvent evaporation, the Acetone extract of the sample was maintained in the refrigerator till use. Plant powder was dried to evaporate all the ethanol and used for methanolic extract preparation. A rotary vacuum evaporator dried the extract to evaporate the solvent. The obtained extract was preserved in the fridge till use. For antimicrobial studies, known quantities of dried extracts were solubilized in DMSO.15
Strains and Cultural Conditions
Standard strains Pseudomonas aeruginosa MTCC 2453 (PA01), Chromobacterium- violaceum MTCC 2656 were purchased from Microbial type culture collection (MTCC), Chandigarh. Cultures received as freeze-dried forms and rejuvenated under the purchase center’s policy.
Assay for Quorum Sensing Inhibition
The qualitative disc diffusion method was applied to estimate the anti-quorum sensing ability of Impatience balsamina extract.16 Selected 0.5 and 1mg of each Chloroform, Acetone, and Ethanol extract on the plant’s leaf, stem, and root since the Minimum Inhibitory Concentration (MIC) on Chromobacterium violaceum is 3.125mg/ ml. The extracts were loaded onto 6mm diameter sterile paper disks placed on the Luria Bertani Agar plates over layered with an overnight culture of C. violaceum MTCC 2656, and the plates were kept at 30°C overnight incubation. A culture plate was observed after overnight incubation to spot the colorless zone around the disc to examine the anti-quorum sensing activity of the extracts.
Determination of Minimum Inhibitory Concentration
All the plant extracts were tested according to CLSI guidelines (2015).17 Among all the extracts, only the Acetone leaf of Impatience balsamina showed potent anti-quorum sensing activity in the growth of Pseudomonas aeruginosa PA01. An overnight liquid culture of the Pseudomonas aeruginosa PA01 was grown in different concentrations of Acetone leaf extract ranging from 25mg/ ml to 0.012mg/ ml. The MIC and sub-MIC were fixed by recording the absorbance (OD600) following 24 hours of incubation at 37°C.
Assay on Pyocyanin Production
Pseudomonas aeruginosa PA01 was cultured for 24 hours in different concentrations of extract starting from sub- MIC (1.52mg/ml- 0.012mg/ml). Pseudomonas aeruginosa broth without extract was kept as the positive control. Culture supernatant was collected and then filtered using a 0.2µm filter, and 3ml of supernatant was mixed with 5ml chloroform. 1ml 0.2 M HCl was added to collect the organic phase, and absorbance was measured at OD 520nm to evaluate the pyocyanin concentration18 (Biorad, smart spec 3000).
Assay on Protease Activity
The Las-A protease activity was assayed using the azocasein method19 by monitoring TCA soluble-azo coupled peptides’ release rate at 440 nm. The reaction mixture contained 0.125 mL 0.3% azocasein (w/v) and 0.125 mL enzyme extract appropriately diluted in 25 mmol L-1 potassium phosphate buffer at pH 8.0. The addition of the enzyme aliquot started the reaction. After incubating at 37°C for 4 hours, the reaction was arrested by adding 0.75 mL of 5% TCA (w/v). The reaction mixture was centrifuged at 8000×g for 10 minutes to collect the supernatant containing TCA-soluble azo-coupled peptides. Neutralize the supernatant with the addition of 0.5M NaOH in a 1:1 ratio of the reaction mix. The absorbance of the supernatant at 440 nm (Biorad, smart spec 3000) was measured against the corresponding blank without an enzyme. One unit of protease activity was defined as the amount of enzyme required to produce an absorbance change of 0.1 in a 1cm cuvette under the assay conditions.
Assay on Chitinase Activity
A modified chitin azure assay measured chitinase activity. P. aeruginosa PAO1 was cultivated with and without the sub-MIC 1.563mg/ml of extract for 48 hours and centrifuged. The culture supernatant procured was filtered using a 0.2 μm filter, and 0.5 ml of the supernatant was mixed with 0.5 ml of sodium citrate buffer (0.1 M, pH 5) supplemented with chitin azure (0.75% chitin azure in sodium citrate buffer). Chitinase activity was determined at OD 570 after 16 hours of incubation at 37°C20. (Biorad, wise spec 3000).
Assay for Biofilm Inhibition
The concentrations used to check biofilm formation were from sub- MIC (1.52mg/ml- 0.012mg/ml). Pseudomonas aeruginosa PA01 was cultured in trypticase soy broth with varying concentrations below MIC value, and the broth of Pseudomonas aeruginosa PA01without extract was kept as the control. The biofilm was imagined after staining with 0.1% crystal violet, and it is solubilized in ethanol and quantified spectrophotometrically at 570nm to check the biofilm formation21(Biorad, smart spec 3000).
Phytochemical Investigation
Mobile phase- A: 0.1% Formic acid in water.
Mobile phase- B: Acetonitrile
Injection volume: 5µL
Gradient program:
Time Flow %A %B
Initial 0.400 90.0 10.0
5.00 0.400 90.0 10.0
12.00 0.400 5.0 95.0
16.00 0.400 5.0 95.0
18.00 0.400 90.0 10.0
20.00 0.400 90.0 10.0
UPLC Make: Waters, USA
Model: Acquity UPLC
MS Make: Waters, USA
Model: Xevo G2- XS QT of Column: Accucore C18, 50x 4.6, 2.6u
Capillary Voltage: 3.0 KV
Collision Energy: 20V
Ramp Collision Energy: 30.90V
Source Temp: 150°C
Desolvation Temp: 450°C
Cone Gas: 50L/ Hr
Desolvation Gas Flow: 800L/Hr
Statistical Analysis
Data are presented as the mean ± standard deviation from experiments analysis in triplicate. Statistical analyses were performed using Pearson’s product-moment correlation test to find the correlation between the concentration of extract and optical density of bacteria, biofilm, and pyocyanin production by bacteria.
Here the hypothesis is as follows.
H0: There is no significant correlation between the concentration of extract and the optical density of bacteria, biofilm, and pyocyanin production by bacteria.
H1: There is a significant correlation between the concentration of extract and the optical density of bacteria, biofilm, and pyocyanin production by bacteria.
The p-value is less than 0.05. Hence, we reject the null hypothesis and conclude that a very high negative correlation exists between the concentration of extract and the optical density of bacteria, biofilm, and pyocyanin production by bacteria.
Antiquorum sensing activity of the plant extract was assessed against Violacein production by Chromobacterium violaceum MTCC 2656 and Pyocyanin production, biofilm production, Las A protease activity, and Chitinase activity of Pseudomonas aeruginosa PA01.
Detection of Anti-Quorum Sensing and Violacein Inhibition Activity
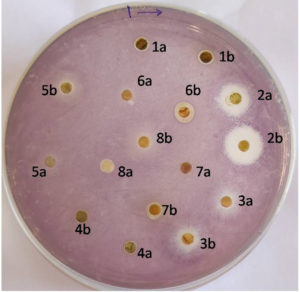
Antiquorum sensing activity of the extract was indicated by the colorless growth of Chromobacterium violaceum MTCC 2656 around the disc impregnated with 0.5, 1mg/ml acetone extract of the leaf (2a, 2b, respectively), which had an enormous effect on other solvent extracts of Impatience balsamina (Figure 1).
Figure 1. Antiquorum sensing activity of the Acetone extract of Impatience balsamina leaf (2a, 2b at 0.5 and 1mg/ml respectively)
Suppression of Violacein production of Chromobacterium violaceum MTCC 2656 gave an initial indication of the extract’s anti-quorum sensing property. It was similar to the report of the anti-quorum sensing property of 5- HMF, cinnamon, peppermint, and lavender oil.19 An equivalent report was observed with Tremella fuciformis extract that it arrested quorum-sensing regulated violacein pigment production in Chromobacterium violaceum CV026 without hindering its growth.22 A similar reflection of action was claimed by essential oils of Melaleuca alternifolia and Rosmarinus Officinalis L at lower concentrations in 0.25µl/ml and 0.125µl/ml respectively, although extracts of Resveratrol (20µg/ml) and Pomegranate granatum L (40µg/ml) also described the same conclusion at rather high concentrations.23
Determination of Minimum Inhibitory Concentration
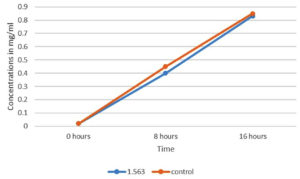
The MIC and sub- MIC of the extract were set at 3.125mg/ml and 1.563mg/ml, respectively. Sub-MIC did not affect the growth curve of Pseudomonas aeruginosa PA01 (Figure 2), so it was decided to select sub-MIC and the below concentrations for the assessment of the effect of the extract on biofilm and other virulence characteristics of Pseudomonas aeruginosa PA01.
Figure 2. Growth curve of Pseudomonas aeruginosa PA01 treated with sub- MIC of extract (1.563mg/ml) and the Pseudomonas aeruginosa PA01 suspension without extract as control added at 0h, 8h and 16 h
Effect on the Production of Virulence Characteristics
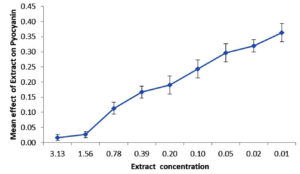
Extract manifested an impressive effect on quorum sensing regulated Las A protease and Chitinase activity by inhibiting 100% and 78.42% of Las A protease and Chitinase production by Pseudomonas aeruginosa PA01 on treatment with the sub-MIC concentration of extract, respectively. There was a remarkable reduction in the Pyocyanin production of Pseudomonas aeruginosa PA01 in the presence of the extract below the Minimum inhibitory concentration; it was reduced up to 93.33% during the presence of Acetone extract of the leaf (Figure 3).
Figure 3. Effect of the Acetone extract of Impatience balsamina leaf on Pyocyanin production by Pseudomonas aeruginosa PA01
A phenomenal inhibition of pyocyanin production of the extract is compatible with the action of 5- HMF by its inhibition of 65.34% on Pyocyanin and the hindrance action of 50µM of eugenol on Pyocyanin by 56%.19 A compatible intimation was found with the dichloromethane extract from root barks of Cordia gilletii in pyocyanin production.24 As observed in the present study, considerable repression of pyocyanin pigment production was documented with plant extracts of Aegle marmelos (51.85%), Azadirachta indica (25. 46%), Cynodon dactylon (19.35%), Eucalyptus globules (5.46%) and Ocimum tenuiflorum (5.25%). In agreement with the present data, reduction of total protease activity was remarked with Eucalyptus globule (2.5units/ml), Aegle marmelos (11.7 units/ml), Cynodon dactylon (20.1 units/ml), Azadirachta indica (49.5 units/ml) and Ocimum tenuiflorum (47.5units/ml).25
This study showed that Acetone extract of leaf of Impatience balsamina on Las A proteolytic activity and Chitinase activity and virtually corresponds to the action of 5- HMF in the manner that 77. 92%, 84.49%, and 57.71% on Las A staphylolytic activity, total protease, and chitinase activity, respectively.19
Effect on Biofilm Formation of Pseudomonas Aeruginosa PA01
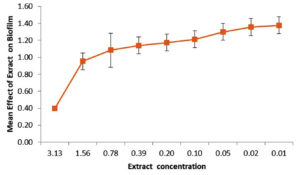
The extract exhibited a relevant inhibitory action on biofilm production of Pseudomonas- aeruginosa PA01 in the sub-MIC concentration of the extract. There were 30.75% of inhibition was recorded with the presence of Acetone extract from the leaf (Figure 4).
Figure 4. Effect of the Acetone extract of Impatience balsamina leaf on Biofilm production by Pseudomonas aeruginosa PA01
In chronic infection, Pseudomonas aeruginosa lives as biofilm, a large number of bacteria seen together in adjacent contiguity. They can survive in unfavorable conditions like host responses and antibacterial agents.26 It is possible to overcome this condition by disturbing the cell- to cell signaling mechanism, which is associated with the management of biofilm formation.27 To examine the anti-quorum sensing activity of the extract, anti-biofilm activity was carried out with the sub- MIC concentration of the extract, and it showed a significant reduction up to 30.75%, the almost related report was observed with 5- HMF, which reduced biofilm formation in Pseudomonas aeruginosa PA01 by 29.80%.19 Another study conducted with Eucalyptus globules, Aegle marmelos, Ocimum- tenuiflorum, Cynodon- dactylon, and Azadirachta indica, showed percentages of biofilm inhibition of 99.77, 99.05, 97.92, 97.47 and 95.26% respectively.25 Dichloromethane extract from root barks of Cordia gilletii also reduces the biofilm formation by PA01.24 A comparable finding was reported with Conocarpus erectus, Bucida baceras, and Callistemon viminalis extracts by significantly inhibiting las A protease and biofilm formation.28
Phytochemical Investigation of the Extract
Plant products such as secondary metabolites are associated with the defense mechanisms of plants and can be utilized as natural bioactive molecules instead of antibiotics to overcome antibiotic-resistant strains29. The most common and widely distributed phytochemicals are flavonoids in fruits, vegetables, and beverages. Flavonoids are responsible for pigmentation in flowers, and they have been classified into Apigenin, Galangin, Flavone and Flavonol glycosides, Isoflavones, Flavones, and Chalcones.30 These chemicals act as natural defense factors for plants by their toxic effect on microorganisms. Several in vitro studies reported that the Flavones, the subgroup of Flavonoids, have higher antibacterial, antioxidant and anti-inflammatory effects.31
In a qualitative phytochemical analysis of the crude extract, the color tests to screen for alkaloids, triterpenoids, flavones, flavanones, tannins, phenols, cardiac glycosides, resins, saponins, proteins/ peptides, and sugars were conducted (Table 1). The crude acetone extract represents 0.374±0.013% of the leaf.
Table (1):
Phytochemical Analysis of Acetone Extract of Impatiens balsamina Leaf.
| Test | Observation | Inference | ||
|---|---|---|---|---|
| Test for Alkaloids | ||||
| Dragendroff’s Test | No Precipitate is Observed | Alkaloids are Absent/ No Detectable Concentration | ||
| Wagner’s Test | No Precipitate is Observed | Alkaloids are Absent | ||
| Test for Sterols | ||||
| Salkowski Test | No Characteristic Observation | Sterols are Absent | ||
| Test for Flavonoids | ||||
| Ferric Chloride Test | Green Color is Observed | Flavonoids are Present | ||
| Aq. Naoh Test | Yellow Color is Observed | Flavones are Present | ||
| Conc. H2so4 Test | Crimson Red Color is Observed | Flavonones are Present | ||
| Test for Saponins | ||||
| Foam Test | No Foam Persisted | Saponins are Absent | ||
| Test for Cardiac Glycosides | ||||
| Kellar Killiani Test | No Brown Ring | Cardiac Glycosides are Absent | ||
| Naoh Reagent Test | No Yellow Color is Observed | Cardiac Glycosides are Absent | ||
| Test for Triterpenoids | ||||
| Salkowski Test | No Red-Brown Colour At Interface | Triterpenoids Absent or No Detectable Concentration | ||
| Test for Tannins | ||||
| Fecl3 Test | Green Precipitate is Observed | Tannins are Present | ||
| Test for Resins | ||||
| Hcl Test | No Pink Colour | Resins Absent | ||
| Test for Phenols | ||||
| Ellagic Acid Test | No Precipitate is Observed | Phenols are Absent | ||
| Test for Proteins | ||||
| Biuret Test | No Characteristic | Proteins are Absent | ||
| Test for Carbohydrates | ||||
| Benedict’s Test | Brown Precipitate with Green Solution | Carbohydrates are Present | ||
Liquid chromatography-mass spectrometry (LC-MS) of the sample gave eight prominent peaks. Each one’s molecular weight is compared with the existing mass database to know the compound (Table 2).
Table (2):
Constituents present in Acetone extract of Impatience balsamina leaf.
Name of the compound |
Class of compound |
Molecular formula |
Molecular weight |
Resembling standard compounds |
|---|---|---|---|---|
5,3’- Dihydroxy flavone |
Natural/Artificial |
C15H10O4 |
254.057909 |
– |
Rutin |
Natural product, Flavonoid. |
C27H30O16 |
610.15338 |
– |
Kaempferol- 3- Glucoside- 6”-p- coumaroyl |
Flavonoid |
C30H26O13 |
594.13734 |
– |
Luteolin- 4’- O- glucoside |
Flavonoid O- glycosides |
C21H20O11 |
448.38 |
– |
Isorhamnetin -3- Galactoside – 6”- Rhamnoside |
Flavonoid |
C28H32O16 |
624.16903 |
1. Isor- 3- Gal- 6”- Rha 2. Isorhamnetin- 3- Galactoside- 6- Rhamnoside |
Quercetin |
Natural product; flavonoid. |
C15H10O7 |
302.04265 |
– |
Kaempferol |
Natural product |
C15H10O6 |
286.04774 |
3,5,7- trihydroxy-2- (4-hydroxyphenyl) chromen- 4- one |
Vinconidine |
Strychnos alkaloids |
C19H20N2O2 |
308.381 |
– |
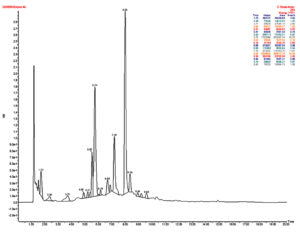
A total of 18 peaks were observed between 1.72min to 9.6min retention time. Ten peaks were negligible, with an area of ±1%. There are six significant peaks; the peak at the retention time of 8min occupies a major percent area (34.99%), and the peak at the retention time of 5.74min occupies a 24.72% area. Other peaks are 7.19min with 11.67% area, 5.53min peak with 6.87% area, 1.72min peak with 4.53% area, and 8.36min peak with 3.29%area. The molecular weight determined by HRMS was analyzed with the MassBank database for eight peaks with more than 1.5%area (Figure 5).
The present study describes the anti-quorum sensing activity of Acetone extract of leaf of Impatience balsamina. The study has been carried out against Pseudomonas aeruginosa PA01. Phytochemical analysis of Acetone leaf extract led to identifying compounds in the extract. LC-MS method was done to check the aggregate analysis of the compounds.
Pseudomonas aeruginosa is a Gram-negative bacillus connected with most infections acquired in hospitals. It is a common persistent colonizer of medical apparatus such as catheters, nebulizers, humidifiers, etc. It is associated with severe infections like healthcare-associated pneumonia, ventilator-associated pneumonia, chronic respiratory failure, cystic fibrosis complications, catheter-associated urinary tract infections, bacteremia, and burn patients. Approximately 7.1%- 7.3% of prevalence are shown by Pseudomonas aeruginosa among all the healthcare-associated infections.32
Resistance to most of the antibacterial agents is the most crucial complication in tackling infections caused by Pseudomonas aeruginosa, causing increased vulnerability to infections resulting in dreadful consequences and is currently identified as a significant hazard globally.33 A regular source of death in burn patients and a massive percentage of sepsis deaths in pediatric burn ICUs result from multidrug resistance in Pseudomonas aeruginosa. A large-scale death rate of bloodstream infections attributable to Pseudomonas aeruginosa is 43.2-58.8%, which is an elevated proportion compared to other terrifying bacterial infections.34
The pathogenicity of these organisms is based on their ability to produce a variety of toxins and proteases and their ability to resist phagocytosis. High mortality due to Pseudomonas aeruginosa is ascribed to its minimal sensitivity to most antibacterial agents. This resistance characteristic is owing to its intrinsic virulence properties.35
There is a professional need for alternative medicine to prevent the ceaseless emergence of multidrug resistance in bacteria. Natural products are considered the best to prevent antibiotic resistance as they can reduce the virulence of bacteria without killing them. Hence inflammation and tissue damage are minimal. The presence of active compounds responsible for the anti-quorum sensing activity of the extract may be helpful for the development of anti-infective drugs. Natural compounds such as Impatience balsamina can be used with antibiotics to control infections effectively. The present study is carried out with the crude extract of Impatience balsamina; it shows promising activities. So, researchers decided to take detailed studies with the pure molecule in the future.
ACKNOWLEDGMENTS
The authors would like to thank HOD, Department of Microbiology, Saveetha Medical College, Saveetha University, Chennai, Tamilnadu for providing laboratory facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
VR carried out the experiments. BG and SC supervised the experiments and guided in drafting the manuscript. VR drafted the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All data sets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Ningthoujam D, Soibam A. To study the anti-inflammatory properties of aqueous extract of leaves of Impatience balsamina in experimental Animal models. IJPSR. 2022;13(5):2039-2047.
- Song YX, Peng S, Mutie FM, et al. Evolution and Taxonomic Significance of seed Micromorphology in Impatience (Balsaminaceae). Front Plant Sci. 2022;13:835943.
Crossref - Singh P, Singh R, Sati N, Ahluwalia V, Sati O P. Phytochemical and Pharmacological Significance of Genus: Impatience. Int J Life Sci Sci Res. 2017;3(1):868-881.
Crossref - Chadha J, Harjai K, Chhibber S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: a chronicle through the perspective of quorum sensing. Environ Microbiol. 2022;24(6):2630-2656.
Crossref - Lister P D, Wolter D J, Hanson N D. Antibacterial Resistant Pseudomonas aeruginosa: Clinical impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin Microbiol Rev. 2009;22(4): 582-610.
Crossref - Pachori P, Gothalwal R, Gandhi P. Emergence of antibiotic resistance Pseudomonas- aeruginosa in intensive care unit, a critical review. Genes Dis. 2019;6(2):109-119.
Crossref - Pang Z, Raudonis R, Glick B R, Lin T J, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37:177-192.
Crossref - Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999-1007.
Crossref - Lau G W, Hassett D J, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10(12):599-606.
Crossref - Papenfort K, Bassler B. Quorum- Sensing Signal Response Systems in Gram- Negative Bacteria. Nat Rev Microbiol. 2016;14(9):576-588.
Crossref - Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6(1):56-60.
Crossref - Pearson JP, Gray KM, Passador L, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U. S. A. 1994;91(1):197-201.
Crossref - Kiratisin P, Tucker KD, Passador L. LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J Bacteriol. 2002;184(17):4912-4919.
Crossref - Ochsner UA, Koch AK, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol. 1994;176(7):2044-2054.
Crossref - Azwanida NN. A review on the Extraction Methods Use in Medicinal Plants, principle, strength and limitation. Medicinal and Aromatic Plants. 2015;4(3):1000196.
Crossref - Choi O, Kang DW, Cho SK, et al. Anti-quorum sensing and anti-biofilm formation activities of plant extracts from South Korea. Asian Pac J Trop Biomed. 2018;8(8):411-417.
Crossref - Rahman MRT, Lou Z, Yu F, Wang P, Wang H. Anti- quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J Biol Sci. 2017;24(2):324- 330.
Crossref - Aybey A, Demirkan. Inhibition of quorum sensing controlled virulence factors in Pseudomonas aeruginosa by human serum paraoxonase. J Med Microbiol. 2016;65(2):105-113.
Crossref - Rajkumari J, Borkotoky S, Reddy D, et al. Anti-quorum sensing and anti-biofilm activity of 5-hydroxymethylfurfural against Pseudomonas aeruginosa PA 01: Insights from in vitro, in vivo and in silico studies. Microbiol Res. 2016;226:19-26.
Crossref - Husain F M, Ahmad I, Asif M, Tahseen Q. Influence of Clove oil on certain quorum-sensing regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas- hydrophila. J Biosci. 2013;38(5):835-844.
Crossref - Zhou JW, Luo HZ, Jiang H, Jian TK, Chen ZQ, Jia AQ. Hordenine: A Novel Quorum Sensing Inhibitor and Antibiofilm Agent against Pseudomonas aeruginosa. J Agri Food Chem. 2018;66(7):1620-1628.
Crossref - Zhu H, Sun SJ. Inhibition of Bacterial Quorum sensing- Regulated Behaviors by Tremella fuciformis Extract. Curr Microbiol. 2008;57(5):418-422.
Crossref - Alvarez MV, Moreira MR, Ponce A. Antiquorum sensing and antimicrobial activity of natural agents with potential use in food. J Food Safety. 2012;32(3):379-387.
Crossref - Okusa PN, Rasamiravaka T, Vandeputte O, Stevigny C, Jaziri ME, Duez P. Extracts of Cordia gillettii de wild (Boraginaceae) quench the quorum sensing of Pseudomonas aeruginosa PA01. J Intercult Ethnopharmacol. 2014;3(4):138-143.
Crossref - Namasivayam SKR, Vivek JM. Antiquorum sensing activities of medicinal plant extracts against quorum sensing mediated virulence factors of Pseudomonas- aeruginosa. Der Pharmacia Lettre. 2016;8(8):412- 423.
- Zhou L, Zheng H, Tang Y, Yu W, Gong Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol Lett. 2013;35(4):631-637.
Crossref - Asif M, Acharya M. Quorum sensing: A nobel target for antibacterial agents. Avicenna J Med. 2012: 2(4): 97- 99.
Crossref - Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52(1):198-203.
Crossref - Khan MS, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to Clove oil. Lett Appl Microbiol. 2009;49(3):354-360.
Crossref - Noumi E, Snoussi M, Merghni A, et al. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L, methanolic extracts. Microbial Pathog. 2017;109:169-176.
Crossref - Mierziak J, Kostyn K, Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19(10):16240-16265.
Crossref - Yigit D, Yigit N, Mavi A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Pranus armeniaca L.) kernels. Braz J Med Biol Res. 2009;42(4):346-352.
Crossref - Reynolds D, Kollef M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An update. Drugs. 2021;81(18):2117-2131.
Crossref - Parkins MD, Somayaji R, Waters VJ. Epidemiology, Biology and Impact of Clonal Pseudomonas aeruginosa Infections in Cystic fibrosis. Clin Microbiol Rev. 2018;31(4):1-38.
Crossref - Ciofu O, Nielsen TT. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents- How Pseudomonas aeruginosa can Escape Antibiotics. Front Microbiol. 2019;10:913.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.