Zinc (Zn) is a vital element for the growth of plants. However, soils often suffer from its deficiency, which adversely affects crops. Zn supplementation using chemical fertilizers is ineffective and negatively affects the environment. Zn is converted from an insoluble state to a soluble state by ZSB which improves the absorption of Zn by plants and promotes overall plant health. Integrating these microbes into agricultural practices through seed inoculation, soil amendment, and foliar sprays offers a sustainable solution to Zn deficiency, promoting healthier crops and contributing to food security. Field trials provide empirical evidence of the extent to which Zinc Solubilizing Bacteria enhances both the quality and quantity of the crops. ZSB into agricultural practices can improve agricultural land productivity, also food security, and promote environmentally sustainable farming practices. This review examines the potential of zinc solubilizing bacteria as an effective alternative for enhancing plant growth and increasing the availability of Zn.
Brassica juncea L., Biostimulants, Zinc Solubilizing Bacteria, Sustainable Agriculture
Biostimulants are compounds or microorganisms that improve plant growth, development, and overall plant health. They improve plant physiological processes to increase nutrient uptake, stress tolerance, and yield. Among the most frequent bio-stimulants used in plant cultivation are: Organic compounds such as humic and fulvic acids promote nutrient availability, water retention capacity, and soil structure.1,2 They enhance root development, nutrient uptake, and plant vigor. Seaweed extracts are another important biostimulant that is derived from marine algae, and they contain various growth-promoting compounds, such as auxins, cytokinins, and microelements.3 They boost nutrient absorption, enhance stress tolerance, and encourage plant development.4 Microbial Inoculants: Beneficial microorganisms like mycorrhizal fungi and Rhizobacteria that promote plant development (PGPR) are known as bio-stimulants. They increase the availability of nutrients, encourage the growth of roots, guard against diseases, and boost the general health of plants.5 Amino acid-based bio-stimulants provide a readily available source of organic nitrogen and stimulate plant metabolic processes. They promote root growth, flowering, and fruit development. Various plant extracts, such as extracts from algae, herbs, or plant tissues, are used as bio-stimulants. They contain natural growth-promoting compounds that improve nutrient uptake, photosynthesis, and stress tolerance. Chitin and Chitosan which are mainly derived from crustacean shells, chitin and chitosan bio-stimulants enhance plant growth, induce defence responses, and improve Nutrient absorption and abiotic stress resistance.6 Silicon Bio-stimulants: Silicon bio-stimulants increase plant resistance to pests, diseases, and abiotic stressors.7 They improve cell strength, photosynthesis, and nutrition uptake. Enzymes: Enzyme-based Biostimulants improve nutrient availability and soil fertility. They boost nutrient cycling, break down organic materials, and encourage root growth. Seed treatments, foliar sprays, soil drenches, and fertigation systems can all be used to apply bio-stimulants. Specific application methods and dosages are determined on the crop, growth stage, and product instructions.8 Their application is often complementary to good agricultural practices, including proper nutrient management, irrigation, and pest control. However, Zn shortage is a ubiquitous problem that reduces crop output and nutritional quality, particularly in Zn-deficient soils. Traditional methods to address Zn deficiency, such as applying Zn fertilizers, have limitations, including environmental concerns and cost implications. These bacteria employ various mechanisms to solubilize Zn, including producing Siderophores chelating agents, and organic acids.9 For instance, organic acids such as gluconic acid and citric acid lower the pH of the soil microenvironment, thereby increasing Zn solubility. Conversely, Siderophores are high-affinity iron-chelating compounds that can also bind to Zn, facilitating plant mobilization and uptake.10 Applying ZSB as bioinoculants has shown promising results in enhancing Zn uptake and improving plant growth and yield.11 Studies have demonstrated that inoculating crops with ZSB can significantly increase Zn concentration in plant tissues, leading to better growth performance and higher nutritional quality of the produce.12 Furthermore, ZSB can boost plant growth by creating phytohormones including indole-3-acetic acid (IAA) and gibberellins, which stimulate root formation and general plant vigour.13
Vital macro and micro nutrients: the cornerstones of optimal plant growth
The number of micro and macro nutrients in soil varies greatly based on factors such as soil type, geographical location, land use, and management approaches (Table 1). It is hard to offer exact tabular statistics without taking these aspects into account.14,15 It’s crucial to remember that these ranges are general guidelines, and specific nutrient levels can vary depending on factors such as soil type, climate, fertilization practices, and cropping history. Soil testing is the most accurate way to determine the actual nutrient levels in a particular soil sample.16 Professional soil testing laboratories can provide detailed reports of nutrient concentrations in soil, helping to guide nutrient management and fertilization practices. The ranges below are estimates that may change based on particular soil conditions.
Table (1):
Micro and macro nutrients typical range in soil and impact of its deficiency on plants
No. |
Micro and Macro Nutrients |
Typical Range in Soil (mg/kg) |
Impact of Nutrients Deficiency on Plants |
Ref. |
|---|---|---|---|---|
1. |
Iron (Fe) |
20-1000 |
Interveinal chlorosis in young leaves, reduced chlorophyll production, poor photosynthesis |
[14,17] |
2. |
Manganese (Mn) |
10-1000 |
Yellowing between veins, poor photosynthesis, weak enzyme activation |
[18] |
3. |
Zinc (Zn) |
1-100 |
Shortened stem length, small leaves, reduced hormone production, poor growth |
[19] |
4. |
Copper (Cu) |
0.5-100 |
Wilting, dieback of shoots, poor enzyme activity, weak stem structure |
[19] |
5. |
Boron (B) |
0.2-10 |
Death of growing points, brittle leaves, poor cell division, reduced root growth |
[18] |
6. |
Molybdenum (Mo) |
0.01-10 |
Yellowing of older leaves, poor nitrogen fixation, reduced growth |
[19] |
7. |
Nickel (Ni) |
0.01-10 |
Reduced seed germination, leaf distortion, impaired enzyme activity, stunted growth |
[16] |
8. |
Nitrogen (N) |
0.1-10 |
Yellowing of older leaves, stunted growth, reduced yield |
[14,16,20] |
9. |
Phosphorus (P) |
0.01-2 |
Poor root development, delayed maturity, weak stems |
[16,14] |
10. |
Potassium (K) |
0.5-50 |
Yellow or brown leaf margins, poor fruit development |
[14,16,19] |
11. |
Calcium (Ca) |
1-50 |
Deformed new leaves, blossom end rot in fruits, weak cell walls |
[19] |
12. |
Magnesium (Mg) |
0.2-10 |
Yellowing between leaf veins, poor photosynthesis |
[21] |
13. |
Sulfur (S) |
0.1-10 |
Yellowing of young leaves, reduced growth |
[14,16] |
14. |
Nitrogen (N) |
20-1000 |
Interveinal chlorosis in young leaves, reduced chlorophyll production, poor photosynthesis |
[14,17] |
Brassica juncea L.: A resilient oilseed crop with multifaceted agricultural significance
India is home to the world’s fourth-largest oilseed economy. Rapeseed-mustard, one of the Second only to groundnuts, which account for 27.8% of India’s oilseed industry, seven edible oilseeds are grown there, accounting for 28.6% of the nation’s total oilseed production.22 Many types of rapeseed-mustard are cultivated all throughout India, and the agroclimatic conditions in the mustard-growing regions vary greatly. Under limited resource conditions, rapeseed-mustard agriculture becomes less profitable for farmers.23 As a result, there is a significant imbalance between mustard demand and supply in India. As a result, Site-specific control of nutrients based on soil-test recommendations should be implemented to increase the current production levels attained by farmers in their fields.24 Boosting and maintaining the yield of rapeseed and mustard and production will necessitate efficient natural resource management, spreading rapeseed-mustard agriculture to additional regions under different cropping methods, and using an integrated approach to plant-water, nutrition, and pest management.25
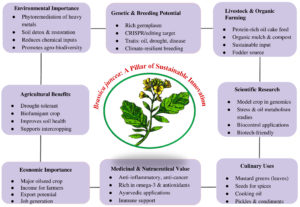
Brassica juncea L., commonly known as Indian mustard or mustard greens, is a crop that is sensitive to zinc deficiency. Several studies have investigated the use of ZSB as bio-stimulants to enhance zinc availability and improve the growth and productivity of Brassica juncea L. The application of zinc solubilizing bacteria can lead to several beneficial effects on plants. Firstly, these bacteria can enhance zinc uptake by increasing its solubility in the rhizosphere, which is the soil region influenced by plant roots. Improved zinc uptake can then promote various physiological processes in plants, including enzyme activities, hormone synthesis, and carbohydrate metabolism.26 Furthermore, zinc-solubilizing bacteria can help promote plant development by producing chemicals including indole-3-acetic acid (IAA), gibberellins, and cytokinins which can promote root development, improve nutrient absorption, and boost overall plant vigour. Figure 1 shows an overview of Brassica juncea L. and its importance in agriculture, environment, and medicine.. Overall, the use of zinc solubilizing bacteria as bio-stimulants for Brassica juncea L. holds promise for improving zinc availability and promoting plant growth. It’s crucial to remember, though, that the precise bacterial strain, soil type, and crop management techniques can all affect how successful ZSB is.
Reviving Micronutrient Dynamics: The plant growth-promoting potential of ZSB
Zinc solubilising bacterial bio-stimulants, beneficial bacteria, promote sustainable Brassica juncea plant growth by improving nutrient availability naturally, replacing chemical fertilizers that can harm the environment.27 Zinc solubilizing bacteria (ZSB) can aid Brassica juncea producers in reducing zinc shortage, increasing nutrient uptake, and promoting crop growth by solubilizing zinc in soil.28 Impact of zinc solubilizing bacteria on various plants (Table 2). Crop specificity is crucial for Brassica juncea, as it has high zinc demand for chlorophyll synthesis, enzyme activation, and hormone regulation. Enhancing zinc solubility and availability can address these requirements. Currently Scientists studying zinc-solubilizing bacteria, particularly Brassica juncea, to increase availability of zinc and stimulate plant growth, with extensive research on their isolation, identification, and characterization.29-31 To increase the current production levels in farmers’ fields, site-specific fertilizer management based on soil test recommendations should be implemented.24 The expansion of rapeseed-mustard cultivation to newer areas under various cropping systems, an integrated approach to plant-water, nutrient, and pest management, and effective natural resource management will all contribute to the growth and stabilization of rapeseed-mustard production and productivity. Biostimulants based on zinc-sulfur improve the zinc-deficient crop Brassica juncea L. By promoting physiological functions and raising zinc production and availability, these biostimulants boost root growth, nutrient absorption, and plant vigor, suppress weeds, control soil erosion, and benefit traditional medicine.32
Table (2):
Impact of zinc solubilizing bacteria on various plants
No. |
Zinc Solubilizing Bacteria (ZSB) |
Insoluble Zinc Compound |
Impact on different plant |
Ref. |
|---|---|---|---|---|
1. |
Azospirillum spp. |
Zinc oxide (ZnO) |
Enhanced growth and zinc uptake in wheat, maize, and rice. |
[29,33] |
2. |
Pseudomonas spp. |
Zinc phosphate Zn3 (PO4)2 |
Improved zinc uptake and growth in soybean and tomato. |
[33] |
3. |
Bacillus spp. |
Zinc carbonate (ZnCO3) |
Increased zinc availability and plant growth in various crops. |
[34-36] |
4. |
Enterobacter spp. |
Zinc silicate (ZnSiO3) |
Enhanced zinc acquisition and growth in sunflower and mustard. |
[37,38] |
5. |
Rhizobium spp. |
Zinc sulfate (ZnSO4) |
Improved zinc uptake and growth in legumes |
[39,40] |
6. |
Bacillus paramycoides |
Zinc oxide (ZnO) |
Enhanced growth and zinc uptake in rice. |
[41] |
7. |
Microbacterium oxydans |
Zinc phosphate Zn3 (PO4)2 |
Enhanced growth and zinc uptake in wheat, vegetable. |
[20] |
8. |
Enterococcus hirae |
Zinc oxide (ZnO) |
Enhancing the quality of wheat grains. |
[42] |
9. |
Stenotrophomonas maltophilia |
ZnO and ZnCO3 |
Increased zinc availability and plant growth in chickpea. |
[43] |
10. |
Burkholderia and Acinetobacter |
Zinc sulfate (ZnSO4) |
Improved growth and zinc uptake in grains, etc. |
[44] |
Factors influencing the interaction between zinc-solubilizing bacteria and different crops for enhanced plant growth promotion
Environmental factors, such as soil properties, and local conditions, can impact ZSB-Brassica juncea interactions. Research and field trials in relevant agricultural environments should be conducted to determine optimal conditions for plant development.45 The sensitivity of Brassica juncea to ZSB varies across geographical regions, making it essential to refer to specific research papers and field experiments for comprehensive information on its effects on this crop.10,46 The response of Brassica juncea to ZSB may vary based on geographical conditions, so it’s recommended to refer to specific research studies and field trials for context-specific information on the effects of ZSB on various crop, like wheat, Rice, Maize, Soyabean, Tomato, Potato, and Spinach.47-51 Bacillus sp. (SS9) and Enterobacter sp. (SS7) inoculation reduced Zn toxicity, promoting plant development and mobilizing Zn, N, and P to plant parts.36 Quantitative real-time reverse transcription PCR was used to examine the function of the zinc-solubilizing bacterial strain Enterobacter cloacae strain ZSB14 in the regulation of iron (Fe)-regulated transporter-like protein (ZIP) genes and Zn-regulated transporters in rice under iron-deficient and iron-sufficient conditions. Zinc oxide in the growth medium boosted the expression of all ZIP genes in rice seedling roots and shoots. ZSB was inoculated into rice seedlings cultured in growth medium containing insoluble zinc oxide.52 Inoculation of B. juncea plants with these strains increased plant growth and Pb uptake in metal-contaminated soil. A greenhouse experiment with Brassica juncea analysed bacterial inoculation’s impact on heavy metal uptake from Pb-Zn mining tailings, revealing beneficial bacteria that boost plant growth and protect against metal toxicity.53,54 Rhizobacteria infection reduced metal concentrations in plant tissues but increased above-ground biomass and soil metal bioavailability, enhancing phytoextraction efficiency compared to control treatments. A study found ZSB in wild legume root nodules, with Bacillus sp. and Enterobacter sp. isolates SS9 and SS7 effective in tolerating 1 g Zn. Inoculation plants showed greater mung bean plant growth and biomass, with reduced Zn toxicity resulting in better plant development and mobilization.36,55 Because of the abundance of nutrients available in the form of root exudates, the rhizosphere is a dynamic environment where microbe-microbe and microbe-plant interaction is at its greatest.56,57 There are many other species of Zinc Solubilizing Bacteria that may have an impact on different plant species. Some Environmental Factors given below:
- Soil pH: Acidic soil conditions, with a pH range of 5.0-6.5, are generally favourable for zinc solubilization by ZSB, promoting plant uptake.58
- Soil organic matter content: Soil organic matter enhances ZSB growth and activity by providing carbon and contributing to the production of organic acids, promoting zinc solubilization.59
- Moisture and water availability: Soil moisture is crucial for zinc solubilization and activity of ZSB, while drought stress can negatively affect populations; hence, proper irrigation management is essential.
- Temperature: Temperature significantly influences ZSB growth and activity, with mesophilic temperatures (25-30 °C) generally promoting ZSB activity and plant growth for each bacterial strain.
- Nutrient availability: Essential nutrients like phosphorus, nitrogen, and potassium are crucial for ZSB activity and Brassica juncea’s nutrient status, while temperature significantly influences ZSB growth and activity.
- Heavy metal contamination: Heavy metals in soil can affect ZSB populations and activity, with some strains showing tolerance, potentially aiding in phytoremediation of contaminated soils.
- Pesticide and chemical application: Chemical inputs like pesticides can negatively impact ZSB populations, necessitating careful consideration to maintain beneficial interactions between ZSB and Brassica juncea.60
Rhizospheric Revolution: Mechanisms of zinc-solubilizing bacteria driving plant vitality
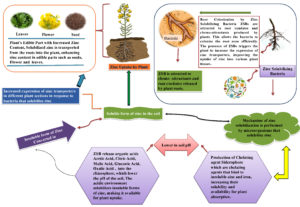
Zinc solubilizing bacteria (ZSB) enhance plant development and zinc uptake on Brassica juncea by converting insoluble zinc compounds into soluble forms and increasing soil nutrient availability.61 ZSB’s organic acids enhance nutrient mobilization, growth, and communication with Brassica juncea roots, overcoming zinc shortage and promoting symbiotic interaction, thereby boosting plant growth.47 Brassica juncea’s zinc uptake enhances growth, development, and yield, while its unique Defence Mechanism Induction mechanism induces systemic resistance against infections and creates antimicrobial compounds in rice (Figure 2). The activation of a plant’s immune system enhances its defence against diseases and stress conditions, thereby promoting overall plant health Zinc solubilizing bacteria (ZSB) can be used as bio-stimulants to promote plant growth in Brassica juncea, depending on factors like soil conditions and bacterial strains.62 Combination with Fertilisers or Bio-stimulants ZSB can be enhanced by blending it with other bio-stimulants, such as micronutrient-enriched fertilisers, to boost plant growth, depending on the crop, stage, soil conditions, and local practices.63,64 To optimize ZSB’s use as bio-stimulants in agriculture, it’s crucial to assess its compatibility with other inputs, bacterial culture formulation, and treatment rates through field trials and consultations.65 Zinc is an essential micronutrient for the growth and development of Brassica juncea (Indian mustard). While zinc is crucial for plant health, excessive or deficient levels of zinc can have adverse effects on Brassica juncea.66 Here are some potential side effects of zinc on Brassica juncea.67-70 Zinc poisoning in Brassica juncea plants can cause chlorosis, slowed growth, diminished root development, necrosis, and impair nutrient uptake, leading to total plant stress and yield loss.67 Zinc deficiency in Brassica juncea can lead to nutritional imbalances and deficient symptoms due to the interference of excess zinc with the absorption of other metals. Zinc deficiency in Brassica juncea can hinder photosynthesis, seed germination, and physiological processes, leading to reduced growth and compromised plant well-being. This can be due to soil conditions, zinc availability, and plant genotype. Zinc levels can also disrupt hormone regulation, enzyme activity, and cellular functions, affecting overall plant health.71
ZSB interact with other bio-stimulants and fertilizers in soil
Zinc Solubilizing Bacteria (ZSB) interact with other bio-stimulants and fertilizers in various ways, enhancing Increased plant growth and soil health (Table 3). ZSB primarily increase the bioavailability of zinc by solubilizing it from insoluble compounds in the soil, which enhances plant zinc uptake. This process can synergise with other bio-stimulants and fertilizers, promoting overall nutrient availability and plant health.
Table (3):
List of Various Zinc-Solubilizing Bacterial Strains and Their Efficacy on Plant Growth
No. |
Isolated Microbial Strains |
Area and Sites of Isolation |
Plants |
Impact |
Ref. |
|---|---|---|---|---|---|
1. |
Pseudomonas aeruginosa |
Soil, water |
Wheat, Maize, Rice Potato, Apple, Grapes, Banana |
Enhanced zinc solubilization aiding overall growth, flowering, and fruit development. |
[76,77] |
2. |
Pseudomonas fra |
Environmental sources, spoiled foods |
Wheat, Maize, Potato, Apple, Grapes |
Improved zinc availability benefiting plant development. |
[47] |
3. |
Pantoea dispersa |
Soil, plants, water, clinical samples |
Wheat, Maize, Potato, Apple, Grapes, Banana |
Potential positive effect on zinc uptake by plants. |
[71] |
4. |
Pantoea agglomerans |
Plants, soil, water, insects, clinical samples |
Wheat, Maize, Potato, Apple, Grapes |
Potential enhancement of zinc absorption by plants. formation of enzymes and proteins crucial for fruit maturation |
[47,78] |
5. |
E. cloacae |
Soil, water, clinical samples, plants |
Wheat, Maize, Potato, Apple, Grapes, Rice, Tomato |
Influence on zinc availability for better plant growth. |
[47] |
6. |
Rhizobium sp. |
Root nodules of legume plants, soil |
Wheat, Maize, Potato, Apple, Rice, Grapes, Chickpea |
Possible improvement in zinc utilization by wheat, maize. Improve legume crop yield and protein content |
[79,80] |
7. |
Pseudomonas striata, |
Water, soil, plants |
Wheat, Maize, Seed yield and shoot dry mass |
Unknown impact, further research is needed. |
[81] |
8. |
Gluconacetobacterdiazotrophicus |
Sugarcane plants, other crops |
Wheat, Maize, Potato, Apple, Grapes, Tomato, |
Potential enhancement of zinc uptake by these plants. |
[82] |
9. |
Enterobacter cloacae |
Soil, water, clinical samples, plants |
Maize, Potato, Apple, Grapes |
Impact on zinc availability for different plant species. |
[52] |
10. |
Bacillus mycoide |
Soil, water, plants, clinical samples |
Wheat, Maize, Potato, Apple, Grapes |
Improved zinc solubilization for various plants. |
[49] |
11. |
P. megaterium |
Soil, water, plants, clinical samples |
Wheat, Maize, Potato, Grapes |
Enhanced zinc absorption aiding plant growth. |
[83,84] |
12. |
P. aryabhattai |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Potential positive effect on zinc uptake by plants. Increased grain yield and quality. |
[84] |
13. |
Bacillus megaterium |
Soil, water, plants, clinical samples |
Rice, Wheat, Maize, Potato, Apple, Grapes |
Improved zinc availability for different plant species. |
[83] |
14. |
B. thuringiensis |
Soil, plants, insects |
Wheat, Maize, Potato, Apple, Grapes |
Possible enhancement of zinc absorption by these plants. |
[83] |
15. |
B. tequilensis |
Soil, plants |
Maize, Potato, Apple, Grapes, Banana |
Potential improvement in zinc availability for plants. |
[83] |
16. |
B. clausii and B. pumilus |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes, Banana |
Potential positive effect on zinc uptake by plants. |
[85] |
17. |
B. licheniformis |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Influence on zinc uptake by different plant species. |
[86] |
18. |
Enterobacter cloacae |
Soil, water, plants, clinical samples |
Wheat, Maize, Potato, Apple, Grapes, Banana |
Impact on zinc availability for different plant species. |
[52] |
19. |
Enterobacter kobei |
Soil, water, plants, clinical samples |
Wheat, Maize, Potato, Apple, Grapes |
Potential enhancement of zinc absorption by plants. |
[18] |
20. |
E. hormaechei |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Potential positive effect on zinc uptake by plants. |
[19] |
21. |
E. ludwigii |
Soil, water, plants |
Rice, Walnut |
Unknown impact, further research needed. |
[19] |
22. |
E. radicincitans |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Possible improvement in zinc utilization by wheat, maize. |
[87] |
23. |
E. gergoviae |
Soil, water, plants |
Wheat, Maize, Tomato, Banana |
Enhanced zinc solubilization aiding plant growth. |
[87] |
24. |
E. soli |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Improved zinc availability benefiting plant development. |
[20] |
25. |
E. taylorae, |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Potential positive effect on zinc uptake by plants. |
[20] |
26. |
E. turicensis |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes, tomato |
Potential enhancement of zinc absorption by plants. |
[17] |
27. |
E. arachidis |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Influence on zinc availability for better plant growth. |
[17,20] |
28. |
E. asburiae |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Possible improvement in zinc utilization by wheat, maize. |
[88] |
29. |
E. hormaechei |
Soil, water, plants |
Wheat, Maize, Potato, Apple, Grapes |
Impact on zinc availability for different plant species. |
[88] |
Interaction with microbial inoculants
ZSB can improve root and shoot growth, increase nutrient availability, and defend plants from diseases by interacting with other beneficial microbes such as Plant Development-Promoting Rhizobacteria (PGPR) or mycorrhizal biofertilizers.28,72
Combination with organic fertilizers
When combined with organic fertilizers, ZSB can Improve plant nutrient absorption by boosting the solubilization of various nutrients such as phosphorous, through microbial activity.73
Effect of chemical fertilizers
Applying chemical fertilizers can sometimes affect ZSB activity. In some cases, high levels of synthetic inputs may suppress microbial populations, including ZSB. Therefore, balanced use of fertilizers is essential to maintain the beneficial effects of ZSB.74
Synergy with humic and fulvic acids
Organic compounds like humic acid and fulvic acids, which are commonly used as bio-stimulants, can enhance ZSB activity by improving soil structure and providing organic matter, which serves as a source of carbon for microbial growth.
Integration in sustainable farming
Integrating ZSB with micronutrient-enriched fertilizers or other Biostimulants can improve overall soil fertility and plant resilience, promoting sustainable agricultural practices and reducing the need for synthetic fertilizers also maintain the pH of the soil.75
Future prospects and contributes to the existing knowledge on zinc-solubilizing bacteria in agriculture
ZSB Interactions with multiple Crops
The manuscript highlights the variable response of Wheat, Rice, Maize and Brassica juncea to ZSB based on geographical and environmental factors, suggesting the need for site-specific research to optimize ZSB use under different agro-climatic conditions. Future research could focus on field trials across different regions to fine-tune ZSB applications tailored to specific crops and environmental conditions.
Synergistic Use with other bio-stimulants
The potential of combining ZSB with other bio-stimulants (e.g., micronutrient-enriched fertilizers) is mentioned, but this combination needs further research. To assess its effectiveness across various crops, comprehensive field studies are necessary. Investigating the compatibility and efficacy of ZSB with other agricultural inputs can provide a deeper understanding of how these bio-stimulants interact and their optimal use, thereby paving the way for practical application in agriculture.
Environmental and soil health impacts
Since the manuscript discusses ZSB’s positive impact on environmental sustainability by reducing the reliance on chemical fertilizers, future research could delve deeper into its long-term effects on soil health, especially in contaminated or zinc-deficient soils. Exploring its role in phytoremediation and broader environmental benefits could provide new insight.
Scaling up ZSB use in agriculture
The manuscript mentions the role of ZSB in sustainable agriculture, but future research should address the challenges in scaling up its use on a commercial level. Investigations into the formulation, application methods, and cost-effectiveness of ZSB in large-scale farming systems could provide practical solutions for broader adoption.
The study contributes to the knowledge of zinc-solubilizing bacteria (ZSB) in agriculture by offering empirical evidence and in-depth analysis of how ZSBs can enhance zinc availability in crops like Brassica juncea and other crops. It adds to the current understanding of the role ZSBs play in promoting sustainable agriculture through several key aspects:
Reduction of chemical fertilizer dependency
The study emphasizes the potential of ZSBs as a replacement to chemical fertilizers that are frequently damaging to the environment. By converting insoluble zinc compounds into soluble forms, ZSBs make zinc more accessible to plants, improving plant growth while reducing environmental pollution.
Improvement in crop growth and yield
ZSBs promote plants’ zinc absorption, enhancing crop growth, particularly in zinc-deficient soils. This contributes to better yields and overall plant health, especially in crops with high zinc demands, such as Brassica juncea, wheat, maize, rice, etc.
Sustainable agricultural practices
The research underscores how applying ZSBs aligns with and reinforces sustainable farming methods. By promoting nutrient uptake, enhancing soil health and cultivating eco-friendly farming techniques, ZSBs contribute significantly to sustainable agriculture.
Field trials and crop specificity
The manuscript provides data from field trials that demonstrate the effectiveness of ZSBs in specific crops. It also outlines the crop Brassica juncea, wheat, maize, etc., explaining how ZSBs address zinc shortages, which is crucial for chlorophyll synthesis and enzyme activation in this crop.
Environmental and economic benefits
By efficiently facilitating the use of natural resources, ZSBs reduce reliance on synthetic inputs, lowering farmers’ costs and mitigating environmental contamination this contributes to a better balance between agricultural productivity and sustainability.
ZSB innovation: a sustainable alternative to conventional zinc bio-stimulants
This study presents a scientifically innovative and focused technique for boosting the growth, development, and productivity of important food crops such as Brassica juncea, wheat, maize, and rice. Unlike traditional bio-stimulants, such as seaweed extracts, humic substances, and general microbial inoculants, which primarily provide broad-spectrum support for nutrient uptake or stress mitigation, this study focuses on resolving zinc-specific nutrient deficiencies, which are common in zinc-depleted agricultural soils commercially available zinc-solubilizing bacterial formulations (Table 4).
Table (4):
Summarizing commercially available zinc-solubilizing bacteria formulations
No. |
Formulation |
Bacterial Strain |
Commercial Name |
Ref. |
|---|---|---|---|---|
1. |
Liquid |
Bacillus sp. |
BioZinc |
[28] |
2. |
Powder |
Pseudomonas sp. |
ZincSol |
[89] |
3. |
Granules |
Gluconacetobacter sp. |
ZnGrow |
[90] |
4. |
Biofertilizer |
Acinetobacter sp. |
ZnBoost |
[91] |
5. |
Liquid |
Burkholderia sp. |
ZnMax |
[92] |
6. |
Powder |
Enterobacter sp. |
ZnPower |
[93] |
7. |
Granules |
Microbacterium sp. |
ZnMicro |
[94] |
8. |
Biofertilizer |
Rhizobium sp. |
ZnRhizo |
[95] |
9. |
Liquid |
Serratia sp. |
ZnSerr |
[96] |
10. |
Powder |
Thiobacillus sp. |
ZnThio |
[97] |
11. |
Granules |
Agrobacterium sp. |
ZnAgro |
[98] |
12. |
Biofertilizer |
Azospirillum sp. |
ZnAzo |
[99] |
13. |
Liquid |
Klebsiella sp. |
ZnKleb |
[100] |
14. |
Powder |
Ralstonia sp. |
ZnRal |
[101] |
15. |
Granules |
Ericoid mycorrhizal fungi |
ZnFungi |
[102] |
16. |
Biofertilizer |
Bacillus subtilis |
ZnSub |
[103] |
17. |
Liquid |
Pseudomonas fluorescens |
ZnFluo |
[91,104] |
18. |
Powder |
Gluconacetobacterdiazotrophicus |
ZnDiazo |
[105] |
19. |
Granules |
Acinetobacter calcoaceticus |
ZnCalc |
[106] |
20. |
Biofertilizer |
Burkholderia cepacia |
ZnCep |
[39,107] |
The main distinctness of this study is its capacity to use naturally existing ZSB strains to successfully convert insoluble zinc into plant-available forms via biological solubilization. This approach not only solves zinc’s poor bioavailability but also provides an ecologically benign alternative to synthetic zinc fertilizers, which are often ineffectual over time, dangerous to the environment, and unsustainable for long-term agricultural application. Aside from zinc mobilization, the ZSB strains tested in this study display other plant growth-promoting characteristics, such as phytohormone (e.g., auxin) synthesis, phosphate solubilization, and improved root system development. These multifunctional features work together to meet the physiological demands of nutrient-demanding crops like Brassica juncea by increasing nutrient absorption efficiency, plant vigor, and tolerance to abiotic stress.
Most importantly, this study presents empirical, field-based data supporting the use of ZSB as a feasible technique for improving crop production, quality, and soil health. By providing a targeted, sustainable, and crop-specific solution, this study establishes ZSB-based bio stimulants as a next-generation tool in climate-resilient and ecologically aware agriculture.
Furthermore, with the inescapable impacts of abiotic stress caused by soil contamination and climate change, Biostimulants may provide a strategy to mitigate their impact on the farming sector. However, a number of things must be considered that effects can fluctuate between agricultural species, productivity and extraction techniques for Biostimulants and constituent quantities, bioactive and effects might vary, and separate Biostimulants can operate differently in the same species. When there is a zinc deficiency, IAA degrades quickly, carbon dioxide fixation becomes less effective, and tiny organic compounds like amino acids, potassium ions and carbohydrates leak out Zinc solubilizing bacteria (ZSB) are significant in today’s environment for various reasons, including their ability to provide a sustainable strategy to enhance soil fertility and plant nutrition. ZSB reduces the need for fertilizers made with chemicals, which may have significant environmental consequences, by increasing zinc availability. ZSB use can help to promote sustainable agriculture practices by lowering dependency on synthetic fertilizers and reducing environmental pollution by solubilizing zinc compounds, ZSB can help with environmental remediation by helping in the detoxification and removal of excess zinc from contaminated soils. This can aid in the restoration of ecosystem health and functionality. ZSB has potential applications outside of agriculture. We can increase agricultural output, reduce environmental impacts, and address zinc deficiency and contamination issues by leveraging ZSB’s strengths. In today’s world, they provide a natural and sustainable alternative to increase zinc availability, boost food security, and encourage more ecologically friendly agricultural practices.
ACKNOWLEDGMENTS
The authors are grateful to the Sanjeev Agrawal Global Educational University, Bhopal, for providing facilities for this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
UV and GG conceptualized the study. GG performed supervision. UV, AD, DK and GG performed visualization. UV, ST, PN, and GG wrote the original draft. UV, ST and GG wrote and reviewed the manuscript. AD and DK edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Zhang X, Schmidt RE. Hormone Containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000;40(5):1344-1349.
Crossref - Zikankuba VL, Mteremko D, James A. Staple Crops Biofortification Linking Agriculture, Food and Nutrition towards Eliminating Hidden Hunger. Eur J Nutr Food Saf. 2019;9(2):112-121.
Crossref - Rose MT, Patti AF, Little KR, Brown AL, Jackson WR, Cavagnaro TR. A Meta-Analysis and review of Plant-Growth response to humic substances. Advances in Agronomy. 2014:37-89.
Crossref - Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V. Plant Growth Promoting Rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J Microb Biochem Technol. 2015;7(2):1000188.
Crossref - Bora SS, Gogoi R, Sharma MR, Naorem RS, Das J. Plant microbiome engineering: a promising approach to safeguard plant health. In: Microbiome Engineering. CRC Press; 2025:156-164.
- Craigie JS. Seaweed extract stimuli in plant science and agriculture. J Appl Phycol. 2010;23(3):371-393.
Crossref - Tewari S, Arora NK, Miransari M. Plant growth promoting rhizobacteria to alleviate soybean growth under abiotic and biotic stresses. Elsevier eBooks. 2016:131-155.
Crossref - Halpern M, Bar-Tal A, Ofek M, Minz D, Muller T, Yermiyahu U. The use of biostimulants for enhancing nutrient uptake. Advances in Agronomy. 2014:141-174.
Crossref - Upadhayay VK, Singh AV, Khan A, Singh J, Pareek N, Raghav A. FE-SEM/EDX Based Zinc Mobilization Analysis of Burkholderia cepacia and Pantoea rodasii and Their Functional Annotation in Crop Productivity, Soil Quality, and Zinc Biofortification of Paddy. Front Microbiol. 2022;13:852192.
Crossref - Bhatt K, Maheshwari DK. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech. 2020;10(2):36.
Crossref - Tripathi S, Yadav S, Sharma P, Purchase D, Syed A, Chandra R. Plant growth promoting strain Bacillus cereus (RCS-4 MZ520573.1) enhances phytoremediation potential of Cynodon dactylon L. in distillery sludge. Environ Res. 2022;208:112709.
Crossref - G Jeyanthi V, Kanimozhi S. Plant Growth Promoting Rhizobacteria (PGPR) – Prospective and Mechanisms: A Review. J Pure Appl Microbiol. 2018;12(2):733-749.
Crossref - Upadhayay VK, Singh AV, Khan A. Cross Talk between Zinc-Solubilizing Bacteria and Plants: A short tale of Bacterial-Assisted Zinc biofortification. Front Soil Sci. 2022;1:788170.
Crossref - Oenema O, Witzke HP, Klimont Z, Lesschen JP, Velthof GL. Integrated assessment of promising measures to decrease nitrogen losses from agriculture in EU-27. Agric Ecosyst Environ. 2009;133(3-4):280-288.
Crossref - Atafar Z, Mesdaghinia A, Nouri J, et al. Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess. 2008;160(1-4):83-89.
Crossref - Pramod K, Ashok K, Dhyani BP, et al. Soil fertility status in some soils of Muzaffarnagar District of Uttar Pradesh, India, along with Ganga canal command area. Afr J Agric Res. 2013;8(14):1209-1217.
Crossref - Parashar M, Dhar SK, Kaur J, et al. Two Novel Plant-Growth-Promoting Lelliottia amnigena Isolates from Euphorbia prostrata Aiton Enhance the Overall Productivity of Wheat and Tomato. Plants. 2023;12(17):3081.
Crossref - Kumar N, Dubey RC. Plant Growth Promoting Endophytic Bacteria Bacillus australimaris BLR41 and Enterobacter kobei BLR45 Enhance the Growth of Medicinal Plant Barleria lupulina Lindl. J Pure Appl Microbiol. 2022;16(4):2647-2658.
Crossref - Erdem B, Sevim E, Sevim A. Molecular Characterization of Gram Negative Isolated from Walnut (Juglans regia L.) Rhizosphere. BSJ Eng Sci. 2022;5(2):54-61.
Crossref - Jolideh T, Zaefarian F, Bakhshandeh E, Sadegh M. Effect of plant growth promoting bacteria on yield of rice under reduced chemical fertilizers. J Agric Sci Sustain Prod. 2025;35(1):41-55.
Crossref - Montemurro F, Fiore A, Campanelli G, Tittarelli F, Ledda L, Canali S. Organic fertilization, green manure, and vetch mulch to improve organic zucchini yield and quality. HortScience. 2013;48(8):1027-1033.
Crossref - Shekhawat K, Rathore SS, Premi OP, Kandpal BK, Chauhan JS. Advances in Agronomic Management of Indian Mustard (Brassica juncea(L.) Czernj. Cosson): An Overview. International Journal of Agronomy. 2012;2012:1-14.
Crossref - Rai PK, Yadav P, Kumar A, Sharma A, Kumar V, Rai P. Brassica juncea: A Crop for Food and Health. In: Kole, C., Mohapatra, T. (eds) The Brassica juncea Genome. Compendium of Plant Genomes. Springer, Cham. 2022:1-13.
Crossref - Banga SS, Banga SK, Labana KS. Heterosis in Indian mustard (Brassica juncea (L.) Coss.). 1984.
Crossref - Nandni N, Rani S, Dhiman I, Wati L. Biopriming with multifarious sulphur-oxidizing bacteria improve in vitro Vigna radiata L. (mung bean) and Brassica juncea L. (mustard) seed germination. Folia Microbiol. 2024.
Crossref - Jalal-Ud-Din S, Elahi NN, Mubeen F. Significance of zinc-solubilizing plant growth-promoting rhizobacterial strains in nutrient acquisition, enhancement of growth, yield, and oil content of canola (Brassica napus L.). Front Microbiol. 2024;15:1446064.
Crossref - Upadhayay VK, Singh AV, Pareek N. An insight in decoding the multifarious and splendid role of microorganisms in crop biofortification. Int J Curr Microbiol Appl Sci. 2018;7(06):2407-2418.
Crossref - Upadhayay VK, Singh AV, Khan A, Sharma A. Contemplating the role of zinc-solubilizing bacteria in crop biofortification: An approach for sustainable bioeconomy. Front Agron. 2022;4:903321.
Crossref - Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS. Role of soil microorganisms in improving P nutrition of plants. Plant Soil. 2002;245(1):83-93.
Crossref - Bahadur I, Maurya BR, Kumar A, Meena VS, Raghuwanshi R. Towards the Soil Sustainability and Potassium-Solubilizing Microorganisms. In: Meena, V., Maurya, B., Verma, J., Meena, R. (eds) Potassium Solubilizing Microorganisms for Sustainable Agriculture. Springer, New Delhi. 2016:255-266.
Crossref - Rathore SS, Shekhawat K, Kandpal BK, Premi OP. Improvement of physiological and productivity traits of Indian mustard (Brassica juncea) through micro-irrigation and fertigation under hot semi-arid eco-region. Indian J Agric Sci. 2017;87(9).
Crossref - Asghar H, Zahir Z, Arshad M, Khaliq A. Relationship between In vitro production of auxins by rhizobacteria and their growth-promoting activities in Brassica juncea L. Biol Fertil Soils. 2002;35(4):231-237.
Crossref - Dayo MH, Shah PAN, Kaleri AA, et al. Integrated effect of seed priming and inoculation on growth and yield of maize (Zea mays L.). Dialogue Soc Sci Rev (DSSR). 2025;3(1):1149-1158
- Nadeem SM, Zahir ZA, Naveed M, Arshad M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can J Microbiol. 2007;53(10):1141-1149.
Crossref - Vessey JK, Chemining’wa GN. The genetic diversity of Rhizobium leguminosarum bv. viciae in cultivated soils of the eastern Canadian prairie. Soil Biol Biochem. 2005;38(1):153-163.
Crossref - Shreya D, Amaresan N, Supriya NR. Zinc solubilizing Bacillus sp (SS9) and Enterobacter sp (SS7) promote mung bean (Vigna radiata L.) growth, nutrient uptake and physiological profiles. Lett Appl Microbiol. 2022;76(2):ovac063.
Crossref - Shaharoona B, Arshad M, Zahir Z, Khalid A. Performance of Pseudomonas spp. containing ACC-deaminase for improving growth and yield of maize (Zea mays L.) in the presence of nitrogenous fertilizer. Soil Biol Biochem. 2006;38(9):2971-2975.
Crossref - Ali A, Mannan A, Hussain I, Hussain I, Zia M. Effective removal of metal ions from aquous solution by silver and zinc nanoparticles functionalized cellulose: Isotherm, kinetics and statistical supposition of process. Environ Nanotechnol Monit Manag. 2018;9:1-11.
Crossref - Mahmood A, Shahzad T, Hussain S, et al. Evaluation of Symbiotic Association between Various Rhizobia, Capable of Producing Plant-Growth-Promoting Biomolecules, and Mung Bean for Sustainable Production. Sustainability. 2021;13(24):13832.
Crossref - Bergmann GT, Bates ST, Eilers KG, et al. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol Biochem. 2011;43(7):1450-1455.
Crossref - Singh A, Mishra S, Choudhary M, et al. Rhizobacteria improve rice zinc nutrition in deficient soils. Rhizosphere. 2022;25:100646.
Crossref - Singh D, Geat N, Rajawat MVS, et al. Prospecting endophytes from different Fe or Zn accumulating wheat genotypes for their influence as inoculants on plant growth, yield, and micronutrient content. Annals of Microbiology. 2018;68(12):815-833.
Crossref - Batool S, Asghar HN, Shehzad MA, et al. Zinc-Solubilizing Bacteria-Mediated Enzymatic and Physiological Regulations Confer Zinc Biofortification in Chickpea (Cicer arietinum L.). J Soil Sci Plant Nutr. 2021;21(3):2456-2471.
Crossref - Gupta G, Snehi SK, Singh V. Role of PGPR in biofilm formations and its importance in plant health. In: Das S, Das S, eds. Biofilms in Plant and Soil Health. Wiley; 2017:27-42
- Arora NK, Fatima T, Mishra I, Verma M, Mishra J, Mishra V. Environmental sustainability: challenges and viable solutions. Environmental Sustainability. 2018;1(4):309-340.
Crossref - Yasmin R, Hussain S, Rasool MH, Siddique MH, Muzammil S. Isolation, Characterization of Zn Solubilizing Bacterium (Pseudomonas protegens RY2) and its Contribution in Growth of Chickpea (Cicer arietinum L.) as Deciphered by Improved Growth Parameters and Zn Content. Dose-Response. 2021;19(3):155932582110367.
Crossref - Ladohia S, Rana N, Srivastava P, Kumar R, Mehta S, Pareek B. Use of zinc solubilizing biofertilizers for increasing the growth and yield of cereals: a review.
J Appl Nat Sci. 2024;16(3).
Crossref - Nimbulkar P, Gupta G, Virkhare U, Althubiani AS, Dutta A, Kher D. Bacterial endophytes and their secondary metabolites: mechanisms of biosynthesis and applications in sustainable agriculture. J Umm Al-Qura Univ Appl Sci. 2025;:1-3.
Crossref - Srithaworn M, Jaroenthanyakorn J, Tangjitjaroenkun J, Suriyachadkun C, Chunhachart O. Zinc solubilizing bacteria and their potential as bioinoculant for growth promotion of green soybean (Glycine max L. Merr.). Peer J. 2023;11:e15128.
Crossref - Hosseini NM. The landuse change detection in Taleghan catchment. Afr J Agric Res. 2012;7(25).
Crossref - Ngole MV, Ekosse GE. Zinc uptake by vegetables: Effects of soil type and sewage sludge. Afr J Biotechnol. 2009;8(22):6258-6266.
Crossref - Ahmad I, Ahmad M, Hussain A, Jamil M. Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: a promising approach for improving cotton growth. Folia Microbiol (Praha). 2021;66(1):115-125.
Crossref - Suleman M, Yasmin S, Rasul M, Yahya M, Atta BM, Mirza MS. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE. 2018;13(9):e0204408.
Crossref - Rai S, Mago Y, Aggarwal G, Yadav A, Tewari S. Liquid Bioformulation: a trending approach towards achieving sustainable agriculture. Mol Biotechnol. 2023;66(10):2725-2750.
Crossref - Bansal K, Raturi A, Katiyar U, Mishra A, Tewari S. Microbial rhizoremediation as a strategy for decontaminating polluted sites and augmenting plant growth. In: Javid A. Parray, Nowsheen Shameem, Dilfuza Egamberdieva (Eds), In Microbiome Research in Plants and Soil, Microbiome Drivers of Ecosystem Function, Academic Press. 2024:181-227.
Crossref - Altaf MM, Khan MSA, Abulreesh HH, Ahmad I. Quorum Sensing in Plant Growth-Promoting Rhizobacteria and Its Impact on Plant-Microbe Interaction. In: Singh, D., Singh, H., Prabha, R. (eds) Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer, Singapore. 2017:311-331.
Crossref - Ahmad F, Husain FM, Ahmad I. Rhizosphere and Root Colonization by Bacterial Inoculants and Their Monitoring Methods: A Critical Area in PGPR Research. In: Ahmad, I., Ahmad, F., Pichtel, J. (eds) Microbes and Microbial Technology. Springer, New York, NY. 2011:363-391.
Crossref - Khan NA, Lone PM. Effects of early and late season defoliation on photosynthesis, growth and yield of mustard (Brassica juncea L.). Braz J Plant Physiol. 2005;17(1):181-186.
Crossref - Yan N, Marschner P, Cao W, Zuo C, Qin W. Influence of salinity and water content on soil microorganisms. International Soil and Water Conservation Research. 2015;3(4):316-323.
Crossref - Hussain MA, Hossain MS, Bhuiyan MSR, Zeba N, Mohsin SM. Field Performance and Genetic Analysis in Some Advanced Lines of Mustard (Brassica rapa L.). The Agriculturists. 2016;14(1):112-121.
Crossref - Devi SNP, Kumari KS, Vasandha S. Assessment of competence of the Pseudomonas aeruginosa to solubilize insoluble form of zinc under various cultural parameters. Arab J Sci Eng. 2015;41(6):2117-2121.
Crossref - Milder JC, Arbuthnot M, Blackman A, et al. An agenda for assessing and improving conservation impacts of sustainability standards in tropical agriculture. Conserv Biol. 2014;29(2):309-320.
Crossref - Dubey RC, Khare S, Kumar P, Maheshwari DK. Combined effect of chemical fertilisers and rhizosphere-competent Bacillus subtilis BSK17 on yield of Cicer arietinum. Archives of Phytopathology and Plant Protection. 2014;47(19):2305-2318.
Crossref - Chauhan AK, Maheshwari DK, Kim K, Bajpai VK. Termitarium-inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 colonizing Curcuma longa L.: isolation, characterization, and evaluation of their biocontrol and plant-growth-promoting activities. Can J Microbiol. 2016;62(10):880-892.
Crossref - Cakmak I, Kutman UB. Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci. 2017;69(1):172-180.
Crossref - Ebbs SD, Kochian LV. Toxicity of zinc and copper to brassica species: Implications for phytoremediation. J Environ Qual. 1997;26(3):776-781.
Crossref - Willis MS, Monaghan SA, Miller ML, et al. Zinc-Induced Copper deficiency. Am J Clin Pathol. 2005;123(1):125-131.
Crossref - Plum LM, Rink L, Haase H. The essential toxin: Impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342-1365.
Crossref - Maares M, Haase H. A Guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12(3):762.
Crossref - Zaborowska M, Wyszkowska J, Borowik A, Kucharski J. Effect of separate and combined toxicity of bisphenol A and zinc on the soil microbiome. Int J Mol Sci. 2022;23(11):5937.
Crossref - Yang WT, Yi YJ, Xia B. Unveiling the duality of Pantoea dispersa: A mini review. Sci Total Environ. 2023;873:162320.
Crossref - Chaudhary T, Dixit M, Gera R, et al. Techniques for improving formulations of bioinoculants. 3 Biotech. 2020;10(5).
Crossref - Singh S, Chhabra R, Sharma A, Bisht A. Harnessing the power of Zinc-Solubilizing Bacteria: a catalyst for a sustainable agrosystem. Bacteria. 2024;3(1):15-29.
Crossref - Kumar A, Dewangan S, Lawate P, Bahadur I, Prajapati S. Zinc-Solubilizing Bacteria: A Boon for Sustainable Agriculture. In: Sayyed, R., Arora, N., Reddy, M. (eds) Plant Growth Promoting Rhizobacteria for Sustainable Stress Management . Microorganisms for Sustainability, vol 12. Springer, Singapore. 2019:139-155.
Crossref - Yadav RC, Sharma SK, Varma A, et al. Modulation in biofertilization and biofortification of wheat crop by inoculation of Zinc-Solubilizing rhizobacteria. Front Plant Sci. 2022;13.
Crossref - Ali MA, Naeem F, Tariq N, Ahmed I, Imran A. Bioactive Nutrient fortified fertilizer: a novel hybrid approach for the enrichment of wheat grains with zinc. Front Plant Sci. 2021;12.
Crossref - Allard-Massicotte R, Tessier L, Lecuyer F, et al. Bacillus subtilis Early Colonization of Arabidopsis thaliana Roots Involves Multiple Chemotaxis Receptors. mBio. 2016;7(6):mBio.01664-16.
Crossref - Ditta A, Ullah N, Imtiaz M, et al. Zn biofortification in crops through Zn-solubilizing plant growth-promoting rhizobacteria. In: Sustainable Plant Nutrition Under Contaminated Environments. Cham: Springer International Publishing; 2022:115-133.
Crossref - Ayyaz M, Khan Z, Tabassam N, Sultan T, Saeed A, Shan M. Isolation and Characterization of Plant Growth Promoting Rhizobacteria for Growth Promotion of Rice (Oryza sativa L.) and Wheat (Triticum aestivum). Pak J Biochem Biotechnol. 2021;2(2):177-194.
Crossref - Singh S, Gupta G, Khare E, Behal KK, Arora NK. Phosphate solubilizing rhizobia promote the growth of chickpea under buffering conditions. Int J Pure Appl Biosci. 2014;2(5):97-106.
Crossref - Sah S, Krishnani S, Singh R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr Res Microb Sci. 2021;2:100084.
Crossref - Delaporte-Quintana P, Lovaisa NC, Rapisarda VA, Pedraza RO. The plant growth promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. Plant Growth Regulation. 2020;91(2):185-199.
Crossref - Bhakat K, Chakraborty A, Islam E. Characterization of zinc solubilization potential of arsenic tolerant Burkholderia spp. isolated from rice rhizospheric soil. World J Microbiol Biotechnol. 2021;37:1-13.
Crossref - Chanchal AK, Singh M, Pradhan AK, et al. Release kinetics and solubilization of insoluble zinc compounds in response to zinc solubilizing bacterial (ZSB) isolates. Scientist. 2022;1(3):1-3
- Dobrzynski J, Jakubowska Z, Dybek B. Potential of Bacillus pumilus to directly promote plant growth. Front Microbiol. 2022;13:1069053.
Crossref - Liu J, Zhang J, Zhu M, et al. Effects of Plant Growth Promoting Rhizobacteria (PGPR) Strain Bacillus licheniformis with Biochar Amendment on Potato Growth and Water Use Efficiency under Reduced Irrigation Regime. Agronomy. 2022;12(5):1031.
Crossref - Leister C, Hugler M. Genome Analysis of Enterobacter asburiae and Lelliottia spp. Proliferating in Oligotrophic Drinking Water Reservoirs and Lakes. Appl Environ Microbiol. 2022;88(14).
Crossref - Abraham J, Silambarasan S. Plant Growth Promoting Bacteria Enterobacter asburiae JAS5 and Enterobacter cloacae JAS7 in Mineralization of Endosulfan. Appl Biochem Biotechnol. 2015;175(7):3336-3348.
Crossref - Haroon M, Khan ST, Malik A. Zinc-Solubilizing Bacteria: An Option to Increase Zinc Uptake by Plants. In: Khan, S.T., Malik, A. (eds) Microbial Biofertilizers and Micronutrient Availability. Springer, Cham. 2021:207-238.
Crossref - Kushwaha P, Kashyap PL, Pandiyan K, Bhardwaj AK. Zinc-Solubilizing Microbes for Sustainable Crop Production: Current Understanding, Opportunities, and Challenges. In: Solanki, M., Kashyap, P., Kumari, B. (eds) Phytobiomes: Current Insights and Future Vistas. Springer, Singapore. 2020:281-298.
Crossref - Hashemnejad F, Barin M, Khezri M, Ghoosta Y, Hammer EC. Isolation and identification of insoluble Zinc-Solubilising bacteria and evaluation of their ability to solubilise various zinc minerals. J Soil Sci Plant Nutr. 2021;21(3):2501-2509.
Crossref - Prakash V, Tripathi S, Sharma S, et al. The contribution of rhizosphere in the supply of zinc to plants. In: Zinc in Plants. Academic Press; 2025:349-367.
Crossref - Chaturvedi H, Singh V, Gupta G. Potential of bacterial endophytes as plant growth promoting factors. J Plant Pathol Microbiol. 2016;7(9):1-6.
Crossref - Pawar SV, Borkar SG. Isolation and characterization of zinc solubilizing bacteria from rhizosphere soil. J Plant Nutr. 2015;38(12):2043-2050.
Crossref - Singh T, Kothari M, Mishra S, et al. Synergistic effect of zinc solubilizing bacteria and consortia on the zinc marker enzymes and gaseous exchange parameters in rice (Oryza sativa L.) for zinc biofortification. Plant Physiol Biochem. 2025;223:109807.
Crossref - Ahmad F, Husain FM, Ahmad I. Rhizosphere and root colonization by bacterial inoculants and their monitoring methods: a critical area in PGPR research. In: Microbes and Microbial Technology: Agricultural and Environmental Applications. New York, NY: Springer New York; 2011:363-391.
- Singh S, Chhabra R, Sharma A, Bisht A. Harnessing the power of zinc-solubilizing bacteria: a catalyst for a sustainable agrosystem. Bacteria. 2024;3(1):15-29.
Crossref - Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163(2):173-181.
Crossref - Mishra P, Mishra J, Bharti C, Arora NK. Salt-tolerant Pseudomonas taiwanensis PWR-1 mediated organic acid production for biofortification of zinc and reducing fertilizer dependency in wheat under saline conditions. J Plant Growth Regul. 2025:1-21.
Crossref - Khalid S, Amanullah, Ahmed I. Enhancing zinc biofortification of wheat through integration of zinc, compost, and zinc-solubilizing bacteria. Agriculture. 2022;12(7):968.
Crossref - Ahirwar NK, Gupta G, Singh V, Rawlley RK, Ramana S. Influence on growth and fruit yield of tomato (Lycopersicon esculentum Mill.) plants by inoculation with Pseudomonas fluorescens (SS5): possible role of plant growth promotion. Int J Curr Microbiol Appl Sci. 2015;4(2):720-730.
- Khanghahi MY, Ricciuti P, Allegretta I, Terzano R, Crecchio C. Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ Sci Pollut Res. 2018;25(25):25862-25868.
Crossref - Kumawat N, Kumar R, Khandkar UR, et al. Silicon (Si)- and zinc (Zn)-solubilizing microorganisms: role in sustainable agriculture. In: Biofertilizers for Sustainable Agriculture and Environment. 2019:109-135.
Crossref - Yahaghi Z, Shirvani M, Nourbakhsh F, Pueyo JJ. Isolation, identification, and application of zinc-solubilizing bacteria exhibiting beneficial traits to promote plant growth and metal uptake. Int J Environ Sci Technol. 2024;1-6.
Crossref - Parameswaran PS, Priya VK, Jayachandran K, Radhakrishnan EK. Plant probiotic potential of rapid zinc-solubilizing Bacillus sp. VR in the presence of exogenously supplemented zinc oxide. Cereal Res Commun. 2025;1-10.
Crossref - Sukhwal A, Jain D, Sharma V, Ojha SN, Jat G, Upadhyay SK, et al. Efficacy evaluation of newly isolated zinc solubilizing bacteria for their potential effect on maize (Zea mays L.) under zinc deficient soil conditions. Land Degrad Dev. 2023;34(16):4912-4923.
Crossref - Warghane A, Thakkar J, Bhardwaj G, Bhatt V, Chopade BA. Isolation and Characterization of Major Cultivable Bacteria from Novel Natural Fertilizer with Comprehensive Nutrient Analysis. J Pure Appl Microbiol. 2025;19(1):197-209.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.