ISSN: 0973-7510
E-ISSN: 2581-690X
The spoilage of muskmelons was rapid due to early maturity of the fruits after immediate harvest before consumption. To minimise the postharvest losses, especially in horticultural crops, food processing and value addition to the produce plays lot of role and the losses can be minimised. Keeping in view, the experiment was planned to prepare muskmelon fruit juice fortified with four different species of lactic acid bacteria viz., Lactiplantibacillus plantarum subsp. plantarum MTCC 9511, Lactobacillus acidophilus MTCC 10307, Lactobacillus delbrueckii subsp. lactis and Lactobacillus casei MTCC 1423. The survivability of lactobacilli and physicochemical parameters were studied during fermentation of the fruit juice. Drop in pH levels from initial pH was recorded in all muskmelon fruit juice samples incubated at two different temperatures (30°C and 37°C) more than 72 hours. But titratable acidity was increased in all muskmelon fruit juice samples incubated at two different temperatures (30°C and 37°C). The fruit juice containing Lactiplantibacillus plantarum subsp. plantarum MTCC 9511 (T4) recorded lower pH levels and maximum titratable acidity, total phenolic content and more viable cells compared to other species of lactobacilli. Sensory evaluation was conducted randomly for all the samples and no significant difference was observed.
Muskmelon Fruit Juice, Lactic Acid Bacteria, pH, Titratable Acidity, Carbohydrates, Total Phenol Concentration, Viable Cell Counts, Sensory Evaluation
Probiotics are often called “good” or “helpful” bacteria since they help in keeping gut healthy. Probiotics have a long history of association with human beings and animals1. More than a century ago, Henry Tissier2 from Pasteur Institute isolated rod shaped bifidobacteria from healthy breast fed infants and reported that these bacteria were dominated in healthy breast fed infants and they were absent from formula fed infants suffering from diarrhoea and pave the way to establish a concept that these bacteria played a role in maintaining gut health. Probiotics are defined by the World Health Organization as “live microorganisms that when administered in adequate amounts confer a health benefit on the host”3. A probiotic food product on an average should contain 106 CFU/mL (colony forming units per mL) as per the guidelines set by World Health Organisation (WHO). Probiotic bacteria are conventionally added to ferment the dairy milk for production of yoghurt, dahi and other similar products making them as functional foods. Many dairy industries are commercially producing probiotic fermented products and being sold throughout the world. Few people have lactose intolerance, allergic to dairy products and presence of high saturated fatty acids in dairy foods making them unfit for consumption4,5. Hence, in recent times non-dairy probiotic foods are gaining importance and several raw materials like cereals, soybean, fruits and vegetables are using for production of non dairy functional foods6. Several probiotic strains have the ability to ferment non dairy foods like fruits and vegetables. Further, fruits and vegetables are good substrates for growth of probiotic lactic acid bacteria7,8,9. In the present scenario, fruit juices with probiotic bacteria are gaining more importance10 attributed to more health benefits by consumption of fruit juice with added probiotics11. Lactic acid bacteria (LAB), generally and widely from the genera Lactobacillus and Bifidobacterium and constitute a maximum proportion of probiotic cultures as nutritional supplements, pharmaceuticals and functional foods12,13.
Cucumis melo L. belongs to Reticulatus group and commonly called as cantaloupe or muskmelon and belongs to Cucurbitaceae family14. This fruit is rich source of phytonutrients like sugars, ascorbic acid, carotene, folic acid and minerals and its sugar content, flavour and texture mainly decides the consumer preference15,16. Hubbard et al17 hypothesised that muskmelon fruits experience a metabolic shift indicated by both physical and compositional changes, such as exocarp netting, mesocarp softening, and the commencement of sucrose accumulation, about halfway through their growth. Postharvest losses of muskmelon in some of the countries accounts for more than 30 percent or 35-40 billion income annually before its consumption after immediate harvest18. Muskmelons are more vulnerable to pathogenic fungi and fungal rots due to high moisture content, easily available nutrients and favourable pH and leads to unsound fruits and not fit for human consumption.
The ripening of muskmelon fruit is rapid and highly coordinated, hereditary characteristics involving a series of irreversible biochemical and physiological changes that leads to the formation of a soft and edible fruit with suited excellent features19. Quality of the muskmelon fruit can be judged by it’s sugar composition and several key enzymes are responsible for sucrose metabolism20. Based on earlier studies by Hubbard et al17, enzymes like sucrose phosphate synthase (SPS) (EC 2.4.1.14) and acid invertase (AI) (EC 3.2.1.26) are associated with sucrose accumulation. But, the activity of sucrose synthase (SS) (EC 2.4.1.13) enzyme in sucrose accumulation is not clearly understood16. Because of quick ripening, the muskmelons spoils quickly within 4-5 days. Technologies to delay ripening process and proper storage facilities are yet to reach small and marginal farmers.
Therefore, the present topic has been undertaken with a view to prepare value added probiotic muskmelon juice supplemented with probiotic cultures like lactic acid bacteria. To give value addition to the muskmelon fruit, maximum returns to the farmers and to curb postharvest losses during superfluity season, there is urgent need to process the excess produce into nutritious value-added products The processing of muskmelon fruits may be converted to different products like fruit leather, jams, pickles, jellies, squashes and juices etc18.
Strains and Cultures
The probiotic bacterial cultures used in this study are Lactobacillus acidophilus MTCC 10307, Lactiplantibacillus plantarum subsp. plantarum MTCC 9511, Lactobacillus casei MTCC 1423. These lactic acid bacteria are obtained from Institute of Microbial Technology (IMTECH), Chandigarh, India. Lactobacillus delbrueckii subsp. lactis was obtained from Department of Agricultural Microbiology, College of Agriculture, University of Agricultural Sciences, Banglore. Lactic acid bacteria were sub cultured on MRS agar (De Man Rogosa and Sharpe Agar medium) (Himedia, India) and were maintained in 20% glycerol stock solutions and stored at -20°C for further studies.
Preparation of Probiotic Muskmelon Juice
Healthy muskmelon fruits purchased from local market and washed with clean tap water thoroughly to remove extraneous material. The fruit pulp and water were mixed in 1:1 ratio and 50 g of sugar was added per kilogram of pulp for preparation of muskmelon fruit juice.
Total soluble solids (TSS) were measured by hand refractometer and it was 8%. Approximately, 150 ml of muskmelon juice is filled to conical flasks and pasteurized to remove unfavourable microorganisms. Muskmelon juice was inoculated with one ml of probiotic strains (Lactobacillus acidophilus MTCC 10307, Lactiplantibacillus plantarum subsp. plantarum MTCC 9511, Lactobacillus casei MTCC 1423, Lactobacillus delbrueckii subsp. lactis) and allowed for fermentation at 30oC and 37oC. To know the effect of cold storage (4oC) on viable lactobacilli, the fruit juice samples were stored at cold conditions (refrigerated conditions i.e., 4-6oC). All the samples in different treatments were maintained in triplicates.
Physico Chemical Analysis during the Fermentation of Muskmelon Juice
pH values for muskmelon juice samples were recorded by a digital pH meter. The titratable acidity of the fermented probiotic muskmelon fruit juice samples were determined titrating against 0.1M of sodium hydroxide (NaOH) in the presence of phenolphthalein indicator. To the 5ml of sample, add 6-7 drops of indicator and titrated against 0.1M NaOH (50ml) in burette till colour change noticed.
Viable Cell Count
Viable cell counts of lactobacilli for all juice samples were isolated on de Mans Rogosa agar (MRS agar) medium by the standard plate count (SPC) method. All the MRS plates were kept for incubation at 30°C for 48 hours to check the lactobacilli colonies21.
Quantitative Analysis of Carbohydrates
Carbohydrates like glucose, fructose and sucrose HPLC (Shimadzu model LC6A) according to Casterline et al22. Ten μl (10 μl) of sample extract was injected to HPLC column to detect the concentration of sugars in the samples. Carbohydrates like glucose, fructose and sucrose are separated by isocratic method with mobile phase consists of acetonitrile: water (80:20 (v/v)) at a flow rate of 1 ml/min. The flow rate was kept at 1 ml/min. Standard sugars (sucrose, fructose and glucose) were used for calibration of HPLC and standards were purchased from Sigma chemicals, USA.
Total Phenolic Concentration (TPC)
Total phenols in the probiotic fruit juice samples was determined using the Folin–Ciocalteu method, as described by Anand et al23. Diluted juice samples (0.5 ml) were pipetted into test tubes and 5 ml of distilled water is added followed by 0.5 ml of Folin–Ciocalteu reagent, and the mixture was kept for reaction for 3 minutes, later, 1.0 ml of 20% sodium carbonate (Na2CO3) solution was added and mixed well and allowed to stand for one hour at room temperature for colour development. Absorbance was measured at 750 nm using a spectrophotometer (MODEL UV–10, Thermo Fisher Scientific, USA) and was expressed as milligrams of gallic acid equivalent per 100 ml). Standard graph was plotted by using different ranges of standard solutions of gallic acid.
Sensory Evaluation
The sensory evaluation was carried for probioticated muskmelon juices by using 9-point hedonic scale24 to know the consumer preference sensory characteristics like taste, aroma, flavour, colour and overall acceptability. Muskmelon juice samples which were stored at refrigerated conditions for four weeks were randomly served for sensory evaluation. The average mean scores of all the sensory parameters were calculated and presented in the results.
Statistical Analysis
The treatments were kept in three replications and the experimental results were subjected to statistical analysis by one-way ANOVA. The analysis of variance and interpretation of the data was done as per the methods given by Gomez and Gomez25.
Effect of Lactic Acid Bacteria on Physical Parameters of Probiotic Muskmelon Juice
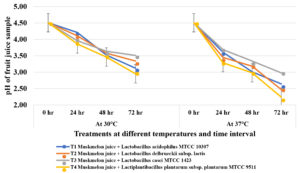
The pH levels in all fruit samples were slowly decreased during fermentation by lactic acid bacteria (Figure 1). The initial pH was recorded as 4.51 for all samples and the pH was slowly decreased in all probiotic muskmelon juice samples during fermentation by lactobacilli incubated at two different temperatures (30oC and 37oC) for 72 hours. At 30oC, T4 (Lactiplantibacillus plantarum subsp. plantarum MTCC 9511) recorded lower pH levels (2.95) after 72 hours of incubation. followed by T1 containing L. acidophilus MTCC 10307 (3.12) at 72 hours respectively. Juice samples inoculated with L. casei MTCC 1423 recorded less decrease in pH (3.51). At 37oC, the T1 (L. acidophilus MTCC 10307) recorded less pH (2.55) followed by T4 (2.65) after 72 hours of incubation. Less reduction in pH level was noticed in juice sample inoculated with T3 (Lactobacillus casei MTCC 1423).
Figure 1. Influence of pH by lactic acid bacteria on muskmelon juice incubated at 30°C and 37°C temperatures at different time intervals
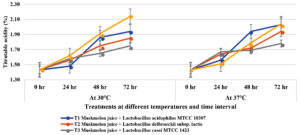
Increase in titratable acidity was observed in probiotic muskmelon juice when incubated at 30oC and 37oC for 72 hours. Maximum titratable acidity was observed with Lactiplantibacillus plantarum subsp. plantarum MTCC 9511 (1.62, 1,91 and 2.14 at 30oC and 1.51, 1.79, 2.04 at 37oC at 24, 48, 72 hours of incubation respectively) followed by L. acidophilus MTCC 10307 (1.48, 1.86, 1.94 at 30oC and 1.56, 1.94, 2.03 at 37oC at 24, 48, 72 hours of incubation respectively) and Lactobacillus delbrueckii subsp. Lactis ( 1.56, 1.65, 1.75 at 30oC and 1.67, 1.69, 1.78 at 37oC at 24, 48, 72 hours of incubation respectively). Less reduction in titratable acidity was recorded in muskmelon juice samples supplemented with Lactobacillus casei MTCC 1423 (Figure 2).
Figure 2. Influence of Titratable Acidity by lactic acid bacteria on muskmelon juice incubated at 30°C and 37°C time intervals
All four probiotic cultures (Lactobacillus acidophilus MTCC 10307, Lactiplantibacillus plantarum subsp. plantarum MTCC 9511, Lactobacillus casei MTCC 1423, Lactobacillus delbrueckii subsp. lactis) were effective in reducing pH of the muskmelon juice samples. The nutrients available in muskmelon juice were utilized by all lactic acid bacteria effectively for cell metabolism and produced more organic acids after hours of incubation and this could be the reason for lowering in pH levels of muskmelon juice4. However, some studies reported that lactic acid bacteria became more sensitive to lower pH levels of 2.5–3.726. Many fruit juice samples inoculated with lactic acid bacterial cultures especially Lactobacillus acidophilus MTCC 10307 recorded higher viable cell counts and leads to lowering of pH in juice samples with more acid production. The results are in accordance with other fruit juices like pomegranate27, peach28, mango29 and apple juice. Acid tolerance by probiotic lactic acid bacteria in probioticated fruit or vegetable juice sample is an important trait. In general, fruit and vegetable juice fermentations carried with probiotic lactic acid bacteria have resulted in decreased levels of pH and increased levels of acidity. Lowering in titratable acidity in the probiotic muskmelon juice may be attributed to the rapid consumption of available carbohydrates by the probiotic bacteria and releasing the end products in the medium. Probiotic bacterial cultures have potential ability to grow in the fruit and vegetable juices and they have the ability to with stand the stress conditions that exist in fruit juices like acidic environment.
Sugar Consumption and Total Phenolic Concentration
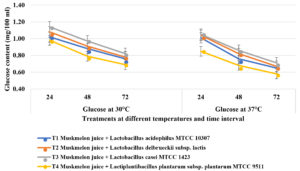
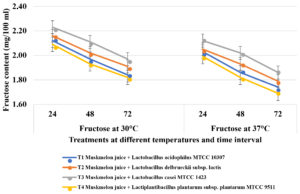
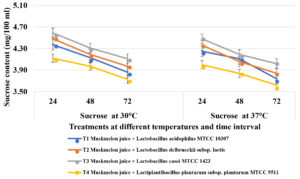
Fruit juice samples have total sugar content of 9-12 g/100 ml/kg. Simultaneously, simple sugars were released from the juice due to saccharification, so that these simple sugars favours bacterial growth. At 30oC, after 24 hours of incubation, highest glucose content was utilised in T4 (Muskmelon juice containing Lactiplantibacillus plantarum subsp. plantarum MTCC 9511) and decreased trend was noticed from 0.98 mg to 0.79 mg at 48 hours and 0.69 mg after 72 hours of incubation followed by T1 (Lactobacillus acidophilus MTCC 10307) which recorded 1.12 mg, 0.88 mg and 0.76 mg at 24, 48 and 72 hours of incubation respectively (Figure 3). Least glucose utilization was noticed in T3 (Lactobacillus casei MTCC 1423). Similarly, highest fructose was utilized in samples containing T4 (Lactiplantibacillus plantarum subsp. plantarum MTCC 9511) where 2.09 mg, 1.93 mg and 1.82 mg at 24, 48 and 72 hours of incubation respectively followed by T1 (L. acidophilus MTCC 10307) which recorded 2.12 mg, 1.97 mg and 1.84 mg at 24, 48 and 72 hours of incubation respectively. Less fructose was utilized by in T3 (Lactobacillus casei MTCC 1423). Similar trend was noticed in sucrose utilization where highest sucrose was consumed by T4 (Lactiplantibacillus plantarum subsp. plantarum MTCC 9511) whereas 4.12 mg, 3.97 mg and 3.73 mg at 24, 48 and 72 hours of incubation respectively, followed by 1 (L. acidophilus MTCC 10307) which recorded 4.37 mg, 4.13 mg and 3.86 mg at 24, 48 and 72 hours of incubation respectively. The fruit juice samples containing Lactobacillus casei MTCC 1423 (T3) was recorded less sugar utilization compared to other lactic acid bacteria. The concentrations of sugars like sucrose, fructose and glucose were reduced in all muskmelon juice samples after 72 hours of fermentation due to varied carbohydrate utilisation by lactobacilli and earlier findings reported that the metabolism of carbohydrates varies with species to species of lactic acid bacteria and depends on the sugar substrate and fermentation time30-32.
Figure 3a. Estimation of Glucose in probioticated muskmelon juice at different temperature and time intervals
Figure 3b. Estimation of Fructose in probioticated muskmelon juice at different temperature and time intervals
Figure 3c. Estimation of Sucrose in probioticated muskmelon juice at different temperature and time intervals
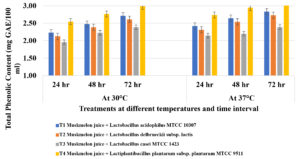
Total phenolic concentration (TPC) was increased significantly in probioticated muskmelon juice during 72 hours of fermentation. Muskmelon juice with Lactiplantibacillus plantarum subsp. plantarum MTCC 9511 (T4) recorded 2.98 µg GAE/100 ml in 72 hours of fermentation followed by L. acidophilus MTCC 10307 (2.72 µg GAE/100 ml) and Lactobacillus casei MTCC 1423 (2.61 µg GAE/100 ml) (Figure 4).
Figure 4. Estimation of Total Phenolic concentration in probioticated muskmelon juice after 72 hours of fermentation.
Probioticated muskmelon juice samples have recorded progressive increase in total phenolic content (TPC) with increase in fermentation time. Our results have close conformity with other research findings1,26,33 where total phenolic contents of probiotic fruit juices have increased compared to controls. Increase in total phenol (214–264 mg GAE/100 mL) content was noticed in Cornelian cherry juice fermented with immobilized cells of probiotic cultures in all the samples stored at refrigerated conditions compared to fermented juice with free cells (165–199 mg GAE/100 mL) and non-fermented juice (135–169 mg GAE/100 mL)34. Fig fruit juice when fermented with Lactobacillus delbrueckii subsp. lactis recorded highest total phenolic contents (TPC=0.45%) and the significant antioxidant activity (IC50=76.55 ppm) compared to the unfermented ones (TPC=0.09%; IC50=76.7 ppm)27,35.
Fermentation of muskmelon fruit juice samples with lactic acid bacteria resulted significant increase in total phenolic content (TPC) and this increase may be attributed to the formation of formation of biochemical compounds like ascorbic acids, carotenoids and other phenolics during fermentation, which have been reported to have many beneficial health properties. These results have close proximity with raw or non probioticated sapota juice28. In general, DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity was significantly higher in probioticated sapota fruit juices compared to probioticated fruit juices.
Viable Cell Counts at Different Time Intervals
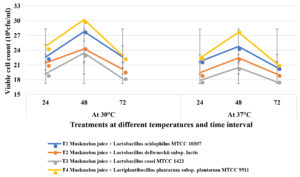
The fermented juice samples were incubated for 72 hours at two different temperatures 30oC and 37oC to know the survivability of lactic acid bacteria (viable cell counts). Increase in viable cell counts in juice samples which were incubated at both temperatures 30oC and 37oC up to 48 hours and decline in cell counts was observed after 72 hours of incubation and did not fall below 106cfu/ml (Figure 5).
Figure 5. Effect of cold storage (4°C) on lactic acid bacterial population in muskmelon juice incubated at different temperatures at different weeks intervals
Decline in viable cell counts were noticed in the juice samples after 72 hours of fermentation and this reduction may be due to consumption of available nutrients by the cells, reduction in pH and oxygen levels during stationary phase and occurrence of autolysis of cells in death phase36. Significant number of probiotic populations in finished product plays key role to confer better health benefits. The ability of probiotic lactobacilli to with stand stressful conditions like acidic environments is an important trait to maintain optimum viable counts in probiotic fruit juice to confer the desired health benefits. The Lactiplantibacillus plantarum subsp. plantarum MTCC 9511 and Lactobacillus acidophilus MTCC 10307 cultures survived and grew well at the lower pH levels in muskmelon juice compared to L. bulgaricus subsp. lactis and Lactobacillus casei MTCC 1423. Similar studies with fruit juices indicated the growth of Lactiplantibacillus plantarum subsp. plantarum MTCC 9511, which resulted in a viable count of 8.0×108 CFU/ml after 72 hours of fermentation29. Shukla et al8 reported that whey pine apple juice, initial viable count decreased to 3.8×107 cfu/ml at refrigerated storage, but the viable count of Lactobacillus acidophilus MTCC 10307 did not fall below 106 cfu/ml. Further, it is observed that during storage at 30 ± 1°C, the viable count of Lactobacillus acidophilus MTCC 10307 declined to 2.9×107 cfu/ml after 120 hours from the initial population (9.5×108 at 48 hours).
Maintaining the viable cells and the activity of probiotic bacteria till the end of shelf life are two important criteria to be fulfilled in juices, where low pH represents a drawback37. Several strains of Lactobacillus acidophilus MTCC 10307, Lactiplantibacillus plantarum subsp. plantarum MTCC 9511, Lactobacillus casei MTCC 1423, Lactobacillus delbrueckii subsp. lactis can grow in fruit matrices due to their tolerance to acidic environments. The main factors for loss of viability of probiotic organisms have been attributed to the decrease in the pH of the medium and accumulation of organic acids during fermentation growth38,39. Minimum recommended probiotic population should be at least greater than 107 CFU/mL at the end of shelf life to confer the health benefits,40-43. The effect of cold storage temperatures on survivability of L. acidophilus MTCC 10307 in probiotic yogurt samples after 20 days of storage revealed that the more viability was found at 2ºC, whereas viability of B. lactis has the highest viability at 8ºC31. Saccaro et al33 recorded reduction of L. acidophilus MTCC 10307 in the range of 2 log 10 cycles /mL at the end of the storage period in yogurt changes like taste, appearance and overall acceptability of the product have no change and also no adverse effects were noticed. Further, L. acidophilus MTCC 10307 it is able to survive till the consumption of the product44.
Sensory Evaluation
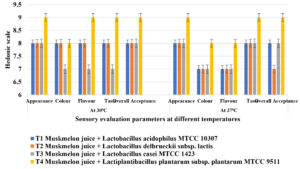
Probioticated muskmelon juices recorded good sensory scores and overall acceptability. significant differences was not found among the treatments regarding sensory scores of probioticated muskmelon juice samples (Figure 6) and the influence of fermentation by lactic acid bacteria on juice texture, taste, flavours and overall acceptance was non significant. Earlier works on probiotic fruit juices like pineapple juice containing Lactobacillus reuteri,7 apple juice containing Lactobacillus casei MTCC 142345 reported that these lactic acid bacteria did not affect sensory evaluation and overall acceptability. Similarly, the results are in close conformity with the results from36 who recorded that carrot flavoured milk remained in healthy condition for 4 days under cold storage. The present studies revealed that the fruit juices can be prepared with lactic acid bacterial cultures and named as probioticated fruit juices for health benefits especially to the people who have lactose intolerance and allergic to milk products.
Briefly, the results showed that the fruit juice containing Lactiplantibacillus plantarum subsp. plantarum MTCC 9511 (T4) recorded lower pH levels (2.95) and maximum titratable acidity, total phenolic content and more viable cells compared to other species of lactobacilli. In fruit juice, the contents of compounds namely glucose, fructose and sucrose were decreased as time of treatment increased.
In present experiment, the treatments with Lactiplantibacillus plantarum subsp. plantarum MTCC 9511 showed higher sensorial acceptability whereas lower sensorial acceptability was observed in treatments treated with Lactobacillus casei MTCC 1423.
ACKNOWLEDGMENTS
The authors would like to thank Acharya NG Ranga Agricultural University, India for providing necessary budget to complete the research project.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by Acharya N G Ranga Agricultural University, Lam, Guntur, Andhra Pradesh, India.
DATA AVAILABILITY
All the datasets generated or analysed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM. Health benefits of probiotics: a review. ISRN Nutr. 2013; 481651.
Crossref - Tissier H. Recherchers sur la flora intestinale normale et pathologique du nourisson. 1900 Doctoral Disserttion. 1900. Univerity of Paris.
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London Ontario, Canada.2002.
- Kumar BV, Sreedharamurthy M, Reddy OVS, Probiotication of tomato juice by lactic acid bacteria. Nutrafoods. 2015; 14: 97-106.
Crossref - Sharma V. Mishra HN. Fermentation of vegetable juice mixture by probiotic lactic acid bacteria. Nutrafoods. 2013; 12 (1): 17-22.
Crossref - Dey G. Non-dairy Probiotic Foods: Innovations and Market Trends. In: Innovations in Technologies for Fermented Food and Beverage Industries; Panda, S., Shetty, P., Eds.; Springer, Cham: Basel, Switzerland. 2018; 159–173.
Crossref - Perricone M, Corbo MR, Sinigaglia M, Speranza B, Bevilacqua A. Viability of Lactobacillus reuteri in fruit juices. J. Funct. Foods. 2014; 10: 421–426.
Crossref - Shukla M, Jha YK, Admassu S. Development of probiotic beverage from whey and pineapple juice. J. Food Proc. Technol. 2013; 4(206): 1-4.
- Tezcan F, Gultekin-Ozguven M, Diken T, Ozcelik B, Erim FB. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009; 115:873–877.
Crossref - Roberts D, Reyes V, Bonilla F, et al. Viability of Lactobacillus plantarum NCIMB 8826 in fermented apple juice under simulated gastric and intestinal conditions. LWT – Food Sci. Technol. 2018; 97:144-150.
Crossref - Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med CellLongev.2016; 7432797.
Crossref - Mattila ST, Myllrinen P, Crittenden R, Mogensen G, Fond R, Saarela M, Technological challenges for future probiotic foods. Int. Dairy J. 2002.12: 173-182.
Crossref - Del PM, Morelli L, Strozzi GP, Allesina S, Barbab M. Probiotics from research to consumer. Dig Liver Dis. 2006; 38: S248–S255.
Crossref - Bailey LH, Bailey EZ. A Concise Dictionary of Plants Cultivated in the U.S. and Canada. Hortus Third “Revised by Staff of the L. H. Bailey Hortium”. The Macmillan Publishing Company. New York. 1976.
- Lester GE. Antioxidant, sugar, mineral, and phytonutrient concentrations across edible fruit tissues of orange fleshed Honeydew melon (Cucumis melo L.). J. Agric. Food Chem. 2008. 56: 3694-3698.
Crossref - Ranadheera C, Vidanarachchi J, Rocha R, Cruz A, Ajlouni S. Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation. 2017; 3(4): 67.
Crossref - Hubbard NL, Huber SC, Pharr DM. Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiol. 1989; 91: 1527-1534.
Crossref - Parveen S, Bushra I, Humaira K, Shazia S, Azhar MA, Value addition: A tool to minimize the postharvest losses in horticultural crops. Greener. J. Agric. Sci. 2014; 4(5):195-198.
Crossref - Prasanna V, Prabha TN, Tharanthan RN. Fruit ripening phenomena- An overview. Crit. Rev. Food Sci. Nutr. 2007; 47: 1-19.
Crossref - Lingle SE, Dunlap JR. Sucrose metabolism in netted muskmelon fruit during development. Plant Physiol.1987; 84: 386- 389.
Crossref - Zhang D. Wang Y. β-amylase in developing apple fruits: activities, amounts and subcellular localization. Sci China C Life Sci. 2002;45(4):429-440.
Crossref - Casterline JR, James L, Carolyne J, Oles YKU. Measurement of sugars and starches in foods by a modification of the AOAC total dietary fiber method. J. AOAC Int.1999; 83:759–765.
Crossref - Anand P, Kularni RS, Policegoudra ASM. Chemical composition and antioxidant activity of sapota (Achras sapota linn.) fruit. J. Food Biochem. 2007; 31:399–414.
Crossref - Peryam DR, Pilgrim FJ. Hedonic scale method of measuring food preferences. Food Technol. 1957; 11: 9–14.
- Gomez KA, Gomez AA. Statistical procedures for agricultural research, 2nd edn. John Wiley and Sons, New York. USA. 1984.
- Sheehan VM, Ross P, Fitzgerald GF. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov Food Sci Emerg Technol. 2007; 8:279–284.
Crossref - Mousavi Z, Mousavi S, Razavi S, Emam DZ, Kiani H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011; 27:123–128.
Crossref - Pakbin B, Razavi SH, Mahmoudi R, Gajarbeygi P. Producing probiotic peach juice. J. Innov. Res. Health Sci. Biotechnol. 2014;1(3): 24683.
Crossref - Reddy LV, Min JH, Wee YJ. Production of probiotic mango juice by fermentation of lactic acid bacteria. J. Microbiol. Biotechnol. 2015. 43: 120-125.
Crossref - Tabasco R, Sanchez PF, Monagas M, Bartolome B, Moreno AMV., Pelaez C, Requena T. Effect of grape polyphenols on lactic acid bacteria and Bifidobacteria growth: resistance and metabolism. Food Microbiol. 2011; 28(7): 1345-1352.
Crossref - Hou JW, Yu RC, Chou CC. Change in some components of soymilk during fermentation with Bifidobacteria. Int. Food Res. J. 2000; 33:393–397.
Crossref - Lester GE, Hodges DM. Antioxidants associated with fruit senescence and human health: Novel orange fleshed non-netted honey dew melon genotype comparisons following different seasonal productions and cold storage durations. Postharvest Biol. Technol. 2008; 48: 347-354.
Crossref - Saccaro DM, Tamime AY, Pilleggi ALOPS, Oliveira MN. The viability of three probiotic organisms grown with yoghurt starter cultures during storage for 21 days at 4ºC. Int. J. Dairy Technol. 2009; 62(3):397-404.
Crossref - Mantzourani I, Nouska C, Terpou A, et al. Production of a novel functional fruit beverage consisting of cornelian cherry juice and probiotic bacteria. Antioxidants.2018; 7(11):163.
Crossref - Wijayanti ED, Setiawan NCE, Cristi JP. Effect of lactic acid fermentation on total phenolic content and antioxidant activity of fig fruit juice (Ficus carica). Adv. Health Sci. Res. 2017; 2: 282-289.
Crossref - Daneshi M, Ehsani MR, Razavi SH, Labbafi M. Effect of refrigerated storage on the probiotic survival and sensory properties of milk/carrot juice mix drink. Electron. J. Biotechnol.2013; 16(5).
Crossref - Yoon KY, Woodams EE, Hang YD. Probiotication of tomato juice by lactic acid bacteria. J. Microbiol. 2004; 42 (4) 315-318.
- Sutton KH. Considerations for the successful development and launch of personalised nutrigenomic foods. Mutat. Res. 2007; 622: 117–121.
Crossref - Vasudha S. Mishra HN. Non dairy probiotic beverages. Intl. Food Res. J. 2013; 20(1):7.
- Nualkaekul S. Charalampopoulos D. Survival of Lactobacillus plantarum in model solutions and fruit juices. Int. J. Food Microbiol. 2011. 146: 111–117.
Crossref - Corbo MR, Bevilacqua A, Petruzzi L, Casanova FP, Sinigaglia M. Functional beverages: The emerging side of functional foods commercial trends, research, and health implications. Compr. Rev. Food Sci. Food Saf. 2014; 13:1192–1206.
Crossref - Tripathi MK, Giri SK. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods. 2014; 9: 225–241.
Crossref - Mortazavian AM, Ehsani MR, Mousavi SM, Rezaei K, Sohrabvandi S, Reinheimer JA, Effect of refrigerated storage temperature on the viability of probiotic microorganisms in yogurt. Int. J. Dairy Technol. 2007; 60 (2):123-127.
Crossref - Hoppe C, Larsen CN, Fondén R, Commercially available human probiotic microorganisms. In: Handbook of Probiotics and Prebiotics. John Wiley & Sons, Inc. 2008; 441-445.
Crossref - Chiranjivi S, Grewal KS, Sharma HK. Effect of incorporation of carrot juice in the preparation of flavoured milk. J. Food Sci. Technol. 2006; 43(1): 80-82.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.